Abstract
Aim: Although recent advances in endovascular devices have markedly improved clinical outcomes of femoropopliteal endovascular therapy, lesions located in the popliteal artery are still a major challenge. This study aimed to determine the association of cardiovascular risk factors, including smoking, diabetes mellitus, and dialysis-dependent renal failure, with the location of atherosclerotic lesions in femoropopliteal artery disease.
Methods: We used a multicenter prospective study database registering patients with symptomatic femoropopliteal artery disease undergoing drug-coated balloon treatment. The analysis included 1912 patients withde novo femoropopliteal lesions. The association of clinical characteristics with popliteal lesions was investigated using the logistic regression model. In addition, the femoropopliteal artery was divided into six segments (the proximal, middle, and distal portions of the superficial femoral artery and P1, P2, and P3 segments of the popliteal artery), and the association of clinical characteristics with the presence of atherosclerotic lesions in the respective arterial segments was investigated.
Results: Smoking and dialysis-dependent renal failure showed a statistically significant inverse and positive association with the presence of popliteal lesions, respectively (adjusted odds ratio, 0.66 [95% confidence interval, 0.51–0.85] and 2.01 [1.62–2.49];P=0.001 andP<0.001), whereas diabetes mellitus did not (P=0.17). The subsequent per-segment analysis presented similar results.
Conclusions: Smoking was inversely associated with popliteal lesions, whereas renal failure on dialysis was positively associated in patients with symptomatic femoropopliteal artery disease who underwent drug-coated balloon treatment. Diabetes mellitus was not significantly associated.
Keywords: Femoropopliteal artery disease, Popliteal lesion, Smoking, Diabetes mellitus, Renal failure on dialysis
See editorial vol. 30: 1305-1306
Introduction
Recent advances in endovascular devices have markedly improved clinical outcomes of femoropopliteal endovascular therapy 1 - 5) . However, lesions located in the popliteal artery are still a major challenge. Although stenting strategies have demonstrated superior clinical outcomes to conventional plain balloon angioplasty in the superficial femoral artery (SFA) 6 - 8) , the popliteal artery, doomed to be biomechanically affected by repetitive knee flexions, is conventionally avoided for stent deployment for fear of stent fracture, in-stent restenosis, and reocclusion 9 , 10) . Furthermore, the popliteal segment has a smaller vessel diameter, which is disadvantageous for patency after angioplasty, even with drug-coated balloons (DCBs). Patients with popliteal artery disease are labeled as a challenging population in femoropopliteal endovascular therapy. However, the differences in clinical features between femoropopliteal artery diseases with and without popliteal lesions remain unclear.
It is well recognized that some cardiovascular risk factors are correlated with a lower-extremity atherosclerosis distribution pattern; the most familiar examples are smoking, diabetes mellitus, and dialysis 11 - 13) . Smokers are likely to have aortoiliac lesions rather than infrapopliteal lesions, whereas patients with diabetes mellitus and those on dialysis often have infrapopliteal lesions rather than aortoiliac lesions 11 - 13) . It has been roughly illustrated that smoking is related to more proximal lesion distribution and that diabetes mellitus and renal failures are related to more distal lesion distribution. However, it remains unknown whether this simplified illustration, that is, linking smoking with proximal lesions and linking diabetes mellitus and renal failure with more distal lesions, would be true of the lesion distribution pattern within the femoropopliteal artery. We hypothesized that smoking would be inversely associated with the involvement of the popliteal artery in femoropopliteal lesions. In contrast, diabetes mellitus and dialysis-dependent renal failure would be positively associated with involvement.
Aim
This study aimed to determine the association of cardiovascular risk factors, including smoking, diabetes mellitus, and dialysis-dependent renal failure, with the location of atherosclerotic lesions in femoropopliteal artery disease.
Methods
Study Population
We used the database of the PrOsPective multiCenter registry Of dRug-coated ballooN for femoropopliteal disease (POPCORN). POPCORN is an ongoing prospective multicenter observational study that registered a total of 2507 adult patients (≥ 20 years) undergoing DCB treatment for femoropopliteal lesions (either de novo or restenotic) of symptomatic lower-extremity artery disease (Rutherford category 2–5) at 64 cardiovascular centers across Japan. The patients were registered between March 2018 and December 2019. The study was conducted in accordance with the Declaration of Helsinki and was approved by the institutional review boards of the participating centers. Informed consent was obtained from the participants or, if not possible, from their families. After excluding 595 patients with restenosed femoropopliteal lesions (i.e., with a history of revascularization) from the 2507 registered patients, this study included the remaining 1912 patients with de novo femoropopliteal lesions (i.e., without any history of revascularization). All patients were diagnosed with atherosclerotic lower-extremity artery disease. Patients with acute limb ischemia 14) were not included. In patients with bilateral limb involvement, the limb that was first registered was selected as the representative limb.
Definitions
The location of lesions was evaluated based on angiography before endovascular revascularization, and involvement of the popliteal artery was determined. The femoropopliteal artery was further divided into the following six segments, including three portions of the SFA and three of the popliteal artery: (1) the proximal SFA segment; (2) the middle SFA segment; (3) the distal SFA segment; (4) the first popliteal (P1) segment (the popliteal artery from the adductor hiatus to the proximal edge of the patella); (5) the second popliteal (P2) segment (the popliteal artery from the proximal edge of the patella to the center of the knee joint space); and (6) the third popliteal (P3) segment (the popliteal artery from the center of the knee joint space to the bifurcation of the anterior tibial artery and tibioperoneal trunk) 15) .
The determination of cardiovascular risk factors was based on the clinical diagnosis according to domestic clinical guidelines. In brief, the presence of hypertension was defined as (1) having antihypertensive treatment, (2) systolic blood pressure of ≥ 140 mmHg, or (3) diastolic blood pressure of ≥ 90 mmHg 16) . Dyslipidemia was defined as (1) having antihyperlipidemic treatment, (2) triglyceride levels of ≥ 150 mg/dl, (3) low-density lipoprotein cholesterol levels of ≥ 140 mg/dl, (4) high-density lipoprotein cholesterol levels of <40 mg/dl, or (5) non-high-density lipoprotein cholesterol levels of ≥ 170 mg/dl 17) . Diabetes mellitus was defined as (1) having antidiabetic treatment, (2) fasting plasma glucose levels of ≥ 126 mg/dl, (3) casual plasma glucose levels of ≥ 200 mg/dl, or (4) hemoglobin A1c levels of ≥ 6.5% 18) . Dialysis dependence, that is, end-stage renal disease on dialysis, included both hemodialysis and peritoneal dialysis. Old age was defined as age of ≥ 75 years 19) . The body mass index was calculated as the weight in kilograms divided by the height in meter squared. The cutoff value of 25 kg/m2 was used to refer to excessive body weight (overweight) in a Japanese population 20 , 21) . Smoking was judged by whether patients currently smoked.
Statistical Analyses
Data are presented as the means±standard deviations or medians (interquartile ranges) for continuous variables and as counts (percentages) for categorical variables, unless otherwise mentioned. A two-sided P value of <0.05 was considered statistically significant. Baseline characteristics were compared between patients with and without popliteal lesions using Welch’s t test for continuous variables, the Mann–Whitney U test for ordinal variables, and the chi-squared test for other discrete variables.
The association of clinical characteristics with popliteal lesions was investigated using the logistic regression model. Their association with lesions in respective arterial segments was also investigated using the logistic regression model. These associations of clinical characteristics with arterial lesions were presented as odds ratios and 95% confidence intervals (CIs). The trend of the odds ratios in the six segments of the femoropopliteal artery was assessed by a linear relationship between arterial segments (consecutively numbered from 1 [representing proximal SFA] to 6 [representing P3]) and their corresponding log-transformed odds ratios, which was statistically tested using 2,000 bootstrapping iterations. The association of clinical characteristics with aortoiliac lesions and the number of diseased infrapopliteal runoffs (the anterior and posterior tibial arteries and the peroneal artery) was analyzed using the logistic regression model and the cumulative link model with a logit link function, respectively, to supplementally check whether this study population could reproduce this classical association. All statistical analyses were performed using R version 4.1.1 (R Development Core Team, Vienna, Austria).
Results
The clinical characteristics of the study population are summarized in Table 1 . The prevalence of smoking, diabetes mellitus, and renal failure on dialysis was 21.7%, 64.6%, and 27.5%, respectively. A total of 687 patients (35.9%) had popliteal lesions. This sample size was calculated to be sufficient to detect an adjusted odds ratio of 1.5 (or its reciprocal 1/1.5=0.67) between smoking, diabetes mellitus, or dialysis-dependent renal failure and the presence of popliteal lesions, with a statistical power of more than 90% (to be exact, 92.0%, 97.5%, and 95.7%, respectively), under an assumption of the observed prevalence and correlation among covariates. Patients with popliteal lesions were more likely to be female, less likely to smoke, and more likely to have renal failure requiring dialysis ( Table 1 ) .
Table 1. Clinical characteristics of the study population.
| Overall population (n=1912) | Patients with popliteal lesions (n=687) | Patients without popliteal lesions (n=1225) | P values | |
|---|---|---|---|---|
| Age (years) | 75±9 | 75±9 | 74±9 | 0.16 |
| Old age (≥ 75 years) | 1029 (53.8%) | 383 (55.7%) | 646 (52.7%) | 0.22 |
| Female sex | 657 (34.4%) | 289 (42.1%) | 368 (30.0%) | <0.001 |
| Overweight | 435 (22.8%) | 140 (20.4%) | 295 (24.1%) | 0.072 |
| Smoking | 414 (21.7%) | 107 (15.6%) | 307 (25.1%) | <0.001 |
| Hypertension | 1625 (85.0%) | 580 (84.4%) | 1045 (85.3%) | 0.65 |
| Dyslipidemia | 1618 (84.6%) | 568 (82.7%) | 1050 (85.7%) | 0.089 |
| Diabetes mellitus | 1235 (64.6%) | 457 (66.5%) | 778 (63.5%) | 0.20 |
| Renal failure on dialysis | 526 (27.5%) | 255 (37.1%) | 271 (22.1%) | <0.001 |
| Symptom of peripheral artery disease | <0.001 | |||
| Intermittent claudication | 1341 (70.1%) | 372 (54.1%) | 969 (79.1%) | |
| Rest pain | 176 (9.2%) | 91 (13.2%) | 85 (6.9%) | |
| Tissue loss | 395 (20.7%) | 224 (32.6%) | 171 (14.0%) | |
| Ankle brachial index | 0.61±0.23 | 0.58±0.26 | 0.64±0.21 | <0.001 |
| Aortoiliac lesion | 411 (21.8%) | 118 (17.5%) | 293 (24.2%) | 0.001 |
| Femoropopliteal lesion | ||||
| Proximal segment of the superficial femoral artery | 843 (44.1%) | 182 (26.5%) | 661 (54.0%) | <0.001 |
| Middle segment of the superficial femoral artery | 1214 (63.5%) | 263 (38.3%) | 951 (77.6%) | <0.001 |
| Distal segment of the superficial femoral artery | 1165 (60.9%) | 463 (67.4%) | 702 (57.3%) | <0.001 |
| First popliteal (P1) segment | 587 (30.7%) | 587 (85.4%) | 0 (0.0%) | <0.001 |
| Second popliteal (P2) segment | 427 (22.3%) | 427 (62.2%) | 0 (0.0%) | <0.001 |
| Third popliteal (P3) segment | 211 (11.0%) | 211 (30.7%) | 0 (0.0%) | <0.001 |
| Popliteal segment (from P1 to P3) | 687 (35.9%) | 687 (100.0%) | 0 (0.0%) | <0.001 |
| Infrapopliteal lesion | <0.001 | |||
| No diseased infrapopliteal runoff | 395 (20.7%) | 95 (13.8%) | 300 (24.6%) | |
| 1 diseased infrapopliteal runoff | 664 (34.8%) | 206 (30.0%) | 458 (37.5%) | |
| 2 diseased infrapopliteal runoffs | 611 (32.0%) | 269 (39.2%) | 342 (28.0%) | |
| 3 diseased infrapopliteal runoffs | 237 (12.4%) | 117 (17.0%) | 120 (9.8%) |
Data are presented as mean±standard deviation, median (interquartile range), or counts (percentages). P values are for the difference between the groups with and without popliteal lesions. Data were missing on ankle brachial index in 35 patients (1.8%), on aortoiliac lesion in 26 patients (1.4%), and on infrapopliteal lesion in 5 patients (0.3%).
As shown in Table 2 , smoking was inversely associated with the presence of popliteal lesions, with an adjusted odds ratio of 0.66 (95% CI, 0.51–0.85; P=0.001), whereas female sex and renal failure on dialysis were positively associated with the presence of lesions, with adjusted odds ratios of 1.64 (95% CI, 1.34–2.01; P<0.001) and 2.01 (95% CI, 1.62–2.49; P<0.001), respectively. Diabetes mellitus was not significantly associated with popliteal lesions, with an adjusted odds ratio of 1.16 (95% CI, 0.94–1.43; P=0.17).
Table 2. Association of clinical characteristics with popliteal lesion.
| Unadjusted odds ratio | Adjusted odds ratio | |
|---|---|---|
| Old age | 1.13 [0.94–1.36] (P= 0.20) | 1.10 [0.90–1.34] (P= 0.36) |
| Female sex | 1.69 [1.39–2.05] (P<0.001) | 1.64 [1.34–2.01] (P<0.001) |
| Overweight | 0.81 [0.64–1.01] (P= 0.064) | 0.83 [0.66–1.05] (P= 0.12) |
| Smoking | 0.55 [0.43–0.70] (P<0.001) | 0.66 [0.51–0.85] (P= 0.001) |
| Hypertension | 0.93 [0.72–1.21] (P= 0.60) | 0.88 [0.67–1.15] (P= 0.35) |
| Dyslipidemia | 0.80 [0.62–1.03] (P= 0.078) | 0.86 [0.66–1.12] (P= 0.27) |
| Diabetes mellitus | 1.14 [0.94–1.39] (P= 0.19) | 1.16 [0.94–1.43] (P= 0.17) |
| Renal failure on dialysis | 2.08 [1.69–2.55] (P<0.001) | 2.01 [1.62–2.49] (P<0.001) |
Data are presented as odds ratios [95% confidence intervals] (P values) for the presence of popliteal lesions. Unadjusted odds ratios were derived from the univariate logistic regression model in a variable of interest was entered as the explanatory variable, whereas adjusted odds ratios were derived from the multivariate logistic regression model in which all the variables listed in the table were entered as the explanatory variables.
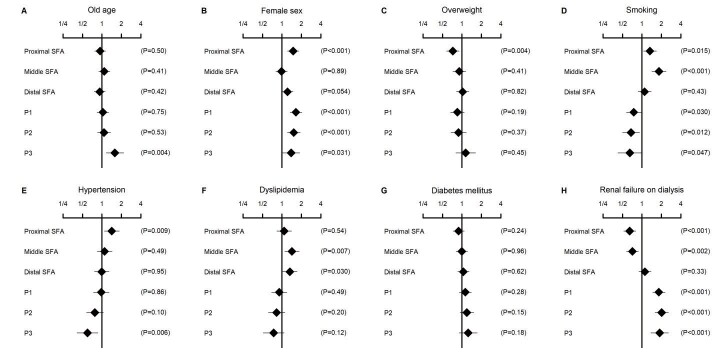
The association of clinical characteristics with more segmented lesion localization is illustrated in Fig.1 . Smoking was positively associated with the lesion involvement of the proximal and middle SFA segments, with adjusted odds ratios of 1.33 (95% CI, 1.06–1.67; P=0.015) and 1.83 (95% CI, 1.43–2.36; P<0.001), respectively; whereas it was inversely associated with the lesion involvement of the P1, P2, and P3 segments, with adjusted odds ratios of 0.75 (95% CI, 0.57–0.97; P=0.030), 0.68 (95% CI, 0.50–0.92; P=0.012), and 0.65 (95% CI, 0.42–0.99; P=0.047), respectively ( Fig.1D ) . In contrast, renal failure on dialysis was inversely associated with the lesion involvement of the proximal and middle SFA segments, with adjusted odds ratios of 0.65 (95% CI, 0.52–0.80; P<0.001) and 0.71 (95% CI, 0.58–0.88; P=0.002), respectively; whereas it was positively associated with the lesion involvement of the P1, P2, and P3 segments, with adjusted odds ratios of 1.82 (95% CI, 1.46–2.27; P<0.001), 2.02 (95% CI, 1.59–2.56; P<0.001), and 1.88 (95% CI, 1.38–2.57; P<0.001), respectively ( Fig.1H ) . Other significant associations were between old age and more involvement of the P3 segment ( Fig.1A ) , between female sex and more involvement of the proximal SFA, P1, P2, and P3 segments ( Fig.1B ) , between overweight and less involvement of the proximal SFA segment ( Fig.1C ) , between hypertension and more involvement of the proximal SFA segment but less involvement of the P3 segment ( Fig.1E ) , and between dyslipidemia and more involvement of the middle and distal SFA segments ( Fig.1F ) (all P<0.05). Diabetes mellitus was not significantly associated with lesions in any of the segments (all P>0.05) ( Fig.1G ) . Neither was the trend of the odds ratios in the six segments statistically significant (P for trend=0.13); the fold increase of the odds ratio per-segment was 1.07 (95% CI, 0.98–1.16). The fold increase to the fifth power, corresponding to the difference in the odds ratio between the proximal SFA and P3, was calculated to be 1.39 (95% CI, 0.90–2.13).
Fig.1. Association of clinical characteristics with affected femoropopliteal segments.
Data are adjusted odds ratios of clinical characteristics (old age [A], male sex [B], overweight [C], smoking [D], hypertension [E], dyslipidemia [F], diabetes mellitus [G], and renal failure on dialysis [H]) for the presence of arterial lesions in each femoropopliteal segment. The values were derived from the multivariate logistic regression model, in which all the variables listed in the figure were entered as the explanatory variables. Error bars indicate 95% confidence intervals. SFA, superficial femoral artery.
We supplementally checked the association of clinical characteristics with aortoiliac and infrapopliteal lesions in this study population ( Table 3 ) . Smoking was positively associated with aortoiliac lesions (adjusted odds ratio, 1.36 [95% CI, 1.04–1.78; P=0.023]) and was inversely associated with infrapopliteal lesions (adjusted odds ratio, 0.81 [95% CI, 0.66–0.997]; P=0.047). On the other hand, both diabetes mellitus and renal failure on dialysis were inversely associated with aortoiliac lesions (adjusted odds ratio, 0.61 [95% CI, 0.48–0.77] and 0.68 [95% CI, 0.51–0.89]; P<0.001 and P=0.005, respectively) and were positively associated with infrapopliteal lesions (adjusted odds ratio, 1.24 [95% CI, 1.03–1.48] and 2.14 [95% CI, 1.77–2.59]; P=0.019 and P<0.001, respectively).
Table 3. Association of clinical characteristics with aortoiliac and infrapopliteal lesions.
| Adjusted odds ratio for aortoiliac lesion | Adjusted odds ratio for infrapopliteal lesion | |
|---|---|---|
| Old age | 0.86 [0.68–1.09] (P= 0.21) | 1.84 [1.55–2.19] (P<0.001) |
| Female sex | 0.70 [0.55–0.90] (P= 0.006) | 1.21 [1.01–1.44] (P= 0.034) |
| Overweight | 0.62 [0.46–0.83] (P= 0.001) | 0.93 [0.76–1.13] (P= 0.46) |
| Smoking | 1.36 [1.04–1.78] (P= 0.023) | 0.81 [0.66–0.997] (P= 0.047) |
| Hypertension | 1.48 [1.06–2.07] (P= 0.021) | 1.09 [0.86–1.37] (P= 0.48) |
| Dyslipidemia | 1.10 [0.80–1.51] (P= 0.55) | 0.99 [0.79–1.25] (P= 0.95) |
| Diabetes mellitus | 0.61 [0.48–0.77] (P<0.001) | 1.24 [1.03–1.48] (P= 0.019) |
| Renal failure on dialysis | 0.68 [0.51–0.89] (P= 0.005) | 2.14 [1.77–2.59] (P<0.001) |
Data are presented as adjusted odds ratios [95% confidence intervals] (P values) for the presence of aortoiliac lesion and the number of diseased infrapopliteal runoffs, derived from the logistic regression model and the cumulative link model with a logit link function, respectively. The unadjusted odds ratios were derived from the univariate model in which a variable of interest was entered as the explanatory variable, whereas the adjusted odds ratios were derived from the multivariate model in which all the variables listed in the table were entered as the explanatory variables.
Discussion
This study demonstrated that smoking and renal failure on dialysis were inversely and positively, respectively, associated with the involvement of the popliteal artery in patients with symptomatic femoropopliteal artery disease undergoing DCB treatment. In contrast, diabetes mellitus had no significant specificity with regard to lesion localization in femoropopliteal artery disease. The subsequent per-segment analyses confirmed that smoking was related to the more proximal lesions, whereas renal failure on dialysis was related to the more distal lesions. Diabetes mellitus was again not associated with the involvement of any arterial segments. To the best of our knowledge, this is the first report investigating the association between cardiovascular risk factors and the distribution pattern of atherosclerotic lesions in femoropopliteal artery disease.
Previous studies on the distribution pattern of atherosclerotic lesions in the lower-extremity arteries, based on a rough division of the arteries into three parts, that is, aortoiliac, femoropopliteal, and infrapopliteal arteries, reported that smoking was linked with aortoiliac rather than infrapopliteal disease, whereas diabetes mellitus and dialysis-dependent renal failure were linked with infrapopliteal rather than aortoiliac lesions 11 - 13) . Our supplemental analyses on aortoiliac and infrapopliteal diseases ( Table 3 ) successfully reproduced those conventional findings, which would suggest an unbiased population in this study.
In this population, smoking was inversely associated with popliteal lesions in the femoropopliteal artery, whereas renal failure on dialysis was positively associated with their presence ( Table 2 ) . The subsequent per-segment analyses ( Fig.1 ) also supported our initial hypothesis that smoking was related to the more proximal femoropopliteal lesion distribution, whereas renal failure on dialysis was related to more distal lesions. These findings align with the conventional results based on trichotomy of the lower-extremity arteries 11 - 13) . Compared with the proximal artery, the distal artery presents more typical features of muscular arteries, characterized as little elastin material, predominant smooth muscle in the tunica media, and a scanty subendothelial layer with a prominent internal elastic membrane. Moreover, the relationship of arterial lumen to wall thickness decreases from proximal to distal, altering arterial flow and shear stress 11) . The graded heterogeneity of these histological and hemodynamic components might modify the susceptibility of respective arterial segments to atherosclerosis induced by smoking and dialysis-dependent renal failure. Uremic toxins and micro-inflammation induce vascular smooth muscle cell dysfunction 22 , 23) , which might partially explain atherosclerotic lesions of more distal, muscular arteries in patients with dialysis-dependent renal failure.
In contrast, diabetes mellitus was not significantly associated with popliteal lesions in femoropopliteal artery disease, which seemed contrary to a familiar diabetes–distality relationship proven by the association between diabetes mellitus and infrapopliteal lesions in lower-extremity artery disease 11 - 13) . Although the true mechanisms of the current findings remained unknown, the presence of elastin in the femoropopliteal artery may be the differentiating factor. Compared with the infrapopliteal artery, the femoropopliteal artery, even the popliteal artery, are more likely to be elastic. The presence of elastin, potentially preventing fibrocellular pathology 24) , might protect against atherosclerotic change by diabetes mellitus. The protective effects could be achieved even if only scanty elastin material exists, as in the distal part of the femoropopliteal artery. Another speculation is a potential protective impact of the arterial lumen size. Not only the SFA but also the popliteal artery, which is proximal to the trifurcation, has a larger vessel diameter than the infrapopliteal artery, distal to the trifurcation. The difference in size might cause the different susceptibility to diabetic atherogenesis between the infrapopliteal artery and the popliteal artery. Our finding indicated that in femoropopliteal artery disease, diabetes mellitus had no remarkable specificity with regard to lesion localization.
Female sex was associated with lesions in the proximal SFA, P1, P2, and P3 segments, whereas dyslipidemia was associated with their presence in the other segments (the middle and distal SFA segments). A previous study, assessing arterial wall compliance among the proximal SFA, the distal SFA, and the middle genicular popliteal artery, reported that the distal SFA was less distensible than the other two 25) . The intermediate portion of the femoropopliteal artery might have different elastic properties, and the difference might affect the sex- and lipid-related acceleration of atherosclerosis. On the other hand, the involvement of the P3 segment was related to old age and hypertension. Its anatomical manifestation, characterized as the direct inflow into the branches plus the repetitive exposure to biomechanical forces accompanied by the knee movement, might be relevant. Similarly, the atherosclerotic lesions in the proximal SFA, the involvement of which was related to being overweight and having hypertension, might be dependent on its anatomical manifestation, such as being directly distal to the bifurcation and being subject to biomechanical forces accompanied by the hip movement.
This study indicates that the pathogenesis of atherosclerotic lesions and the contribution by individual cardiovascular risk factors are heterogeneous among arterial segments within the femoropopliteal artery. More research is required to prove the underlying pathological mechanisms, which will promote the understanding of the pathogeneses of femoropopliteal artery disease. Future studies are also warranted to reveal the influence of individual cardiovascular risk factors on atherosclerotic change in each segment of the femoropopliteal artery. Such studies will contribute to establishing practical strategies in the management of cardiovascular risk factors for the primary and secondary prevention of the disease.
Our study had several limitations. First, our data were cross-sectional, and we could not prove causal relationships between cardiovascular risk factors and affected arterial segments. Respective cardiovascular risk factors per se might be complexly linked with one another 26) . Second, details of cardiovascular risk factors 27 - 30) , medications 31 - 34) , and other general conditions 35 , 36) were not analyzed in this study. Third, our study population was limited to patients undergoing femoropopliteal DCB treatment, which would cause a selection bias. Whether similar findings were observed in patients undergoing other revascularization strategies 3) and in symptom-free patients not requiring revascularization 37 , 38) remains unknown. Fourth, this study was conducted in Japan. Future studies in other countries are necessary to validate the findings.
Conclusion
Smoking was inversely associated with popliteal lesions, whereas renal failure on dialysis was positively associated in patients with symptomatic femoropopliteal artery disease who underwent DCB treatment. On the other hand, diabetes mellitus had no significant specificity with regard to lesion localization in femoropopliteal artery disease.
Acknowledgements
The authors would like to thank the collaborators of the POPCORN study and medical staff at the participating centers.
Notice of Grant Support
This study, analyzing a database of the POPCORN study, was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number JP22K08623, which covered the article processing charge. The POPCORN study is sponsored by the Research Association for Lower Limb Artery Revascularization (LIBERAL). However, the funding body played no role in the study’s design, the enrollment of study participants, their treatment during the study period, the data analysis, or the interpretation of the data.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- 1).Aboyans V, Ricco JB, Bartelink MEL, Bjorck M, Brodmann M, Cohnert T, Collet JP, Czerny M, De Carlo M, Debus S, Espinola-Klein C, Kahan T, Kownator S, Mazzolai L, Naylor AR, Roffi M, Rother J, Sprynger M, Tendera M, Tepe G, Venermo M, Vlachopoulos C, Desormais I and Group ESCSD: 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J, 2018; 39: 763-816 [Google Scholar]
- 2).Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RA, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D and Walsh ME: 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 2017; 135: e726-e779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Iida O, Takahara M and Mano T: Evidence-Experience Gap and Future Perspective on the Treatment of Peripheral Artery Disease. J Atheroscler Thromb, 2021; 28: 1251-1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Kawasaki D, Nakata A, Nishian K, Nishimura M, Fujiwara R, Nakata T and Fukunaga M: Impact of Peak Systolic Velocity Ratio after Drug-Coated Balloon for Femoropopliteal Disease: Three-Month Serial Observation Vessel Echo Study. J Atheroscler Thromb, 2022; 29: 1352-1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Soga Y, Takahara M, Iida O, Suzuki K, Mori S, Kawasaki D, Haraguchi K, Yamaoka T and Ando K: Ten-Year Clinical Follow-Up Following Bare-Nitinol Stent Implantation for Femoropopliteal Artery Disease. J Atheroscler Thromb, 2022; 29: 1448-1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Schillinger M, Sabeti S, Loewe C, Dick P, Amighi J, Mlekusch W, Schlager O, Cejna M, Lammer J and Minar E: Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med, 2006; 354: 1879-1888 [DOI] [PubMed] [Google Scholar]
- 7).Krankenberg H, Schlüter M, Steinkamp HJ, Bürgelin K, Scheinert D, Schulte KL, Minar E, Peeters P, Bosiers M, Tepe G, Reimers B, Mahler F, Tubler T and Zeller T: Nitinol stent implantation versus percutaneous transluminal angioplasty in superficial femoral artery lesions up to 10 cm in length: the femoral artery stenting trial (FAST). Circulation, 2007; 116: 285-292 [DOI] [PubMed] [Google Scholar]
- 8).Gray WA, Feiring A, Cioppi M, Hibbard R, Gray B, Khatib Y, Jessup D, Bachinsky W, Rivera E, Tauth J, Patarca R, Massaro J, Stoll HP, Jaff MR and Investigators SS: S.M.A.R.T. self-expanding nitinol stent for the treatment of atherosclerotic lesions in the superficial femoral artery (STROLL): 1-year outcomes. J Vasc Interv Radiol, 2015; 26: 21-28 [DOI] [PubMed] [Google Scholar]
- 9).Chang IS, Chee HK, Park SW, Yun IJ, Hwang JJ, Lee SA, Kim JS, Chang SH and Jung HG: The primary patency and fracture rates of self-expandable nitinol stents placed in the popliteal arteries, especially in the P2 and P3 segments, in Korean patients. Korean J Radiol, 2011; 12: 203-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Cui C, Huang X, Liu X, Li W, Lu X, Lu M, Jiang M and Yin M: Endovascular treatment of atherosclerotic popliteal artery disease based on dynamic angiography findings. J Vasc Surg, 2017; 65: 82-90 [DOI] [PubMed] [Google Scholar]
- 11).Diehm N, Shang A, Silvestro A, Do DD, Dick F, Schmidli J, Mahler F and Baumgartner I: Association of cardiovascular risk factors with pattern of lower limb atherosclerosis in 2659 patients undergoing angioplasty. Eur J Vasc Endovasc Surg, 2006; 31: 59-63 [DOI] [PubMed] [Google Scholar]
- 12).Wasmuth S, Baumgartner I, Do DD, Willenberg T, Saguner A, Zwahlen M and Diehm N: Renal insufficiency is independently associated with a distal distribution pattern of symptomatic lower-limb atherosclerosis. Eur J Vasc Endovasc Surg, 2010; 39: 591-596 [DOI] [PubMed] [Google Scholar]
- 13).Takahara M, Iida O, Kohsaka S, Soga Y, Fujihara M, Shinke T, Amano T, Ikari Y, J EVT and investigators JP: Diabetes mellitus and other cardiovascular risk factors in lower-extremity peripheral artery disease versus coronary artery disease: an analysis of 1,121,359 cases from the nationwide databases. Cardiovasc Diabetol, 2019; 18: 155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Tsujimura T, Takahara M, Iida O, Kohsaka S, Soga Y, Fujihara M, Mano T, Ohya M, Shinke T, Amano T and Ikari Y: In-Hospital Outcomes after Endovascular Therapy for Acute Limb Ischemia: A Report from a Japanese Nationwide Registry [J-EVT Registry]. J Atheroscler Thromb, 2021; 28: 1145-1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Davaine JM, Querat J, Guyomarch B, Brennan MÁ, Costargent A, Chaillou P, Patra P and Goueffic Y: Incidence and the clinical impact of stent fractures after primary stenting for TASC C and D femoropopliteal lesions at 1 year. Eur J Vasc Endovasc Surg, 2013; 46: 201-212 [DOI] [PubMed] [Google Scholar]
- 16).Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, Horio T, Hoshide S, Ikeda S, Ishimitsu T, Ito M, Ito S, Iwashima Y, Kai H, Kamide K, Kanno Y, Kashihara N, Kawano Y, Kikuchi T, Kitamura K, Kitazono T, Kohara K, Kudo M, Kumagai H, Matsumura K, Matsuura H, Miura K, Mukoyama M, Nakamura S, Ohkubo T, Ohya Y, Okura T, Rakugi H, Saitoh S, Shibata H, Shimosawa T, Suzuki H, Takahashi S, Tamura K, Tomiyama H, Tsuchihashi T, Ueda S, Uehara Y, Urata H and Hirawa N: The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res, 2019; 42: 1235-1481 [DOI] [PubMed] [Google Scholar]
- 17).Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, Umemoto S, Egusa G, Ohmura H, Okamura T, Kihara S, Koba S, Saito I, Shoji T, Daida H, Tsukamoto K, Deguchi J, Dohi S, Dobashi K, Hamaguchi H, Hara M, Hiro T, Biro S, Fujioka Y, Maruyama C, Miyamoto Y, Murakami Y, Yokode M, Yoshida H, Rakugi H, Wakatsuki A, Yamashita S, Committee for E and Clinical Management of A: Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. J Atheroscler Thromb, 2018; 25: 846-984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Araki E, Goto A, Kondo T, Noda M, Noto H, Origasa H, Osawa H, Taguchi A, Tanizawa Y, Tobe K and Yoshioka N: Japanese Clinical Practice Guideline for Diabetes 2019. Diabetol Int, 2020; 11: 165-223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Ouchi Y, Rakugi H, Arai H, Akishita M, Ito H, Toba K, Kai I and Joint Committee of Japan Gerontological Society (JGLS) and Japan Geriatrics Society (JGS) on the definition and classification of the elderly: Redefining the elderly as aged 75 years and older: Proposal from the Joint Committee of Japan Gerontological Society and the Japan Geriatrics Society. Geriatr Gerontol Int, 2017; 17: 1045-1047 [DOI] [PubMed] [Google Scholar]
- 20).Examination Committee of Criteria for ‘Obesity Disease’ in J and Japan Society for the Study of O: New criteria for ‘obesity disease’ in Japan. Circ J, 2002; 66: 987-992 [DOI] [PubMed] [Google Scholar]
- 21).Takahara M, Iida O, Soga Y, Kodama A, Terashi H, Azuma N and investigators Ss: Current and Past Obesity in Japanese Patients with Critical Limb Ischemia Undergoing Revascularization. J Atheroscler Thromb, 2021; 28: 44-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Henaut L, Mary A, Chillon JM, Kamel S and Massy ZA: The Impact of Uremic Toxins on Vascular Smooth Muscle Cell Function. Toxins (Basel), 2018; 10: 218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Castillo-Rodriguez E, Pizarro-Sanchez S, Sanz AB, Ramos AM, Sanchez-Nino MD, Martin-Cleary C, Fernandez-Fernandez B and Ortiz A: Inflammatory Cytokines as Uremic Toxins: “Ni Son Todos Los Que Estan, Ni Estan Todos Los Que Son”. Toxins (Basel), 2017; 9: 114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Karnik SK, Brooke BS, Bayes-Genis A, Sorensen L, Wythe JD, Schwartz RS, Keating MT and Li DY: A critical role for elastin signaling in vascular morphogenesis and disease. Development, 2003; 130: 411-423 [DOI] [PubMed] [Google Scholar]
- 25).Tai NR, Giudiceandrea A, Salacinski HJ, Seifalian AM and Hamilton G: In vivo femoropopliteal arterial wall compliance in subjects with and without lower limb vascular disease. J Vasc Surg, 1999; 30: 936-945 [DOI] [PubMed] [Google Scholar]
- 26).Takahara M, Soga Y, Fujihara M, Kawasaki D, Kozuki A and Iida O: Association of Age with Mortality Rate after Femoropopliteal Endovascular Therapy for Intermittent Claudication. J Atheroscler Thromb, 2022; 29: 474-481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Yanaka K, Akahori H, Imanaka T, Miki K, Yoshihara N, Kimura T, Tanaka T, Asakura M and Ishihara M: Relationship Between Lipoprotein(a) and Angiographic Severity of Femoropopliteal Lesions. J Atheroscler Thromb, 2021; 28: 555-561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Tomoi Y, Takahara M, Soga Y, Fujihara M, Iida O, Kawasaki D, Kozuki A and Ando K: Prognostic Value of the CHA2DS2-VASc Score after Endovascular Therapy for Femoral Popliteal Artery Lesions. J Atheroscler Thromb, 2021; 28: 1153-1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Tsubakimoto Y: New Risk Stratification in Patients with Femoropopliteal PAD. Can We Fight Against the Poor Prognosis? J Atheroscler Thromb, 2021; 28: 1128-1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Higashino N, Iida O, Hata Y, Asai M, Masuda M, Okamoto S, Ishihara T, Nanto K, Kanda T, Tsujimura T, Okuno S, Matsuda Y, Takahara M and Mano T: Impact of Longer Hemodialysis Vintage with Higher Serum Phosphorus Level on Clinical Outcomes in Patients with Chronic Limb-Threatening Ischemia Presenting Tissue Loss after Endovascular Therapy. J Atheroscler Thromb, 2022; 29: 370-378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Golledge J and Drovandi A: Evidence-Based Recommendations for Medical Management of Peripheral Artery Disease. J Atheroscler Thromb, 2021; 28: 573-583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Hata Y, Iida O, Okamoto S, Ishihara T, Nanto K, Tsujimura T, Higashino N, Toyoshima T, Kitano I, Tsuji Y, Takahara M and Mano T: Impact of Guideline-Directed Medical Therapy on 10-Year Mortality after Revascularization for Patients with Chronic Limb-Threatening Ischemia. J Atheroscler Thromb, 2022; doi: 10.5551/jat.63773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Wu CK, Lin CH, Yar N, Kao ZK, Yang YB and Chen YY: Long-Term Effectiveness of Cilostazol in Patients with Hemodialysis with Peripheral Artery Disease. J Atheroscler Thromb, 2022; doi: 10.5551/jat.63404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Matsumoto T, Yoshino S, Furuyama T, Morisaki K, Nakano K, Koga JI, Maehara Y, Komori K, Mori M and Egashira K: Pitavastatin-Incorporated Nanoparticles for Chronic Limb Threatening Ischemia: A Phase I/IIa Clinical Trial. J Atheroscler Thromb, 2022; 29: 731-746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Takahara M, Iida O, Soga Y, Azuma N and Nanto S: Clinical Impact of Measures for Frailty Severity in Poor-Risk Patients Undergoing Revascularization for Chronic Limb-Threatening Ischemia. J Atheroscler Thromb, 2022; 29: 221-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Kodama A, Takahara M, Iida O, Soga Y, Terashi H, Kawasaki D, Izumi Y, Mii S, Komori K and Azuma N: Ambulatory Status Over Time after Revascularization in Patients with Chronic Limb-Threatening Ischemia. J Atheroscler Thromb, 2022; 29: 866-880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Ban S, Sakakura K, Jinnouchi H, Taniguchi Y, Tsukui T, Watanabe Y, Yamamoto K, Seguchi M, Wada H and Fujita H: Association of Asymptomatic Low Ankle-Brachial Index with Long-Term Clinical Outcomes in Patients after Acute Myocardial Infarction. J Atheroscler Thromb, 2022; 29: 992-1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Fu X, Qi Y, Han P, Chen X, Jin F, Shen Z, Mou Y, Qi Z, Zhu J, Chen Y, Zhou W, Zheng Y, Zhang Z, Li M and Guo Q: Relationship Between Physical Performance and Peripheral Arterial Diseases in Different Age Groups of Chinese Community-Dwelling Older Adults. J Atheroscler Thromb, 2022; doi: 10.5551/jat.63697 [DOI] [PMC free article] [PubMed] [Google Scholar]