Abstract
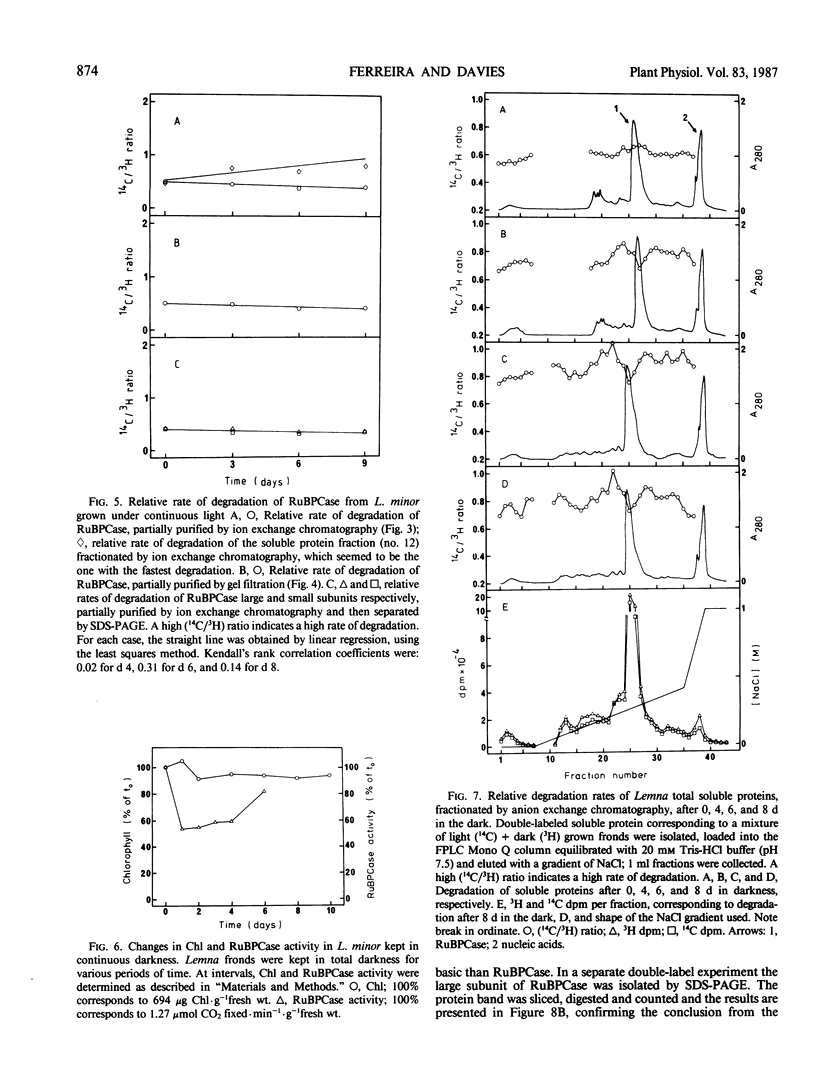
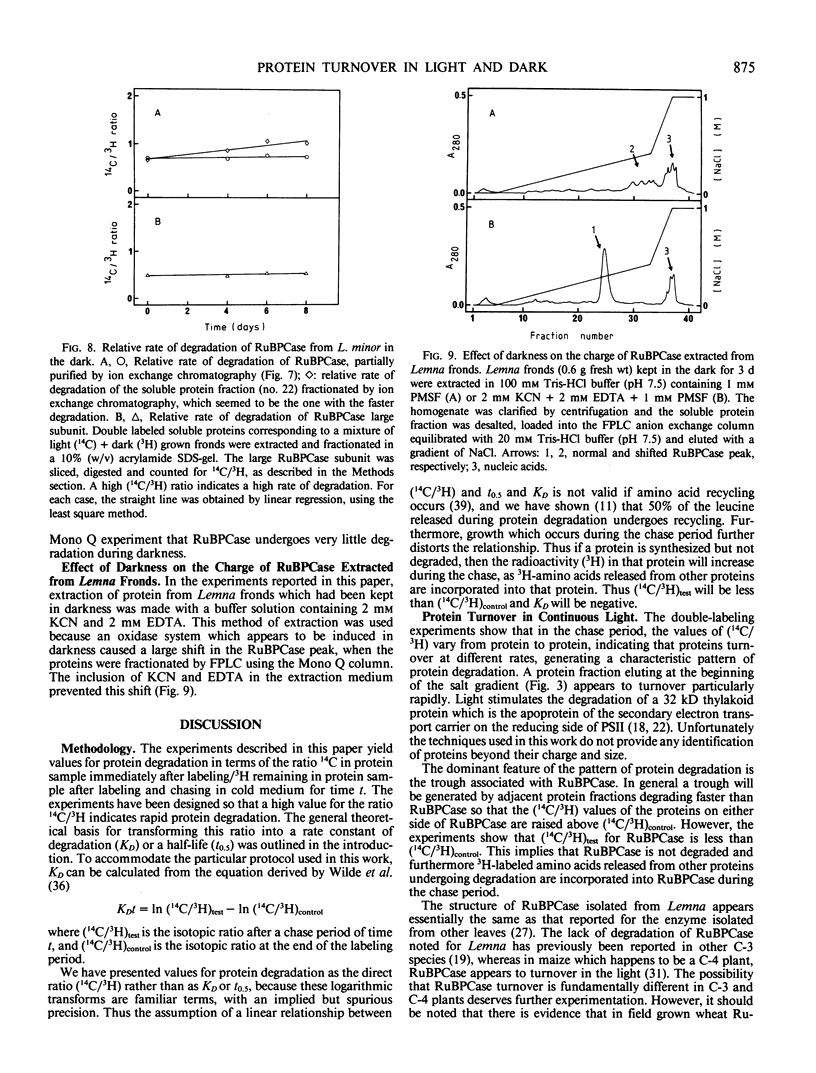
Ribulose bisphosphate carboxylase from Lemna minor resembles the structure reported for the enzyme from other plants. When grown in the light, the enzyme appears to undergo little or no degradation, as measured by a double-isotope method. This situation is similar to that reported for wheat and barley, but is unlike that reported for maize, where the enzyme degrades at the same rate as total protein. Prolonged periods of darkness usually induce leaf senescence, characterized by the rapid degradation of chlorophyll and protein, with ribulose bisphosphate carboxylase undergoing preferential degradation. In L. minor there is selective protein degradation in the dark, but chlorophyll and ribulose bisphosphate carboxylase are stable when fronds are kept in the darkness for up to 8 days. It appears that Lemna is not programmed to senesce, or at least that darkness does not induce senescence in Lemna. Although there is no evidence for in vivo degradation or modification of ribulose bisphosphate carboxylase during prolonged periods of darkness, extracts from fronds which have been kept in the dark for periods in excess of 24 hours convert ribulose bisphosphate carboxylase to a more acidic form. The properties of the dark-induced system which acts on ribulose bisphosphate carboxylase, suggest that it may be a mixed function oxidase. The proposition that the selectivity of protein degradation is genetically determined, so that the rate at which a protein is degraded is determined by its charge or size, was tested for fronds grown in the light or maintained in the dark. There was no significant correlation between protein degradation and either charge or size, in light or dark.
Full text
PDF

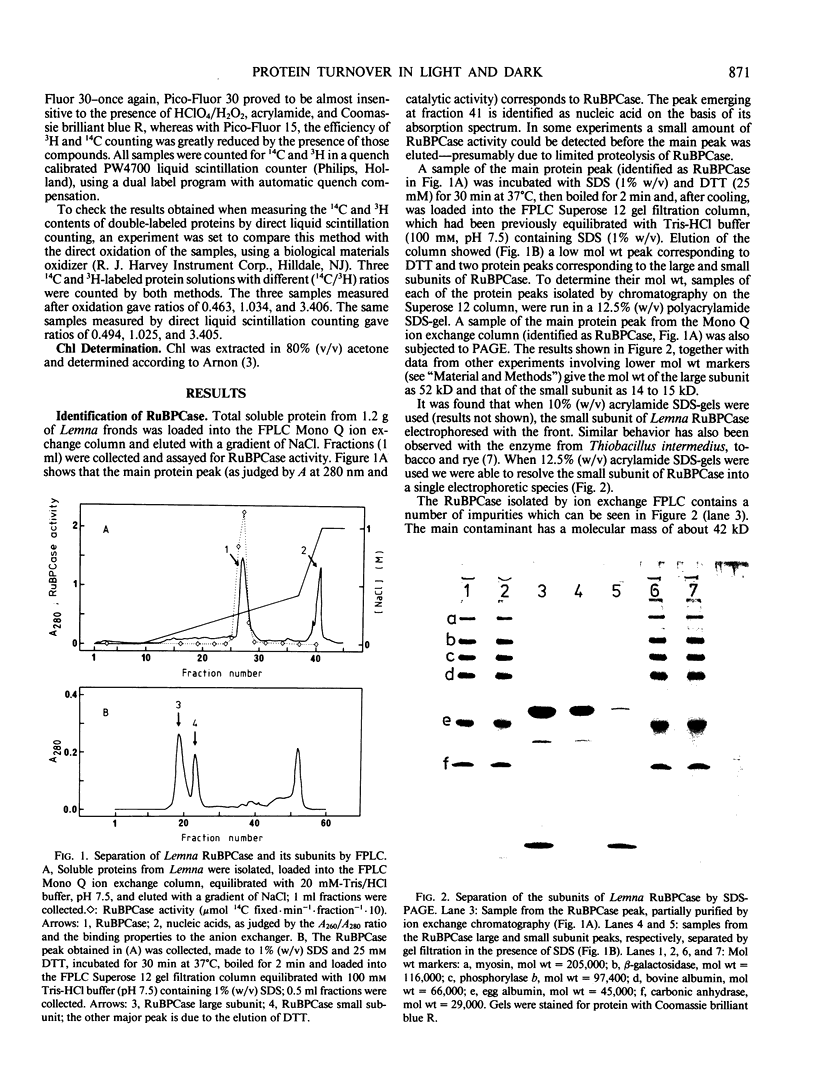
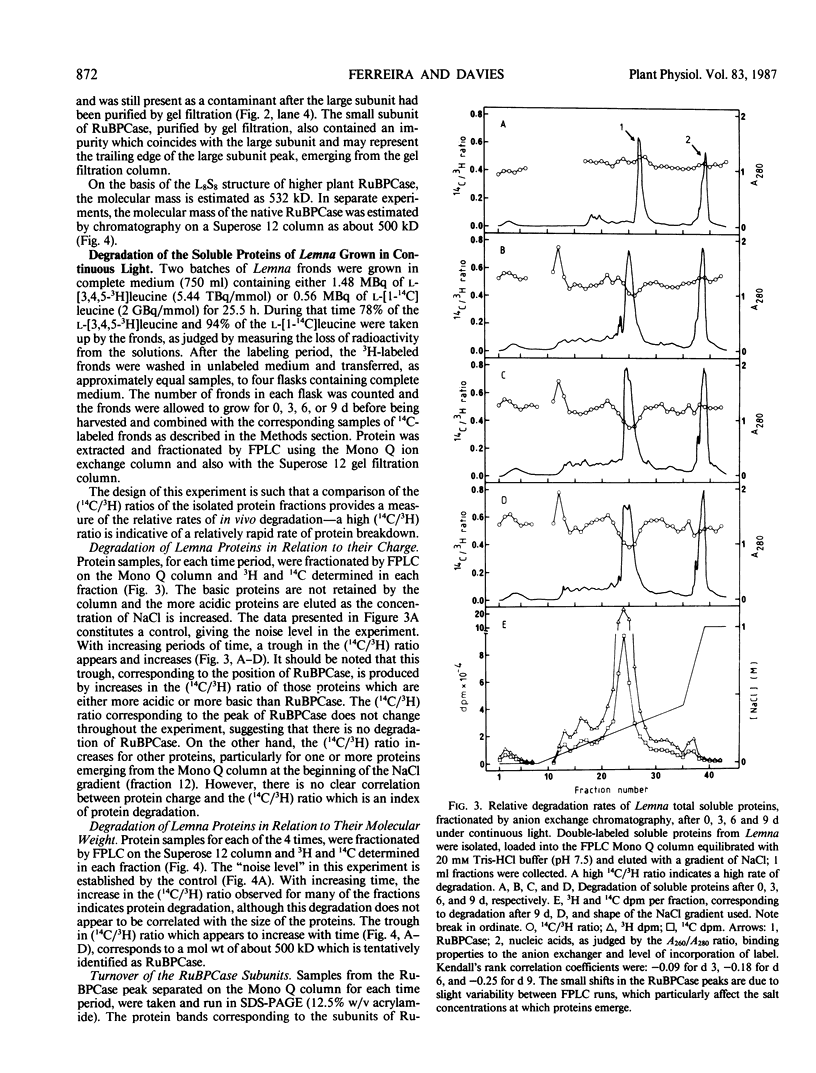
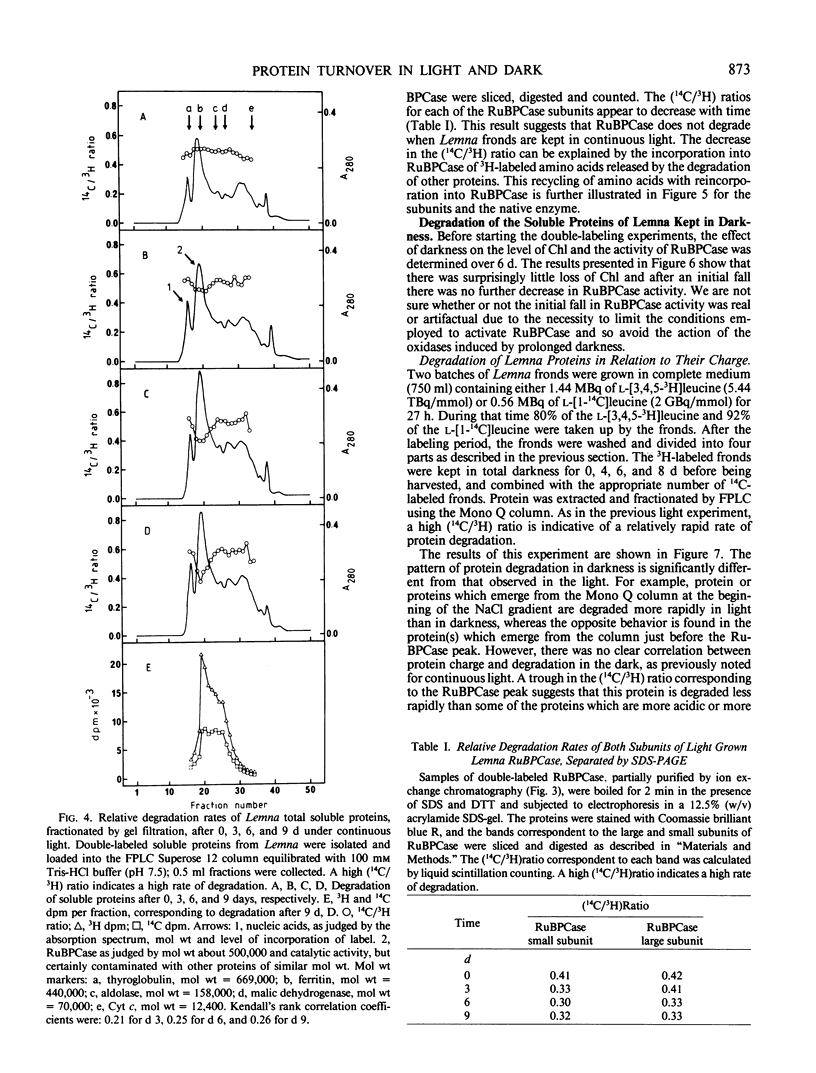


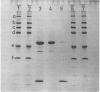
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acton G. J., Gupta S. A releationship between protein-degradation rates in vivo, isoelectric points, and molecular weights obtained by using density labelling. Biochem J. 1979 Nov 15;184(2):367–377. doi: 10.1042/bj1840367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias I. M., Doyle D., Schimke R. T. Studies on the synthesis and degradation of proteins of the endoplasmic reticulum of rat liver. J Biol Chem. 1969 Jun 25;244(12):3303–3315. [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmair A., Finley D., Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986 Oct 10;234(4773):179–186. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- Bennett J. Regulation of photosynthesis by reversible phosphorylation of the light-harvesting chlorophyll a/b protein. Biochem J. 1983 Apr 15;212(1):1–13. doi: 10.1042/bj2120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman L. H., Chollet R. Presence of two subunit types in ribulose 1,5-bisphosphate carboxylase from Thiobacillus intermedius. J Bacteriol. 1980 Feb;141(2):652–657. doi: 10.1128/jb.141.2.652-657.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. J., Davies D. D. General characteristics of normal and stress-enhanced protein degradation in Lemna minor (duckweed). Biochem J. 1980 Nov 15;192(2):499–506. doi: 10.1042/bj1920499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. D., Humphrey T. J. Amino Acid recycling in relation to protein turnover. Plant Physiol. 1978 Jan;61(1):54–58. doi: 10.1104/pp.61.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dice J. F., Dehlinger P. J., Schimke R. T. Studies on the correlation between size and relative degradation rate of soluble proteins. J Biol Chem. 1973 Jun 25;248(12):4220–4228. [PubMed] [Google Scholar]
- Dice J. F., Goldberg A. L. A statistical analysis of the relationship between degradative rates and molecular weights of proteins. Arch Biochem Biophys. 1975 Sep;170(1):213–219. doi: 10.1016/0003-9861(75)90112-5. [DOI] [PubMed] [Google Scholar]
- Dice J. F., Goldberg A. L. Relationship between in vivo degradative rates and isoelectric points of proteins. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3893–3897. doi: 10.1073/pnas.72.10.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dice J. F., Walker C. D., Byrne B., Cardiel A. General characteristics of protein degradation in diabetes and starvation. Proc Natl Acad Sci U S A. 1978 May;75(5):2093–2097. doi: 10.1073/pnas.75.5.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich J. W., Huffaker R. C. Photosynthesis, leaf resistances, and ribulose-1,5-bisphosphate carboxylase degradation in senescing barley leaves. Plant Physiol. 1980 Jun;65(6):1103–1107. doi: 10.1104/pp.65.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Hoffman-Falk H., Mattoo A. K., Marder J. B., Edelman M., Ellis R. J. General occurrence and structural similarity of the rapidly synthesized, 32,000-dalton protein of the chloroplast membrane. J Biol Chem. 1982 Apr 25;257(8):4583–4587. [PubMed] [Google Scholar]
- Humphrey T. J., Davies D. D. A sensitive method for measuring protein turnover based on the measurement of 2-3H-labelled amino acids in protein. Biochem J. 1976 Jun 15;156(3):561–568. doi: 10.1042/bj1560561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinkopf G. E., Huffaker R. C., Matheson A. Light-induced de Novo Synthesis of Ribulose 1,5-Diphosphate Carboxylase in Greening Leaves of Barley. Plant Physiol. 1970 Sep;46(3):416–418. doi: 10.1104/pp.46.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. D-Ribulose-1,5-bisphosphate carboxylase-oxygenase. Improved methods for the activation and assay of catalytic activities. Anal Biochem. 1977 Mar;78(1):66–75. doi: 10.1016/0003-2697(77)90009-4. [DOI] [PubMed] [Google Scholar]
- Peterson L. W., Huffaker R. C. Loss of Ribulose 1,5-Diphosphate Carboxylase and Increase in Proteolytic Activity during Senescence of Detached Primary Barley Leaves. Plant Physiol. 1975 Jun;55(6):1009–1015. doi: 10.1104/pp.55.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L. W., Kleinkopf G. E., Huffaker R. C. Evidence for lack of turnover of ribulose 1,5-diphosphate carboxylase in barley leaves. Plant Physiol. 1973 Jun;51(6):1042–1045. doi: 10.1104/pp.51.6.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D. D., Wilkinson P., Coates D. J. Increased chromosomal mutation rate after hybridization between two subspecies of grasshoppers. Science. 1983 Jun 10;220(4602):1165–1167. doi: 10.1126/science.6407107. [DOI] [PubMed] [Google Scholar]
- Simpson E. Biochemical and genetic studies of the synthesis and degradation of RuBP carboxylase. Basic Life Sci. 1978;11:113–125. doi: 10.1007/978-1-4684-8106-8_8. [DOI] [PubMed] [Google Scholar]
- Simpson E. Measurement of Protein Degradation in Leaves of Zea mays Using [H]Acetic Anhydride and Tritiated Water. Plant Physiol. 1981 Jun;67(6):1214–1219. doi: 10.1104/pp.67.6.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H., Stoddart J. L. Separation of Chlorophyll Degradation from Other Senescence Processes in Leaves of a Mutant Genotype of Meadow Fescue (Festuca pratensis L.). Plant Physiol. 1975 Sep;56(3):438–441. doi: 10.1104/pp.56.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas A. The Turnover of Nucleic Acids in Lemna minor. Plant Physiol. 1970 Jun;45(6):742–751. doi: 10.1104/pp.45.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde C. J., Paskin N., Saxton J., Mayer R. J. Protein degradation during terminal cytodifferentiation. Studies on mammary gland in organ culture. Biochem J. 1980 Oct 15;192(1):311–320. doi: 10.1042/bj1920311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Breakdown of Ribulose Bisphosphate Carboxylase and Change in Proteolytic Activity during Dark-induced Senescence of Wheat Seedlings. Plant Physiol. 1978 Oct;62(4):604–608. doi: 10.1104/pp.62.4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Ribulose Bisphosphate Carboxylase and Proteolytic Activity in Wheat Leaves from Anthesis through Senescence. Plant Physiol. 1979 Nov;64(5):884–887. doi: 10.1104/pp.64.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak R., Martin A. F., Prior G., Rabinowitz M. Comparison of turnover of several myofibrillar proteins and critical evaluation of double isotope method. J Biol Chem. 1977 May 25;252(10):3430–3435. [PubMed] [Google Scholar]