Abstract
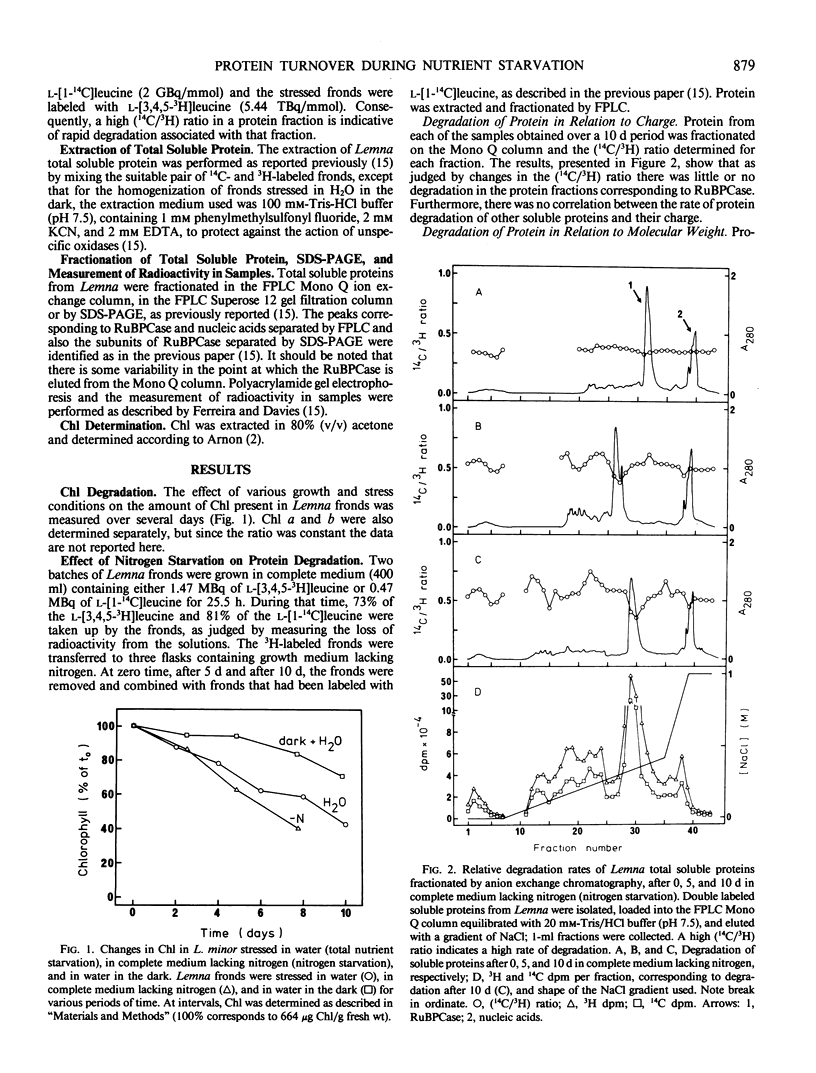
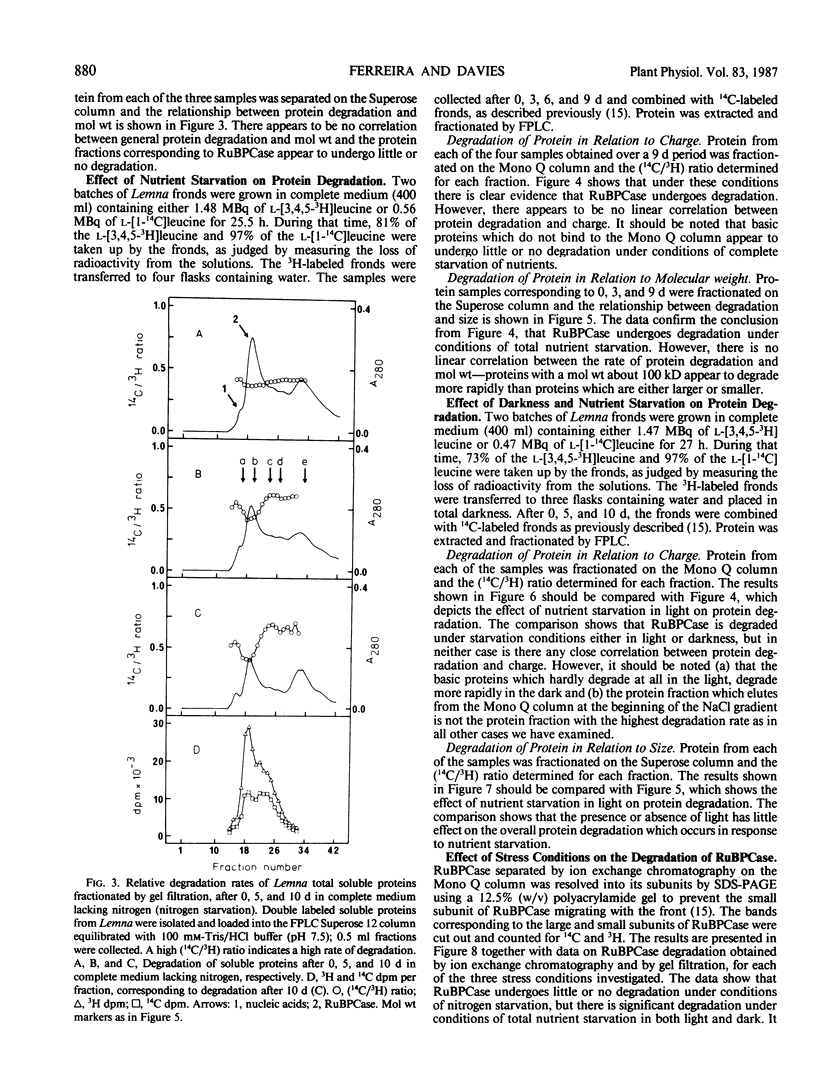
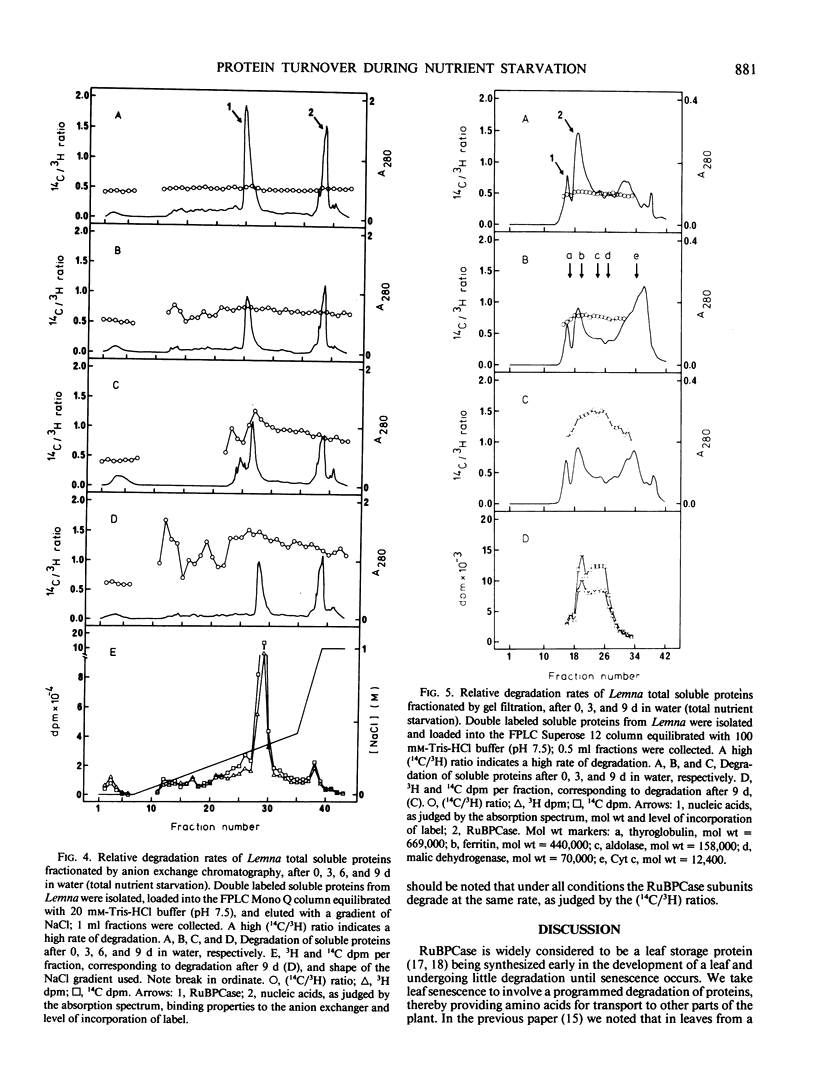
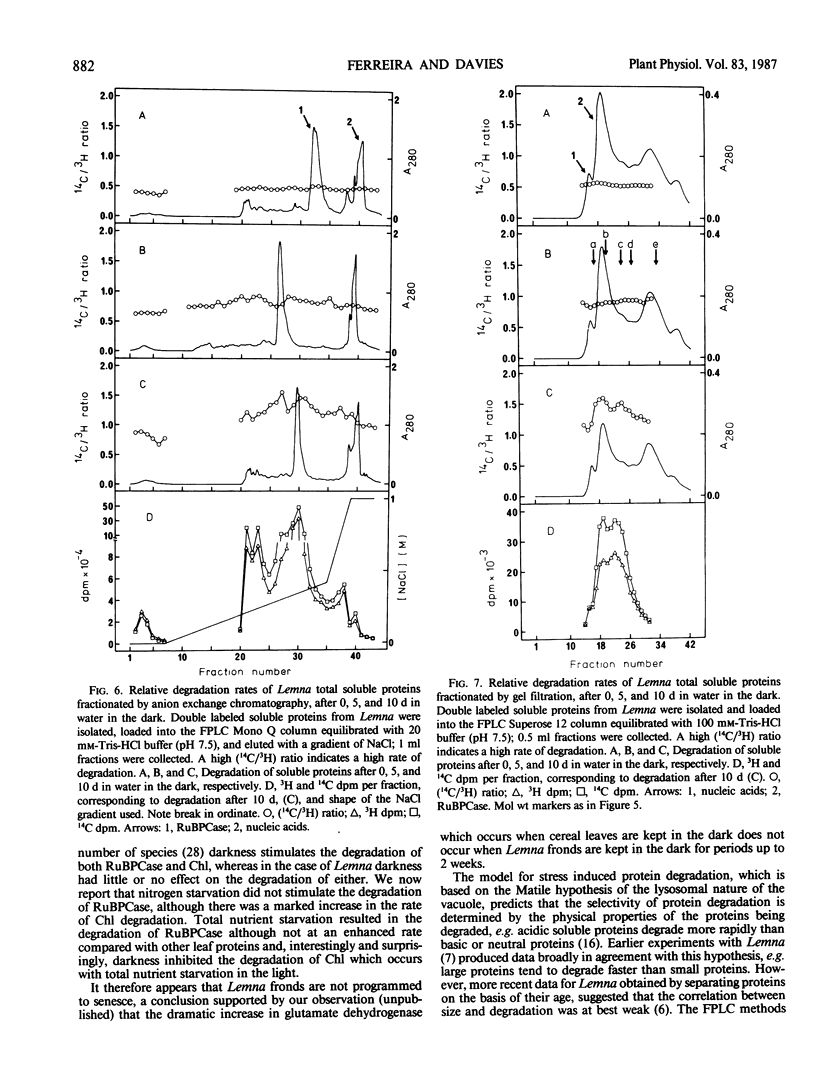
The concept of ribulose bisphosphate carboxylase as a storage protein is not supported in the case of Lemna minor, where the enzyme appears to be particularly stable under conditions of nitrogen starvation. Total nutrient starvation in light and in the dark induced the degradation of this enzyme, but not at an enhanced rate compared with other leaf proteins and, surprisingly, darkness inhibited the degradation of chlorophyll which occurs with total nutrient starvation in the light. The data suggest that Lemna is not programmed to senesce in response to nutrient starvation. Differences in the pattern of protein degradation, which occurred under the stress conditions employed, are not consistent with a simple model of protein degradation in which the degradative system is assumed to be located in the vacuole. The data is best explained by a dual system in which cytosolic proteins are degraded by a vacuolar/lysosomal system and chloroplast proteins are degraded within the chloroplast. Whatever the system of degradation, our data do not support the proposed correlation between the rate of protein degradation and either protein charge or size.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias I. M., Doyle D., Schimke R. T. Studies on the synthesis and degradation of proteins of the endoplasmic reticulum of rat liver. J Biol Chem. 1969 Jun 25;244(12):3303–3315. [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett N. M., Naylor A. W. Amino Acid and protein metabolism in bermuda grass during water stress. Plant Physiol. 1966 Sep;41(7):1222–1230. doi: 10.1104/pp.41.7.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Poole R. J. Kinetics of Ca/H Antiport in Isolated Tonoplast Vesicles from Storage Tissue of Beta vulgaris L. Plant Physiol. 1986 Mar;80(3):727–731. doi: 10.1104/pp.80.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Poole R. J. Na/H Antiport in Isolated Tonoplast Vesicles from Storage Tissue of Beta vulgaris. Plant Physiol. 1985 May;78(1):163–167. doi: 10.1104/pp.78.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Poole R. J. Nitrate storage and retrieval in Beta vulgaris: Effects of nitrate and chloride on proton gradients in tonoplast vesicles. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3683–3687. doi: 10.1073/pnas.82.11.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canut H., Alibert G., Boudet A. M. Hydrolysis of Intracellular Proteins in Vacuoles Isolated from Acer pseudoplatanus L. Cells. Plant Physiol. 1985 Dec;79(4):1090–1093. doi: 10.1104/pp.79.4.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canut H., Alibert G., Carrasco A., Boudet A. M. Rapid Degradation of Abnormal Proteins in Vacuoles from Acer pseudoplatanus L. Cells. Plant Physiol. 1986 Jun;81(2):460–463. doi: 10.1104/pp.81.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. J., Davies D. D. General characteristics of normal and stress-enhanced protein degradation in Lemna minor (duckweed). Biochem J. 1980 Nov 15;192(2):499–506. doi: 10.1042/bj1920499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. J., Oliver J., Davies D. D. Stress and Protein Turnover in Lemna minor. Plant Physiol. 1979 Dec;64(6):1109–1113. doi: 10.1104/pp.64.6.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. J., Roberts K., Davies D. D. Model for Stress-induced Protein Degradation in Lemna minor. Plant Physiol. 1980 Dec;66(6):1119–1122. doi: 10.1104/pp.66.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dice J. F., Dehlinger P. J., Schimke R. T. Studies on the correlation between size and relative degradation rate of soluble proteins. J Biol Chem. 1973 Jun 25;248(12):4220–4228. [PubMed] [Google Scholar]
- Dice J. F., Walker C. D., Byrne B., Cardiel A. General characteristics of protein degradation in diabetes and starvation. Proc Natl Acad Sci U S A. 1978 May;75(5):2093–2097. doi: 10.1073/pnas.75.5.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein D., Elias-Bishko S., Hershko A. Requirement for protein synthesis in the regulation of protein breakdown in cultured hepatoma cells. Biochemistry. 1975 Nov 18;14(23):5199–5204. doi: 10.1021/bi00694a028. [DOI] [PubMed] [Google Scholar]
- Ferreira R. B., Davies D. D. Protein degradation in lemna with particular reference to ribulose bisphosphate carboxylase: I. The effect of light and dark. Plant Physiol. 1987 Apr;83(4):869–877. doi: 10.1104/pp.83.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., Dice J. F. Intracellular protein degradation in mammalian and bacterial cells. Annu Rev Biochem. 1974;43(0):835–869. doi: 10.1146/annurev.bi.43.070174.004155. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Kleinkopf G. E., Huffaker R. C., Matheson A. Light-induced de Novo Synthesis of Ribulose 1,5-Diphosphate Carboxylase in Greening Leaves of Barley. Plant Physiol. 1970 Sep;46(3):416–418. doi: 10.1104/pp.46.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolson M. F., Rea P. A., Poole R. J. Identification of 3-O-(4-benzoyl)benzoyladenosine 5'-triphosphate- and N,N'-dicyclohexylcarbodiimide-binding subunits of a higher plant H+-translocating tonoplast ATPase. J Biol Chem. 1985 Oct 5;260(22):12273–12279. [PubMed] [Google Scholar]
- Miller B. L., Huffaker R. C. Hydrolysis of Ribulose-1,5-bisphosphate Carboxylase by Endoproteinases from Senescing Barley Leaves. Plant Physiol. 1982 Jan;69(1):58–62. doi: 10.1104/pp.69.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L. W., Huffaker R. C. Loss of Ribulose 1,5-Diphosphate Carboxylase and Increase in Proteolytic Activity during Senescence of Detached Primary Barley Leaves. Plant Physiol. 1975 Jun;55(6):1009–1015. doi: 10.1104/pp.55.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. J., Briskin D. P., Krátký Z., Johnstone R. M. Density gradient localization of plasma membrane and tonoplast from storage tissue of growing and dormant red beet : characterization of proton-transport and ATPase in tonoplast vesicles. Plant Physiol. 1984 Mar;74(3):549–556. doi: 10.1104/pp.74.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. J. Development and Characteristics of Sodium-selective Transport in Red Beet. Plant Physiol. 1971 Jun;47(6):735–739. doi: 10.1104/pp.47.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragster L. E., Chrispeels M. J. Autodigestion in crude extracts of soybean leaves and isolated chloroplasts as a measure of proteolytic activity. Plant Physiol. 1981 Jan;67(1):104–109. doi: 10.1104/pp.67.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D. D., Wilkinson P., Coates D. J. Increased chromosomal mutation rate after hybridization between two subspecies of grasshoppers. Science. 1983 Jun 10;220(4602):1165–1167. doi: 10.1126/science.6407107. [DOI] [PubMed] [Google Scholar]
- Thayer S. S., Huffaker R. C. Vacuolar Localization of Endoproteinases EP(1) and EP(2) in Barley Mesophyll Cells. Plant Physiol. 1984 May;75(1):70–73. doi: 10.1104/pp.75.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenhuis M., Douma A., Harder W., Osumi M. Degradation and turnover of peroxisomes in the yeast Hansenula polymorpha induced by selective inactivation of peroxisomal enzymes. Arch Microbiol. 1983 Jun;134(3):193–203. doi: 10.1007/BF00407757. [DOI] [PubMed] [Google Scholar]
- Wittenbach V. A. Breakdown of Ribulose Bisphosphate Carboxylase and Change in Proteolytic Activity during Dark-induced Senescence of Wheat Seedlings. Plant Physiol. 1978 Oct;62(4):604–608. doi: 10.1104/pp.62.4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A., Lin W., Hebert R. R. Vacuolar localization of proteases and degradation of chloroplasts in mesophyll protoplasts from senescing primary wheat leaves. Plant Physiol. 1982 Jan;69(1):98–102. doi: 10.1104/pp.69.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]


