Abstract
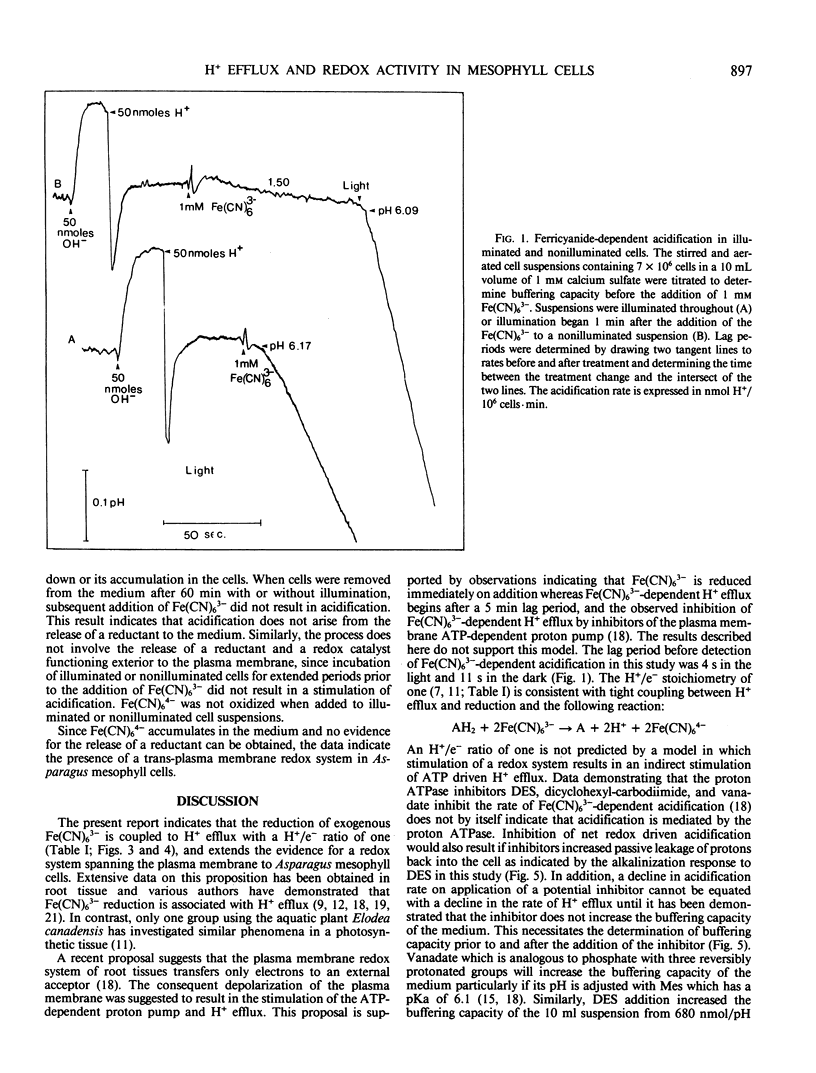
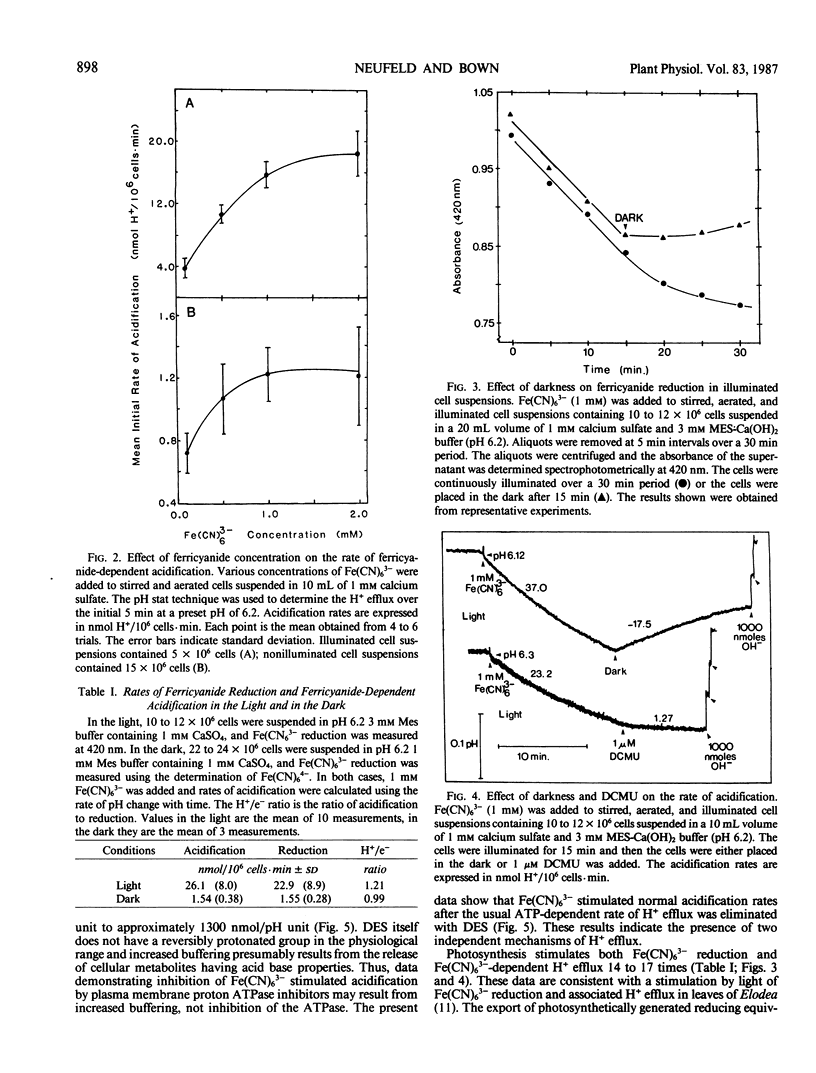
Potassium ferricyanide (K3Fe[CN]6) was added to aerated and stirred nonbuffered suspensions of mechanically isolated photosynthetically competent Asparagus sprengeri Regel mesophyll cells. Rates of Fe(CN)63− reduction and H+ efflux were measured with or without illumination. On the addition of 1 millimolar Fe(CN)63− to nonilluminated cell suspensions acidification of the medium indicated an H+ efflux of 1.54 nanomoles H+/106 cells per minute. Simultaneous Fe(CN)63− reduction occurred at a rate of 1.55 nanomoles Fe(CN)63−/106 cells per minute. Illumination stimulated these rates 14 to 17 times and corresponding values were 26.1 nanomoles H+/106 cells per minute and 22.9 nanomoles Fe(CN)63−/106 cells per minute. These two processes appeared to be tightly coupled and were rapidly inhibited when illuminated suspensions were transferred to darkness or treated with 1 micromolar 3-(3,4-dichlorophenyl)-1,1 dimethylurea. Addition of 0.1 millimolar diethylstilbestrol eliminated ATP dependent H+ efflux in illuminated or nonilluminated cells but had no influence on Fe(CN)63− dependent H+ efflux. Recent reports indicate that a transmembrane redox system spans the plasma membrane of root cells and is coupled to the efflux of H+. The present report extends these observations to photosynthetically competent mesophyll cells. The results indicate a transport process independent of ATP driven H+ efflux which operates with a H+/e− stoichiometry of one.
Full text
PDF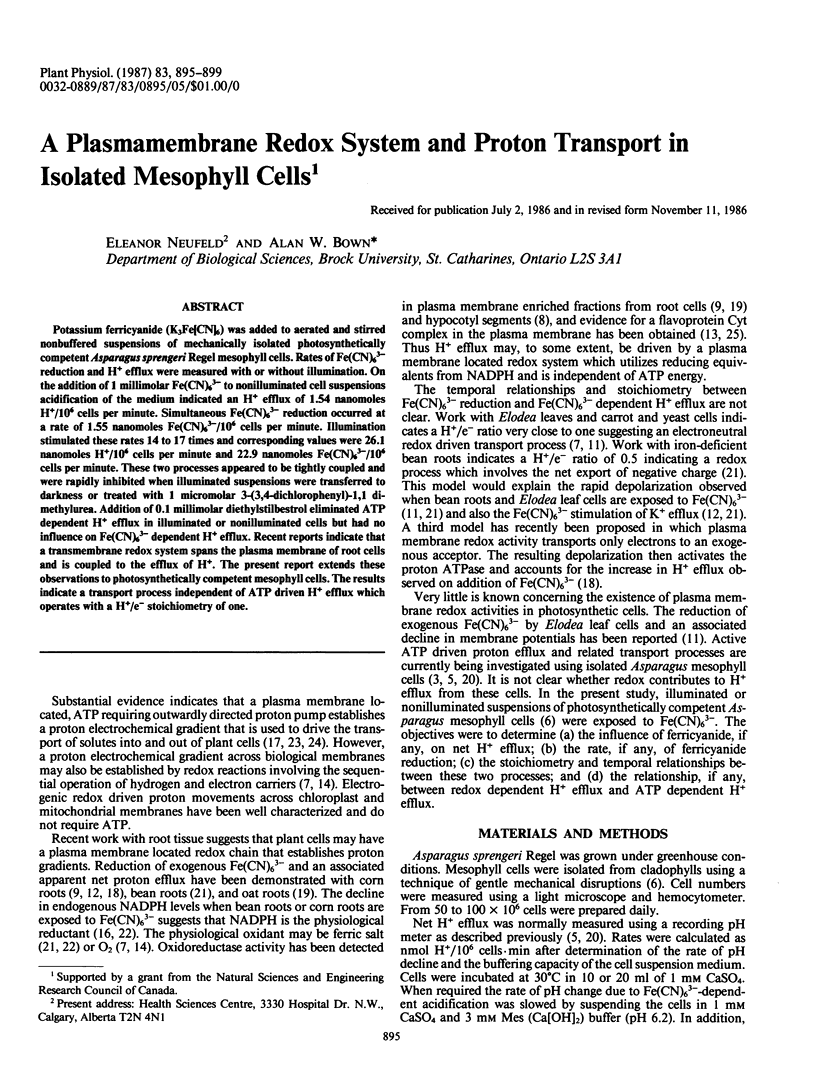


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M., SHAVIT N. A SENSITIVE AND SIMPLE METHOD FOR DETERMINATION OF FERROCYANIDE. Anal Biochem. 1963 Dec;6:549–554. doi: 10.1016/0003-2697(63)90149-0. [DOI] [PubMed] [Google Scholar]
- Bown A. W. An investigation into the roles of photosynthesis and respiration in h efflux from aerated suspensions of asparagus mesophyll cells. Plant Physiol. 1982 Sep;70(3):803–810. doi: 10.1104/pp.70.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bown A. W., Nicholls F. An investigation into the role of photosynthesis in regulating ATP levels and rates of h efflux in isolated meosphyll cells. Plant Physiol. 1985 Dec;79(4):928–934. doi: 10.1104/pp.79.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bown A. W., Pullen J., Shadeed N. M. Disulfiram metabolism in isolated mesophyll cells and inhibition of photosynthesis and cyanide-resistant respiration. Plant Physiol. 1984 Nov;76(3):846–848. doi: 10.1104/pp.76.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane F. L., Sun I. L., Clark M. G., Grebing C., Löw H. Transplasma-membrane redox systems in growth and development. Biochim Biophys Acta. 1985 Aug 1;811(3):233–264. doi: 10.1016/0304-4173(85)90013-8. [DOI] [PubMed] [Google Scholar]
- Federico R., Giartosio C. E. A transplasmamembrane electron transport system in maize roots. Plant Physiol. 1983 Sep;73(1):182–184. doi: 10.1104/pp.73.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian L. V., Lucas W. J. Potassium Transport in Corn Roots : III. Perturbation by Exogenous NADH and Ferricyanide. Plant Physiol. 1985 Feb;77(2):429–436. doi: 10.1104/pp.77.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong T. Y., Briggs W. R. Partial purification and characterization of a blue light-sensitive cytochrome-flavin complex from corn membranes. Plant Physiol. 1981 May;67(5):1042–1046. doi: 10.1104/pp.67.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. Further Characterization on the Transport Property of Plasmalemma NADH Oxidation System in Isolated Corn Root Protoplasts. Plant Physiol. 1984 Feb;74(2):219–222. doi: 10.1104/pp.74.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'neill S. D., Spanswick R. M. Effects of vanadate on the plasma membrane ATPase of red beet and corn. Plant Physiol. 1984 Jul;75(3):586–591. doi: 10.1104/pp.75.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein B., Stern A. I. Relationship of Transplasmalemma Redox Activity to Proton and Solute Transport by Roots of Zea mays. Plant Physiol. 1986 Apr;80(4):805–811. doi: 10.1104/pp.80.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein B., Stern A. I., Stout R. G. Redox activity at the surface of oat root cells. Plant Physiol. 1984 Oct;76(2):386–391. doi: 10.1104/pp.76.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijmons P. C., Lanfermeijer F. C., de Boer A. H., Prins H. B., Bienfait H. F. Depolarization of Cell Membrane Potential during Trans-Plasma Membrane Electron Transfer to Extracellular Electron Acceptors in Iron-Deficient Roots of Phaseolus vulgaris L. Plant Physiol. 1984 Dec;76(4):943–946. doi: 10.1104/pp.76.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijmons P. C., van den Briel W., Bienfait H. F. Cytosolic NADPH is the electron donor for extracellular fe reduction in iron-deficient bean roots. Plant Physiol. 1984 May;75(1):219–221. doi: 10.1104/pp.75.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widell S., Lundborg T., Larsson C. Plasma membranes from oats prepared by partition in an aqueous polymer two-phase system : on the use of light-induced cytochrome B reduction as a marker for the plasma membrane. Plant Physiol. 1982 Nov;70(5):1429–1435. doi: 10.1104/pp.70.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]


