Abstract
Aims: The long-term prognostic value of the bioavailability of L-arginine, an important source of nitric oxide for the maintenance of vascular endothelial function, has not been investigated fully. We therefore investigated the relationship between amino acid profile and long-term prognosis in patients with a history of standby coronary angiography.
Methods: We measured the serum concentrations of L-arginine, L-citrulline, and L-ornithine by high-speed liquid chromatography. We examined the relationship between the L-arginine/L-ornithine ratio and the incidence of all-cause death, cardiovascular death, and major adverse cardiovascular events (MACEs) in 262 patients (202 men and 60 women, age 65±13 years) who underwent coronary angiography over a period of ≤ 10 years.
Results: During the observation period of 5.5±3.2 years, 31 (12%) patients died, including 20 (8%) of cardiovascular death, while 32 (12%) had MACEs. Cox regression analysis revealed that L-arginine/L-ornithine ratio was associated with an increased risk for all-cause death (unadjusted hazard ratio, 95% confidence interval) (0.940, 0.888–0.995) and cardiovascular death (0.895, 0.821–0.965) (p<0.05 for all). In a model adjusted for age, sex, hypertension, hyperlipidemia, diabetes, current smoking, renal function, and log10-transformed brain natriuretic peptide level, cardiovascular death (0.911, 0.839–0.990,p=0.028) retained an association with a low L-arginine/ L-ornithine ratio. When the patients were grouped according to an L-arginine/L-ornithine ratio of 1.16, the lower L-arginine/L-ornithine ratio group had significantly higher incidence of all-cause death, cardiovascular death, and MACEs.
Conclusion: A low L-arginine/L-ornithine ratio may be associated with increased 10-year cardiac mortality.
Keywords: Amino acid, Mortality, Prognosis, Cardiovascular event
See editorial vol. 30: 1311-1312
1.Introduction
Arteriosclerosis increases with age, partly due to lifestyle-related diseases such as hypertension, diabetes, renal dysfunction, and physical dysfunction, and the progression is associated with further cardiovascular events 1 - 5) . Atherosclerosis is caused by endothelial dysfunction due to vascular stiffening and hypercoagulability 6 - 11) . Nitric oxide (NO) plays an important role in the maintenance of vascular endothelial function by alleviating vasoconstriction, vascular smooth muscle cell proliferation, leukocyte adhesion, and platelet aggregation. The sole source of nitrogen for NO is L-arginine, which produces NO via the activity of endogenous NO synthase (NOS). Therefore, endothelial dysfunction due to decreased NO bioavailability is associated with decreased L-arginine bioavailability 12) . L-arginine is obtained from the diet and from the endogenous synthesis and degradation of proteins, and serum L-arginine concentrations are generally maintained at levels sufficient for NO production 13 , 14) .
However, despite normal serum L-arginine concentrations, patients with hypertension, hyperlipidemia, and diabetes mellitus may experience reduced L-arginine bioavailability and associated endothelial dysfunction. This phenomenon has been termed the “arginine paradox” 15) . The global L-arginine bioavailability ratio (GABR) 16) , defined as the ratio of L-arginine levels to the sum of its major metabolite levels (L-arginine / [L-citrulline+L-ornithine]), is an important indicator in a variety of clinical situations, including stable coronary artery disease (CAD) 17 - 24) . Patients with cardiogenic shock due to acute myocardial infarction (AMI) have been reported to have a lower GABR compared with patients with stable CAD 25) . We also previously reported that the GABR is lower in patients with acute coronary syndrome (ACS) 26 - 28) . Thus, many cross-sectional studies have examined amino acid profiles; however, long-term mortality according to the amino acid profile has not been fully investigated.
2.Aim
In this study, we investigated the relationship between the amino acid profile and 10-year mortality in patients with a history of standby coronary angiography.
3.Methods
3.1. Study Patients
We recruited patients who underwent elective cardiac catheterization for suspected or previously diagnosed CAD at the National Defense Medical College (Tokorozawa, Japan) from November 2012 to November 2013 and who had serum amino acid measurements. This study was approved by the Ethics Committee of the National Defense Medical College (no. 1084) and was conducted in accordance with the Declaration of Helsinki. The study protocol was registered on the website of University Hospital Medical Information Network (UMIN) Center in Japan (URL: https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000010380, UMIN000009635). We obtained written informed consent from each patient. The exclusion criteria were age <20 years, ongoing treatment for malignancy, and acute emergent status including acute coronary syndrome and acute heart failure. All patients included in the study were fasting.
3.2. Primary and Secondary Endpoints
The patients were followed prospectively until June 2021. The condition of each patient was assessed from our hospital records by at least two of the authors. The primary endpoints were all-cause death and cardiovascular death. The secondary endpoint was major adverse cardiovascular events (MACEs). We defined MACEs as death from cardiovascular causes, non-fatal myocardial infarction, and non-fatal cerebral stroke.
3.3. Coronary Angiography and Amino Acid Measurements
Coronary angiography was performed using a 4Fr catheter system. We obtained angiograms from four standard projections for each of the right and left coronary arteries. Obstructive stenosis was defined as a narrowing of more than 75% of the lumen; we defined CAD as coronary stenosis in at least one major coronary artery or its branches or a clinical history of myocardial infarction, percutaneous coronary intervention, or coronary artery bypass graft surgery. We collected blood samples in plain tubes from the guide sheath during coronary angiography prior to heparin administration, and we refrigerated the samples immediately. We obtained serum by centrifugation at 3,000 rpm for 10 min at 4℃ and stored the samples at −80℃ until needed for amino acid analysis. Serum L-arginine, L-citrulline, and L-ornithine levels were measured by high-speed liquid chromatography using a Shimadzu RF-20A system (Shimadzu Corporation, Kyoto, Japan) with a Symmetry C18 column (3.9×150 mm, particle size 5 µm; Waters Corp., Milford, MA). The detection method was based on fluorescence derivatization using the AccQ-Fluor™ reagent (Waters Corp.), as previously described 29 , 30) .
3.4. Patient Classification
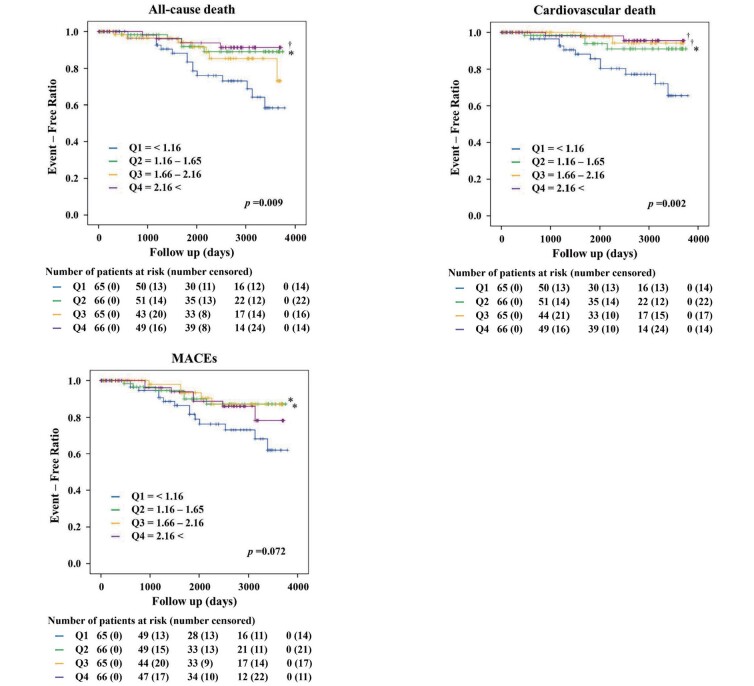
To obtain Kaplan–Meier curves based on survival time analysis, we classified the patients into four groups using the quartile range of the L-arginine/L-ornithine ratio: from Q1 to Q4 for the lowest to the highest quartile range, respectively.
3.5. Coronary Risk Factors
We assessed coronary risk factors using the following definitions. We defined hypertension as blood pressure greater than 140/90 mmHg or a prior diagnosis of hypertension with blood pressure-lowering medication. We defined diabetes mellitus as fasting blood glucose >126 mg/dL or the use of insulin or oral hypoglycemic agents. We defined hyperlipidemia as total cholesterol >220 mg/dL, low-density lipoprotein cholesterol >140 mg/dL, or a prior diagnosis of hyperlipidemia with lipid-lowering medication. We calculated the estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease equation modified for the Japanese population 31 , 32) .
3.6. Statistical Analysis
Summary data are presented as mean±standard deviation and 95% confidence interval (CI) for parametric variables and median (interquartile range) for nonparametric variables. We performed cross-table analysis using the chi-square test or Fisher’s exact test, as appropriate. The comparison of measured variables by four groups was performed using analysis of variance (ANOVA). For nonparametric variables, we used the Kruskal–Wallis test. Residual analysis was performed using Bonferroni’s post-hoc test after ANOVA. In Cox proportional hazards model analysis, we added age and sex as explanatory variables in Model 1. We added hypertension, hyperlipidemia, diabetes mellitus, current smoking, CAD, eGFR, and log10-transformed brain natriuretic peptide level (Log10BNP) to Model 1 for Model 2 because these factors are known to influence vascular endothelial function 33 - 35) . We further added diabetes mellitus, prescription of statin, calcium channel blocker and nitrates, eGFR, and Log10BNP to Model 1 for Model 3 because statins and calcium channel blockers have been reported to improve vascular endothelial function 36 , 37) . We used the Kaplan–Meier method with the log-rank test. We performed most statistical analyses using JMP version 15.0 (SAS Institute, Inc., Cary, NC) or SPSS version 22.0 (SPSS Japan, Tokyo, Japan), while cubic spline analysis was performed using R version 4.1.0 (R Core Team 2020). For all analyses, we considered p<0.05 to be statistically significant. We also considered a two-sided p-value<0.05 to be statistically significant.
4.Results
4.1. Clinical Characteristics of the Patients
We included data from 262 patients (202 men, 60 women; mean age 65±13 years) who underwent elective cardiac catheterization and serum amino acid measurements in the analysis ( Fig.1 ) . Of these patients, 129 (49%) had CAD ( Table 1 ) . The observation period was 5.5±3.2 years. The rank of quartiles of L-arginine/L-ornithine increased with age, CAD, and previous coronary artery bypass surgery. There were differences in eGFR and Log10BNP among the quartile groups.
Fig.1.
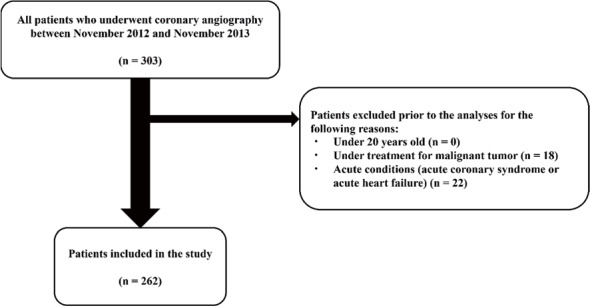
Flowchart of patient inclusion
Table 1. Patient characteristics and laboratory measurements classified by quartiles of the L-arginine/L-ornithine ratio (Q1-Q4).
| L-arginine/L-ornithine | Total (n=280) | Q1 (n=65) | Q2 (n=66) | Q3 (n=65) | Q4 (n=66) | p-value |
|---|---|---|---|---|---|---|
| <1.16 | 1.16 - 1.65 | 1.66 - 2.16 | 2.16< | |||
| Age (years)* | 65±13 | 68±11 | 67±12 | 62±14 | 64±14 | 0.022 |
| Sex (male/female), n (%) | 202/60 (23/77) | 15/50 (23/77) | 14/52 (21/79) | 11/64 (17/83) | 20/46 (30/70) | 0.324 |
| BMI (kg/m2) | 23.5±4.0 | 23.7±3.3 | 23.1±3.5 | 23.4±3.7 | 23.8±5.2 | 0.766 |
| Hypertension, n (%) | 165 (63) | 47 (72) | 45 (68) | 39 (60) | 34 (52) | 0.067 |
| Hyperlipidemia, n (%) | 136 (52) | 41 (63) | 34 (52) | 33 (51) | 28 (42) | 0.129 |
| Diabetes mellitus, n (%) | 88 (34) | 23 (35) | 26 (39) | 19 (29) | 20 (30) | 0.580 |
| Current smoking, n (%) | 75 (29) | 24 (37) | 15 (23) | 20 (31) | 16 (24) | 0.253 |
| Previous conditions | ||||||
| CAD, n (%)*,† | 128 (49) | 39 (60) | 39 (59) | 26 (40) | 24 (36) | 0.007 |
| OMI, n (%) | 47 (18) | 12 (18) | 17 (26) | 9 (14) | 9 (14) | 0.229 |
| CABG, n (%)‡ | 16 (6) | 8 (12) | 5 (8) | 3 (5) | 0 | 0.027 |
| PCI, n (%) | 64 (24) | 17 (26) | 18 (27) | 15 (23) | 14 (21) | 0.843 |
| HCM, n (%) | 27 (10) | 5 (8) | 8 (12) | 9 (14) | 5 (8) | 0.546 |
| DCM, n (%) | 20 (8) | 3 (5) | 4 (6) | 5 (8) | 8 (12) | 0.398 |
| Atrial fibrillation, n (%) | 29 (11) | 9 (14) | 8 (12) | 7 (11) | 5 (8) | 0.704 |
| Medications | ||||||
| Bata-blocker, n (%) | 99 (38) | 28 (43) | 23 (35) | 26 (40) | 22 (33) | 0.635 |
| ACE inhibitor, n (%) | 24 (9) | 7 (11) | 7 (11) | 6 (9) | 4 (6) | 0.770 |
| ARB, n (%) | 121 (46) | 32 (49) | 29 (44) | 25 (38) | 35 (53) | 0.365 |
| Calcium channel blocker, n (%) | 94 (36) | 25 (38) | 28 (42) | 19 (29) | 22 (33) | 0.415 |
| Nitrates, n (%) | 18 (7) | 6 (9) | 8 (12) | 2 (3) | 2 (3) | 0.094 |
| Furosemide, n (%) | 41 (16) | 12 (18) | 9 (14) | 9 (14) | 11 (17) | 0.849 |
| Spironolactone, n (%) | 27 (10) | 9 (14) | 3 (5) | 9 (14) | 6 (9) | 0.237 |
| Statin, n (%) | 126 (48) | 37 (57) | 31 (47) | 34 (52) | 24 (36) | 0.104 |
| Insulin, n (%) | 21 (8) | 5 (8) | 7 (11) | 4 (6) | 5 (8) | 0.818 |
| Warfarin, n (%) | 36 (14) | 14 (22) | 10 (15) | 7 (11) | 5 (8) | 0.109 |
| Hb (g/dL) | 13.4±1.8 | 13.4±1.8 | 13.1±1.8 | 13.7±1.8 | 13.6±1.7 | 0.314 |
| AST (IU/L) | 26±10 | 25±9 | 27±10 | 27±13 | 27±10 | 0.768 |
| ALT (IU/L) | 25±17 | 23±15 | 23±14 | 27±18 | 26±19 | 0.383 |
| LDL cholesterol (mg/dL) | 104±30 | 106±26 | 102±29 | 102±37 | 104±29 | 0.865 |
| TG (mg/dL) | 114 (84. 172) | 127 (79. 185) | 107 (85. 171) | 112 (90. 162) | 114 (77. 164) | 0.212 |
| HDL cholesterol (mg/dL) | 54±17 | 51±12 | 54±16 | 56±17 | 57±21 | 0.179 |
| HbA1c (%) | 5.9±1.0 | 5.7±0.8 | 6.0±1.1 | 6.0±1.2 | 5.9±0.9 | 0.401 |
| Cr (mg/dL)§ | 1.1±1.2 | 1.1±1.1 | 1.5±1.8 | 0.9±0.3 | 0.9±0.7 | 0.020 |
| CRP (mg/dL) | 0.30 (0.30. 0.30) | 0.30 (0.30. 0.50) | 0.30 (0.30. 0.30) | 0.30 (0.30. 0.30) | 0.30 (0.30. 0.30) | 0.930 |
| eGFR (mL/min)‡,§ | 66±22 | 63±24 | 59±23 | 69±18 | 73±23 | 0.001 |
| Log10BNP§ | 1.76±0.66 | 1.76±0.68 | 1.96±0.71 | 1.70±0.63 | 1.63±0.59 | 0.030 |
| Dd (mm) | 50±8 | 48±9 | 51±8 | 51±8 | 49±8 | 0.186 |
| Ds (mm) | 33±10 | 32±9 | 34±11 | 35±11 | 32±9 | 0.331 |
| EF (%) | 61±15 | 62±14 | 60±17 | 59±16 | 62±14 | 0.538 |
| DT (ms) | 218±83 | 230±111 | 200±73 | 215±75 | 227±65 | 0.189 |
| E/E’ | 14.5±8.0 | 13.8±6.4 | 16.2±9.3 | 14.7±10.7 | 13.3±4.2 | 0.285 |
| LAD (mm) | 40±9 | 39±11 | 40±10 | 41±8 | 39±8 | 0.531 |
| L-arginine (μmol/L)‖ | 82.8±24.1 | 76.1±24.5 | 87.0±24.0 | 82.2±22.1 | 85.9±24.9 | 0.041 |
| L-ornithine (μmol/L)§,¶,# | 57.1±29.2 | 90.3±30.8 | 62.2±17.5 | 43.5±12.0 | 32.8±11.0 | <0.001 |
| L-citrulline (μmol/L) | 132.0±75.7 | 142.5±76.0 | 140.6±95.7 | 119.1±55.6 | 125.9±68.6 | 0.220 |
| L-arginine/L-ornithine§,¶,# | 1.73±0.76 | 0.87±0.19 | 1.41±0.15 | 1.90±0.15 | 2.72±0.61 | <0.001 |
| L-arginine/L-citrulline | 0.80±0.48 | 0.66±0.33 | 0.83±0.57 | 0.86±0.51 | 0.83±0.47 | 0.061 |
| GABR§,¶ | 0.50±0.21 | 0.36±0.12 | 0.48±0.18 | 0.56±0.21 | 0.61±0.23 | <0.001 |
Significant p-values are indicated in bold. *p<0.05 Q1,2 vs. Q3; †p<0.05 Q1,2 vs. Q4; ‡p<0.05 Q1 vs. Q4; §p<0.05 Q2 vs. Q3,4; ‖p<0.05 Q1 vs. Q2,4; ¶p<0.01 Q1 vs. Q2,3,4; #p<0.01 Q3 vs. Q4.
ACE, angiotensin-converting enzyme; ALT, alanine aminotransferase; ARB, angiotensin receptor blocker; AST, aspartate aminotransferase; BMI, body mass index; BNP, brain natriuretic peptide; BP, blood pressure; BUN, blood urea nitrogen; CABG, coronary artery bypass graft; CAD, coronary artery disease; Cr, creatinine; CRP, c-reactive protein; DCM, dilated cardiomyopathy; Dd, left ventricular end-diastolic dimension; Ds, left ventricular end-systolic dimension; DT, deceleration time; EF, ejection fraction; eGFR, estimated glomerular filtration rate; GABR, global L-arginine bioavailability ratio; Hb, hemoglobin; HbA1c, hemoglobin A1c; HCM, hypertrophic cardiomyopathy; HDL, high-density lipoprotein; Ht, hematocrit; LAD, left atrial dimension; LDL, low-density lipoprotein; OMI, old myocardial infarction; PCI, percutaneous coronary intervention; TG, triglyceride.
4.2. Association between the Endpoints and Amino Acid Profile
The number of patients with all-cause death, cardiovascular death, or MACEs during the observation period is shown in Table 2 . Thirty-one patients died during the observation period, of which cardiovascular death occurred in 20 patients. MACEs were counted in 32 patients. The lowest ranked group had a higher frequency of death from heart failure and sudden death.
Table 2. All events that occurred in the observation period.
| L-arginine/L-ornithine | Total | 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile |
|---|---|---|---|---|---|
| <1.16 | 1.16 - 1.65 | 1.66 - 2.16 | 2.16< | ||
| All patients, n | 262 | 65 | 66 | 65 | 66 |
| All-cause death, n (%) | 31 (12) | 15 (23) | 5 (8) | 7 (11) | 4 (6) |
| Cancer, n (%) | 6 (2) | 1 (2) | 1 (2) | 2 (3) | 2 (3) |
| Others, n (%) | 5 (2) | 2 (3) | 0 | 3 (5) | 0 |
| Cardiovascular death, n (%) | 20 (8) | 12 (18) | 4 (6) | 2 (3) | 2 (3) |
| Heart failure, n (%) | 7 (3) | 3 (5) | 3 (5) | 1 (2) | 0 |
| Stroke, n (%) | 4 (2) | 2 (3) | 0 | 1 (2) | 1 (2) |
| Aortic dissection, n (%) | 2 (1) | 1 (2) | 0 | 0 | 1 (2) |
| Sudden death, n (%) | 6 (2) | 5 (8) | 1 (2) | 0 | 0 |
| Infective endocarditis, n (%) | 0 | 1 (2) | 0 | 0 | 0 |
| MACEs, n (%) | 32 (12) | 14 (22) | 6 (9) | 5 (8) | 7 (11) |
| Non-fatal AMI, n (%) | 4 (2) | 0 | 1 (2) | 1 (2) | 2 (3) |
| Non-fatal stroke, n (%) | 8 (3) | 2 (3) | 0 | 2 (3) | 4 (6) |
| Survivor of VF, n (%) | 0 | 0 | 1 (2) | 0 | 0 |
There was a patient in Q4 who experienced both non-fatal AMI and non-fatal stroke. AMI, acute myocardial infarction; MACEs, major adverse cardiovascular events.
4.3. Survival Curves
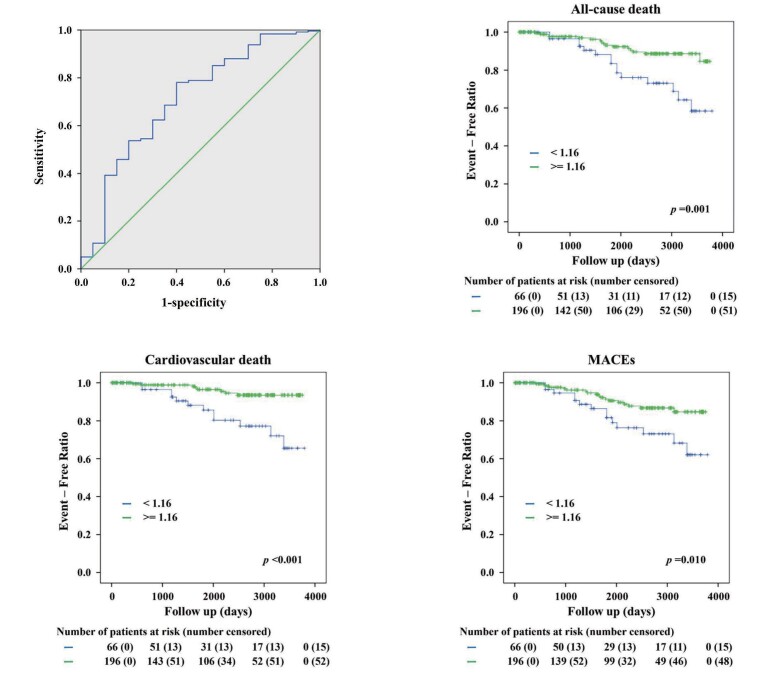
The Kaplan–Meier curves of the quartile groups are shown in Fig.2 . Increased death from all causes and from cardiovascular events were associated with lower L-arginine/L-ornithine ratios. Therefore, dividing the patients into two groups at an L-arginine/L-ornithine ratio of 1.16, which is the highest boundary of the lowest quartile group and suggested to be the most effective cut-off point in the ROC curve, showed that the lower group had a higher incidence of all-cause death, cardiovascular death, and MACEs ( Fig.3 ) .
Fig.2. Kaplan–Meier curves by quartile (Q1–Q4) showing that lower L-arginine/L-ornithine ratios are associated with increased mortality from all-cause death and cardiovascular death.
The p-values in the graphs indicates overall difference among the quartile groups. *p<0.05, †p<0.01 vs. Q1. MACEs, major adverse cardiovascular events.
Fig.3. Optimal L-arginine/L-ornithine ratio (1.16) obtained by receiver-operating characteristic curve; Kaplan–Meier curves classified according to the 1.16 ratio.
For the endpoint of all-cause death, the optimal cut-off value for the L-arginine/L-ornithine ratio was 1.16 (area under the curve 0.657, sensitivity 0.450, specificity 0.854, n=143, p=0.025). This cut-off point was used in the subsequent analyses. Kaplan–Meier curves for two groups classified according to the L-arginine/ L-ornithine ratio of 1.16. The p-values in the graphs indicate the statistical significance of the differences among the groups. MACEs, major adverse cardiovascular events.
4.4. Cox Regression Analysis for the Primary and Secondary Endpoints
The results of the Cox hazard analysis for the L-arginine/L-ornithine ratio for the primary and secondary endpoints are shown in Table 3 . In the univariate Cox analysis, the hazard ratios (HR) (95% CI) were 0.940 (0.888-0.995) for all-cause death and 0.890 (0.821-0.965) for cardiovascular death (p<0.05 for all). This ratio also tended to be associated with the incidence of MACEs but was not significant (p=0.093). In the multivariate Cox analysis of Models 1, 2, and 3, the risk of cardiovascular death decreased with increasing L-arginine/L-ornithine ratio.
Table 3. Unadjusted and adjusted risk for the study endpoints with the L-arginine/L-ornithine ratio.
| Events, n (%) | For All-cause death | For Cardiovascular death | For MACEs | ||||
|---|---|---|---|---|---|---|---|
| 31 (12) | 20 (8) | 32 (13) | |||||
| Scale | |||||||
| L-arginine/L-ornithine | |||||||
| Unadjusted HR (95% CI), p | 0.940 (0.888-0.995) | 0.032 | 0.890 (0.821-0.965) | 0.005 | 0.956 (0.906-1.008) | 0.093 | |
| Adjusted HR (95% CI), p, Model 1 | 0.948 (0.895-1.005) | 0.074 | 0.893 (0.824-0.968) | 0.006 | 0.960 (0.910-1.012) | 0.130 | |
| Adjusted HR (95% CI), p, Model 2 | 0.965 (0.909-1.024) | 0.234 | 0.911 (0.839-0.990) | 0.028 | 0.963 (0.911-1.017) | 0.173 | |
| Adjusted HR (95% CI), p, Model 3 | 0.966 (0.912-1.024) | 0.250 | 0.920 (0.849-0.998) | 0.044 | 0.965 (0.914-1.019) | 0.198 | |
| L-arginine/L-citrulline | |||||||
| Unadjusted HR (95% CI), p | 0.834 (0.724-0.961) | 0.012 | 0.791 (0.651-0.962) | 0.019 | 0.939 (0.846-1.042) | 0.236 | |
| Adjusted HR (95% CI), p, Model 1 | 0.846 (0.726-0.986) | 0.033 | 0.803 (0.650-0.991) | 0.041 | 0.973 (0.870-1.087) | 0.626 | |
| Adjusted HR (95% CI), p, Model 2 | 0.939 (0.815-1.083) | 0.388 | 0.928 (0.769-1.12) | 0.434 | 1.035 (0.933-1.149) | 0.514 | |
| Adjusted HR (95% CI), p, Model 3 | 0.941 (0.816-1.086) | 0.406 | 0.933 (0.775-1.124) | 0.467 | 1.039 (0.935-1.154) | 0.478 | |
| GABR | |||||||
| Unadjusted HR (95% CI), p | 0.704 (0.553-0.896) | 0.004 | 0.598 (0.425-0.842) | 0.003 | 0.850 (0.692-1.043) | 0.119 | |
| Adjusted HR (95% CI), p, Model 1 | 0.723 (0.556-0.941) | 0.016 | 0.597 (0.408-0.871) | 0.008 | 0.897 (0.718-1.121) | 0.341 | |
| Adjusted HR (95% CI), p, Model 2 | 0.888 (0.676-1.165) | 0.390 | 0.784 (0.534-1.151) | 0.214 | 1.036 (0.820-1.309) | 0.768 | |
| Adjusted HR (95% CI), p, Model 3 | 0.894 (0.681-1.173) | 0.418 | 0.813 (0.561-1.176) | 0.271 | 1.043 (0.828-1.315) | 0.719 | |
| Category | |||||||
| L-arginine/L-ornithine (vs. Q4) | |||||||
| Unadjusted HR (95% CI), p | Q3 | 1.854 (0.542-6.348) | 0.325 | 1.087 (0.153-7.728) | 0.934 | 0.716 (0.227-2.258) | 0.569 |
| Q2 | 1.133 (0.303-4.243) | 0.853 | 1.876 (0.342-10.296) | 0.469 | 0.777 (0.260-2.321) | 0.651 | |
| Q1 | 4.048 (1.341-12.221) | 0.013 | 6.517 (1.454-29.199) | 0.014 | 2.026 (0.816-5.029) | 0.128 | |
| (trend) | 0.016 | 0.007 | 0.089 | ||||
| Adjusted HR (95% CI), p, Model 1 | Q3 | 2.297 (0.638-8.275) | 0.203 | 1.766 (0.232-13.471) | 0.583 | 0.946 (0.287-3.117) | 0.927 |
| Q2 | 1.047 (0.275-3.991) | 0.946 | 2.049 (0.365-11.485) | 0.415 | 0.795 (0.261-2.417) | 0.686 | |
| Q1 | 3.760 (1.194-11.843) | 0.024 | 7.685 (1.615-36.575) | 0.010 | 2.091 (0.810-5.398) | 0.127 | |
| (trend) | 0.030 | 0.010 | 0.141 | ||||
| Adjusted HR (95% CI), p, Model 2 | Q3 | 2.346 (0.606-9.073) | 0.217 | 1.822 (0.216-15.385) | 0.581 | 0.934 (0.272-3.207) | 0.914 |
| Q2 | 0.638 (0.154-2.651) | 0.537 | 1.257 (0.201-7.865) | 0.807 | 0.571 (0.179-1.82) | 0.343 | |
| Q1 | 3.016 (0.892-10.192) | 0.076 | 6.320 (1.201-33.258) | 0.030 | 1.996 (0.739-5.386) | 0.173 | |
| (trend) | 0.027 | 0.025 | 0.091 | ||||
| Adjusted HR (95% CI), p, Model 3 | Q3 | 2.193 (0.562-8.550) | 0.258 | 1.523 (0.180-12.859) | 0.699 | 0.802 (0.232-2.771) | 0.727 |
| Q2 | 0.609 (0.142-2.604) | 0.504 | 1.435 (0.214-9.642) | 0.710 | 0.625 (0.190-2.056) | 0.439 | |
| Q1 | 2.650 (0.796-8.818) | 0.112 | 4.989 (0.959-25.950) | 0.056 | 1.756 (0.645-4.784) | 0.271 | |
| (trend) | 0.058 | 0.080 | 0.091 | ||||
| Category | |||||||
| L-arginine/L-ornithine <1.16 | |||||||
| Unadjusted HR (95% CI), p | 3.084 (1.521-6.250) | 0.002 | 4.862 (1.986-11.903) | 0.001 | 2.443 (1.215-4.914) | 0.012 | |
| Adjusted HR (95% CI), p, Model 1 | 2.735 (1.331-5.619) | 0.006 | 4.797 (1.913-12.030) | 0.001 | 2.313 (1.137-4.704) | 0.021 | |
| Adjusted HR (95% CI), p, Model 2 | 2.666 (1.203-5.907) | 0.016 | 4.912 (1.756-13.736) | 0.002 | 2.527 (1.186-5.385) | 0.016 | |
| Adjusted HR (95% CI), p, Model 3 | 2.387 (1.078-5.284) | 0.032 | 3.803 (1.381-10.472) | 0.010 | 2.227 (1.039-4.775) | 0.040 | |
Univariate and multivariate Cox proportional regression analysis of death from any cause, cardiac death, and MACEs. Model 1 adjusted for age and sex. Model 2 includes Model 1 plus hypertension, hyperlipidemia, diabetes mellitus, current smoking, CAD, eGFR, and Log10BNP. Model 3 includes Model 1 plus diabetes mellitus, prescription of statin, calcium channel blocker and nitrates, eGFR, and Log10BNP. Significant p-values are indicated in bold.
BNP, brain natriuretic peptide; CAD, coronary artery disease; CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; MACEs, major adverse cardiovascular events.
In the quartile group, the lowest-ranked group had a significantly higher risk of cardiovascular death compared with the highest-ranked group. In the binary classification, a cutoff point of 1.16 for the L-arginine/L-ornithine ratio was useful for assessing risk for all endpoints. The risk of an L-arginine/L-ornithine ratio less than 1.16 was 2.7 times higher for all-cause death, 4.9 times higher for cardiovascular death, and 2.5 times higher for MACEs compared with the others.
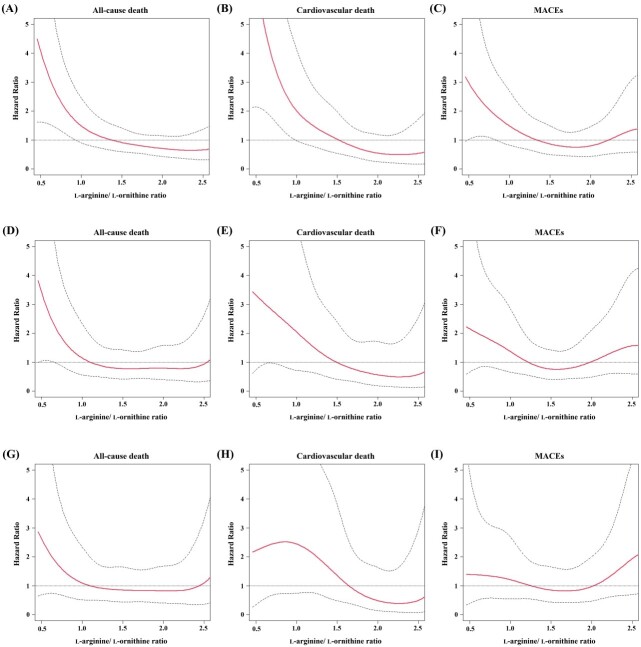
4.5. Cubic Spline Analysis for the Primary and Secondary Endpoints
The results of the cubic spline analysis for the primary and secondary endpoints of the L-arginine/L-ornithine ratio are shown in Fig.4 . In the univariate analysis, HR tended to decrease with increasing L-arginine/L-ornithine ratio for all-cause death, cardiovascular death, and MACEs. In particular, the HR for cardiovascular death decreased with increasing L-arginine/L-ornithine ratio in the multivariate analysis in Model 2.
Fig.4. Cubic spline analysis of the hazard ratio adjusted for the L-arginine/L-ornithine ratio.
The spline curves were described to show the relationships of L-arginine/L-ornithine ratio with hazard ratios for all-cause death (A, D, G), cardiovascular death (B, E, H), and MACEs (C, F, I), respectively. The risks were analyzed in univariate (A-C), multivariate (D-F) adjusting for age, sex, hypertension, hyperlipidemia, diabetes mellitus, current smoking, CAD, eGFR, and Log10BNP, and multivariate (G-I) adjusting for age, sex, diabetes mellitus, prescription of statin, calcium channel blocker and nitrates, eGFR, and Log10BNP. BNP, brain natriuretic peptide; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate; MACEs, major adverse cardiovascular events.
5.Discussion
We found that a low L-arginine/L-ornithine ratio was associated with an increased risk of cardiovascular death after statistical adjustment for the following clinical and laboratory factors: hypertension, hyperlipidemia, diabetes mellitus, current smoking, CAD, eGFR, and Log10BNP. These results suggest that the L-arginine/L-ornithine ratio may be an independent biomarker for assessing long-term mortality from cardiovascular death.
L-arginine is the only source of nitrogen for NO production and is supplied by the body either via the diet or the ornithine circuit. Serum L-arginine concentrations are generally maintained at a level sufficient for NO production 13 , 14) . However, in acute severe conditions (e.g., sepsis), L-arginine concentrations in plasma and intracellular muscle are significantly reduced compared with healthy individuals 38 , 39) . There is no change in plasma L-arginine production in patients with sepsis 40) , suggesting that the decrease in L-arginine concentration in these patients is due to altered arginine metabolism or increased transport across the cell membrane. The arginine transport system is modulated by bacterial endotoxins and inflammatory cytokines, which may upregulate cationic amino acid transporter-2 (CAT-2) and downregulate cationic amino acid transporter-1 (CAT-1) 41 , 42) . As a result, the transport of arginine to inducible NOS-2 is increased, while its transport to NOS-3 (endothelial NOS) is decreased.
In contrast, despite normal L-arginine concentrations in serum, endothelial dysfunction may occur due to the chronically decreased bioavailability of L-arginine 15) . One possible cause is increased arginase activity 43 - 45) . L-arginine is converted to NO and L-citrulline by NOS and to L-ornithine and urea by arginase. Thus, L-arginine is a common substrate for NOS and arginase. Increased arginase activity in endothelial cells may lead to endothelial dysfunction by decreasing the concentration of L-arginine available for NO production. Another possibility may be the decreased bioavailability of arginine by metabolites of L-arginine, given that L-ornithine and L-arginine share the same transporter (CAT-1), which may increase serum L-ornithine levels. When this occurs, L-ornithine competes with L-arginine for CAT-1, thus decreasing the bioavailability of L-arginine to endothelial cells 14) . This deficiency of L-arginine and excess of L-ornithine can have adverse effects such as structural problems in the vasculature, neuronal toxicity, and abnormal growth of tumor cells 46) .
Because there is no net loss of L-arginine and its catabolic metabolites, L-ornithine and L-citrulline, during urea synthesis, a low L-arginine/L-ornithine ratio indicates decreased L-arginine bioavailability with increased arginase activity. In previous cross-sectional studies, arteriosclerosis, CAD, and ACS were found to be strongly associated with the bioavailability of L-arginine 26 , 27 , 47 - 49) . Decreased L-arginine bioavailability is likewise associated with obesity, insulin resistance, and metabolic syndrome 50) . Increased arginase activity causes a relative deficiency of L-arginine 51 , 52) . In addition, recent studies have reported that the inhibition of arginase activity and L-arginine intake increases NO production 44 , 53) . These results may highlight the importance of arginase in endothelial dysfunction.
However, few studies have investigated the effects of relative L-arginine deficiency on long-term mortality. Molek et al. revealed that a decrease in L-arginine concentration was reflective of the clinical impact and severity in AMI patients. A delay in the recovery of L-arginine concentration at 6 months after onset was associated with death, myocardial infarction, or hospital admission for heart failure in the following 5 years 48) . Tang et al. reported that a lower GABR and higher L-citrulline concentrations were associated with an increased risk of developing CAD and MACEs at 3 years 16) . However, to our knowledge, there are no other longer-term studies. Our results suggested that an L-arginine/L-ornithine ratio with a cut-off value of 1.16 was associated with the prognosis of cardiac death up to 10 years. However, the GABR was not associated with such a prognosis in the multivariate analysis including adjustment by eGFR ( Table 3 ) . This may be because serum levels of L-citrulline are more dependent on renal function compared with those of L-arginine and L-ornithine. Another possible mechanism is that renal dysfunction prevents L-citrulline from being metabolized to L-arginine, resulting in an excess of L-citrulline and a decrease in the GABR 54) .
The present study had some limitations that should be considered. The first is the small number of patients. For example, regarding MACEs in this study, most of the events were cardiovascular deaths, whereas there were very few nonfatal AMI and stroke events. As a result, MACEs likely resembled the results for cardiovascular deaths. Therefore, further studies with a larger number of patients are needed. The second limitation is the effect of diurnal variation on amino acid profiles. Circadian rhythms of L-arginine concentrations have been reported to be relatively high in the early morning and to decrease during the day 55) . In the present study, patients underwent cardiac catheterization at different times of the day, but we did not measure time-course changes of the amino acid profiles. The third limitation, which overlaps slightly with the second, is that the amino acid profile was measured only once. The possibility that amino acid profiles may have changed in individual patients during the 10-year prospective follow-up period cannot be ruled out.
6.Conclusion
A low L-arginine/L-ornithine ratio may be associated with increased long-term cardiovascular mortality. Activation of arginase in the urea cycle and decreased bioavailability of L-arginine may also be associated with cardiovascular prognosis.
Acknowledgements
We would like to thank Azusa Onodera of the Department of Internal Medicine, National Defense Medical College, for excellent technical support.
Notice of Gran Support
This work was supported by a grant (to T. Adachi) from the Ministry of Defense of Japan and a grant-in-aid for scientific research from the Ministry of Education, Science, and Culture of Japan (to N. Masaki) (20K08503).
Conflict of Interest
The authors have no conflicts of interest directly relevant to the content of this article.
References
- 1).Wilkinson IB, McEniery CM: Arterial stiffness, endothelial function and novel pharmacological approaches. Clin Exp Pharmacol Physiol, 2004; 31: 795-799 [DOI] [PubMed] [Google Scholar]
- 2).Wang X, Keith JC Jr, Struthers AD, Feuerstein GZ: Assessment of arterial stiffness, a translational medicine biomarker system for evaluation of vascular risk. Cardiovasc Ther, 2008; 26: 214-223 [DOI] [PubMed] [Google Scholar]
- 3).Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE, Carr JJ, Budoff MJ, Allison MA: Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA, 2014; 311: 271-278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Zieman SJ, Melenovsky V, Kass DA: Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol, 2005; 25: 932-943 [DOI] [PubMed] [Google Scholar]
- 5).Brunner EJ, Shipley MJ, Witte DR, Singh-Manoux A, Britton AR, Tabak AG, McEniery CM, Wilkinson IB, Kivimaki M: Arterial stiffness, physical function, and functional limitation: the Whitehall II Study. Hypertension, 2011; 57: 1003-1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Wilkinson IB, Franklin SS, Cockcroft JR: Nitric oxide and the regulation of large artery stiffness: from physiology to pharmacology. Hypertension, 2004; 44: 112-116 [DOI] [PubMed] [Google Scholar]
- 7).McEniery CM, Wallace S, Mackenzie IS, McDonnell B, Yasmin Newby DE, Cockcroft JR, Wilkinson IB: Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension, 2006; 48: 602-608 [DOI] [PubMed] [Google Scholar]
- 8).Namba T, Masaki N, Takase B, Adachi T: Arterial Stiffness Assessed by Cardio-Ankle Vascular Index. Int J Mol Sci, 2019; 20: 3664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Tanaka K, Sueishi K: The coagulation and fibrinolysis systems and atherosclerosis. Lab Invest, 1993; 69: 5-18 [PubMed] [Google Scholar]
- 10).Smith EB, Thompson WD, Crosbie L, Stirk CM: Fibrinogen/fibrin in atherogenesis. Eur J Epidemiol, 1992; 8: 83-87 [DOI] [PubMed] [Google Scholar]
- 11).Naito M, Hayashi T, Kuzuya M, Funaki C, Asai K, Kuzuya F: Effects of fibrinogen and fibrin on the migration of vascular smooth muscle cells in vitro. Atherosclerosis, 1990; 83: 9-14 [DOI] [PubMed] [Google Scholar]
- 12).Förstermann U, Sessa WC: Nitric oxide synthases: regulation and function. Eur Heart J, 2012; 33: 829-837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Luiking YC, Ten Have GA, Wolfe RR, Deutz NE: Arginine denovo and nitric oxide production in disease states. Am J Physiol Endocrinol Metab, 2012; 303: E1177-1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Wu G, Morris SM Jr: Arginine metabolism: nitric oxide and beyond. Biochem J, 1998; 336: 1-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Bode-Böger SM, Scalera F, Ignarro LJ: The L-arginine paradox: Importance of the L-arginine/asymmetrical dimethylarginine ratio. Pharmacol Ther, 2007; 114: 295-306 [DOI] [PubMed] [Google Scholar]
- 16).Tang WH, Wang Z, Cho L, Brennan DM, Hazen SL: Diminished global arginine bioavailability and increased arginine catabolism as metabolic profile of increased cardiovascular risk. J Am Coll Cardiol, 2009; 53: 2061-2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Sourij H, Meinitzer A, Pilz S, Grammer TB, Winkelmann BR, Boehm BO, März W: Arginine bioavailability ratios are associated with cardiovascular mortality in patients referred to coronary angiography. Atherosclerosis, 2011; 218: 220-225 [DOI] [PubMed] [Google Scholar]
- 18).Tripolt NJ, Meinitzer A, Eder M, Wascher TC, Pieber TR, Sourij H: Multifactorial risk factor intervention in patients with Type 2 diabetes improves L-arginine bioavailability ratios. Diabet Med, 2012; 29: 365-368 [DOI] [PubMed] [Google Scholar]
- 19).Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, Hazen SL, Vichinsky EP, Morris Jr SM, Gladwin MT: Dysregulated arginine metabolism, hemolysis associated pulmonary hypertension, and mortality in sickle cell disease. JAMA, 2005; 294: 81-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Shao Z, Wang Z, Shrestha K, Thakur A, Borowski AG, Sweet W, Thomas JD, Moravec CS, Hazen SL, Tang WH: Pulmonary hypertension associated with advanced systolic heart failure: dysregulated arginine metabolism and importance of compensatory dimethylarginine dimethylaminohydrolase-1. J Am Coll Cardiol, 2012; 59: 1150-1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Tang WH, Shrestha K, Wang Z, Troughton RW, Klein AL, Hazen SL: Diminished global arginine bioavailability as a metabolic defect in chronic systolic heart failure. J Card Fail, 2013; 19: 87-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Bersani FS, Wolkowitz OM, Lindqvist D, Yehuda R, Flory J, Bierer LM, Makotine I, Abu-Amara D, Coy M, Renus V, Epel E, Marmar C, Mellon SH: Global arginine bioavailability, a marker of nitric oxide synthetic capacity, is decreased in PTSD and correlated with symptom severity and markers of inflammation. Brain Behav Immun, 2016; 52: 153-160 [DOI] [PubMed] [Google Scholar]
- 23).Ali-Sisto T, Tolmunen T, Viinamäki H, Mäntyselkä P, Valkonen-Korhonen M, Koivumaa-Honkanen H, Honkalampi K, Ruusunen A, Nandania J, Velagapudi V, Lehto SM: Global arginine bioavailability ratio is decreased in patients with major depressive disorder. J Affect Disord, 2018; 15: 145-151 [DOI] [PubMed] [Google Scholar]
- 24).Onmaz DE, Isik K, Sivrikaya A, Abusoglu S, Gezer İA, Abusoglu G, Yerlikaya FH, Unlu A: Determination of serum methylarginine levels by tandem mass spectrometric method in patients with ankylosing spondylitis. Amino Acids, 2021; 53: 1329-1338 [DOI] [PubMed] [Google Scholar]
- 25).Nicholls SJ, Wang Z, Koeth R, Levison B, DelFraino B, Dzavik V, Griffith OW, Hathaway D, Panza JA, Nissen SE, Hochman JS, Hazen SL: Metabolic profiling of arginine and nitric oxide pathways predicts hemodynamic abnormalities and mortality in patients with cardiogenic shock after acute myocardial infarction. Circulation, 2007; 116: 2315-2324 [DOI] [PubMed] [Google Scholar]
- 26).Miyazaki K, Masaki N, Adachi T: Arginine deficiency measured by global arginine bioavailability ratio in patients with acute coronary syndrome. Vasc Fail, 2018; 2: 80-87 [Google Scholar]
- 27).Miyazaki K, Masaki N, Adachi T: Decreased arginine bioavailability in patients with coronary artery disease in an outpatient setting. Vasc Fail, 2019; 3: 31-36 [Google Scholar]
- 28).Miyazaki K, Namba T, Hakuno D, Adachi T: Neurohormonal and metabolic profile of heart failure in obese versus non-obese patients. Vasc Fail, 2020; 4: 16-21 [Google Scholar]
- 29). Heresztyn T, Worthley MI, Horowitz JD: Determination of l-arginine and NG, NG-and NG, NG’ -dimethyl- L-arginine in plasma by liquid chromatography as AccQ-FluorTM fluorescent derivatives. J Chromatogr B Analyt Biomed Life Sci, 2004; B805: 325-329 [DOI] [PubMed] [Google Scholar]
- 30).Teerlink T, Nijveldt RJ, De Jong S, Van Leeuwen PA: Determination of L-arginine, asymmetric dimethyl L-arginine, and symmetric dimethyl L-arginine in human plasma and other biological samples by high-performance liquid chromatography. Anal Biochem, 2002; 303: 131-137 [DOI] [PubMed] [Google Scholar]
- 31).Imai E, Horio M, Nitta K, Yamagata K, Iseki K, Hara S, Ura N, Kiyohara Y, Hirakata H, Watanabe T, Moriyama T, Ando Y, Inaguma D, Narita I, Iso H, Wakai K, Yasuda Y, Tsukamoto Y, Ito S, Makino H, Hishida A, Matsuo S: Estimation of glomerular filtration rate by the MDRD study equitation modified for Japanese patients with chronic kidney disease. Clin Exp Nephrol, 2007; 11: 41-50 [DOI] [PubMed] [Google Scholar]
- 32).Imai E, Horio M, Iseki K, Yamagata K, Watanabe T, Hara S, Ura N, Kiyohara Y, Hirakata H, Moriyama T, Ando Y, Nitta K, Inaguma D, Narita I, Iso H, Wakai K, Yasuda Y, Tsukamoto Y, Ito S, Makino H, Hishida A, Matsuo S: Prevalence of chronic kidney disease (CKD) in Japanese population predicted by the MDRD equation modified by a Japanese coefficient. Clin Exp Nephrol, 2007; 11: 156-163 [DOI] [PubMed] [Google Scholar]
- 33).Panza JA, Quyyumi AA, Brush JE, Epstein SE: Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J M, 1990; 323: 22-27 [DOI] [PubMed] [Google Scholar]
- 34).Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Oshima T, Chayama K: Endothelial function and oxidative stress in renovascular hypertension. N Engl J M, 2002; 346: 1954-1962 [DOI] [PubMed] [Google Scholar]
- 35).Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, Deanfield JE: Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation, 1993; 88: 2149-2155 [DOI] [PubMed] [Google Scholar]
- 36).Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, Sessa WC, Walsh K: The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med, 2000; 6: 1004-1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Dohi Y, Criscione L, Pfeiffer K, Lüscher TF: Angiotensin blockade or calcium antagonists improve endothelial dysfunction in hypertension: studies in perfused mesenteric resistance arteries. J Cardiovasc Pharmacol, 1994; 24: 372-379 [DOI] [PubMed] [Google Scholar]
- 38).Freund H, Atamian S, Holroyde J, Fischer JE: Plasma amino acids as predictors of the severity and outcome of sepsis. Ann Surg, 1979; 190: 571-576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Milewski PJ, Threlfall CJ, Heath DF, Holbrook IB, Irving MH: Intracellular free amino acids in undernourished patients with or without sepsis. Clin Sci (Lond), 1982; 62: 83-91 [DOI] [PubMed] [Google Scholar]
- 40).Argaman Z, Young VR, Noviski N, Castillo-Rosas L, Lu XM, Zurakowski D, Cooper M, Davison C, Tharakan JF, Ajami A, Castillo L: Arginine and nitric oxide metabolism in critically ill septic pediatric patients. Crit Care Med, 2003; 31: 591-597 [DOI] [PubMed] [Google Scholar]
- 41).Reade MC, Clark MF, Young JD, Boyd CAR: Increased cationic amino acid flux through a newly expressed transporter in cells overproducing nitric oxide from patients with septic shock. Clin Sci (Lond), 2002; 102: 645-650 [DOI] [PubMed] [Google Scholar]
- 42).Schwartz D, Schwartz IF, Gnessin E, Wollman Y, Chernichovsky T, Blum M, Iaina A: Differential regulation of glomerular arginine transporters (CAT-1 and CAT-2) in lipopolysaccharide-treated rats. Am J Physiol Renal Physiol, 2003; 284: F788-F795 [DOI] [PubMed] [Google Scholar]
- 43).Toya T, Hakuno D, Shiraishi Y, Kujiraoka T, Adachi T: Arginase inhibition augments nitric oxide production and facilitates left ventricular systolic function in doxorubicin-induced cardiomyopathy in mice. Physiol Rep, 2014; 2: e12130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Pernow J, Jung C: Arginase as a potential target in the treatment of cardiovascular disease: reversal of arginine steal? Cardiovasc Res, 2013; 98: 334-343 [DOI] [PubMed] [Google Scholar]
- 45).Morris SM Jr: Recent advances in arginine metabolism: roles and regulation of the arginases. Br J Pharmacol, 2009; 157: 922-930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Caldwell RW, Rodriguez PC, Toque HA, Narayanan SP, Caldwell RB: Arginase: A Multifaceted Enzyme Important in Health and Disease. Physiol Rev, 2018; 98: 641-665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Shemyakin A, Kövamees O, Rafnsson A, Böhm F, Svenarud P, Settergren M, Jung C, Pernow J: Arginase inhibition improves endothelial function in patients with coronary artery disease and type 2 diabetes mellitus clinical perspective. Circulation, 2012; 126: 2943-2950 [DOI] [PubMed] [Google Scholar]
- 48).Molek P, Zmudzki P, Wlodarczyk A, Nessler J, Zalewski J: The shifted balance of arginine metabolites in acute myocardial infarction patients and its clinical relevance. Sci Rep, 2021; 11: 83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Masaki N, Hakuno D, Toya T, Shiraishi Y, Kujiraoka T, Namba T, Yada H, Kimura K, Miyazaki K, Adachi T: Association between brachial-ankle pulse wave velocity and the ratio of l-arginine to asymmetric dimethylarginine in patients undergoing coronary angiography. J Cardiol, 2015; 65: 311-317 [DOI] [PubMed] [Google Scholar]
- 50).Moon J, Kim OY, Jo G, Shin MJ: Alterations in Circulating Amino Acid Metabolite Ratio Associated with Arginase Activity Are Potential Indicators of Metabolic Syndrome: The Korean Genome and Epidemiology Study. Nutrients, 2017; 9: 740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Bivalacqua TJ, Hellstrom WJ, Kadowitz PJ, Champion HC: Increased expression of arginase II in human diabetic corpus cavernosum: In diabetic-associated erectile dysfunction. Biochem Biophys Res Commun, 2001; 283: 923-927 [DOI] [PubMed] [Google Scholar]
- 52).Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, Caldwell RB, Caldwell RW: Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res, 2008; 102: 95-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).McKnight JR, Satterfield MC, Jobgen WS, Smith SB, Spencer TE, Meininger CJ, McNeal CJ, Wu G: Beneficial effects of l-arginine on reducing obesity: Potential mechanisms and important implications for human health. Amino Acids, 2010; 39: 349-357 [DOI] [PubMed] [Google Scholar]
- 54).Walker V: Ammonia metabolism and hyperammonemic disorders. Adv Clin Chem, 2014; 67: 73-150 [DOI] [PubMed] [Google Scholar]
- 55).Tangphao O, Chalon S, Coulston AM, Moreno H Jr, Chan JR, Cooke JP, Hoffman BB, Blaschke TF: L-arginine and nitric oxide-related compounds in plasma: comparison of normal and arginine-free diets in a 24-h crossover study. Vasc Med, 1999; 4: 27-32 [DOI] [PubMed] [Google Scholar]