Abstract
Secondary metabolites from plants are ubiquitous and have applications in medicines, food additives, scents, colorants, and natural pesticides. Biotechnological production of secondary metabolites that have economic benefits is an attractive alternative to conventional methods. Cell, adventitious, and hairy root suspension cultures are typically used to produce secondary metabolites. According to recent studies, somatic embryos in suspension culture are useful tools for the generation of secondary metabolites. Somatic embryogenesis is a mode of regeneration in several plant species. This review provides an update on the use of somatic embryogenesis in the production of valuable secondary metabolites. The factors influencing the generation of secondary metabolites using somatic embryos in suspension cultures, elicitation methods, and prospective applications are also discussed in this review.
Graphical abstract
Keywords: Bioactive compounds, Bioreactors, Elicitation, Secondary metabolites, Somatic embryogenesis, Scale-up process
Introduction
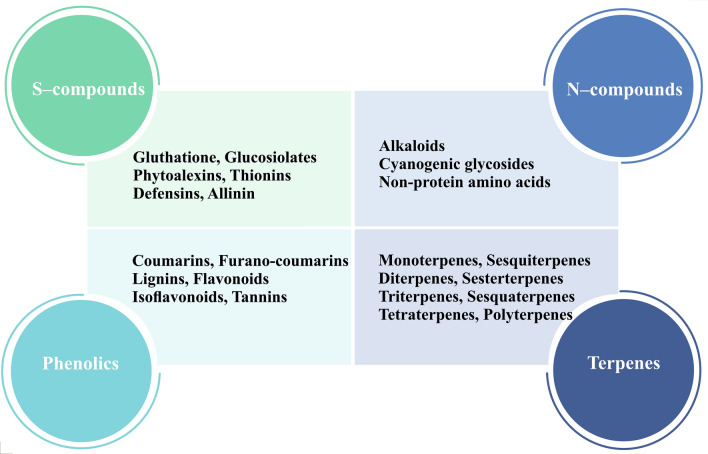
Over their life cycles, plants produce and accumulate a considerable variety of secondary metabolites, which are organic substances derived from primary metabolites. Secondary metabolites have a variety of functions that influence how plants interact with their environment, including protecting them from pathogens, defending them from abiotic stressors such as ultraviolet radiation, luring pollinators, and acting as signaling molecules (Guerrieri et al. 2019; Pang et al. 2021). Among the various plant species, there is a large diversity of these chemicals. Higher plants have been found to have about 200,000 secondary compounds (Fang et al. 2019; Wang et al. 2019). Secondary metabolites are altered after production through a variety of processes, including methylation, glycosylation, and hydroxylation. Phenolics, terpenes, nitrogen-containing compounds, and sulfur compounds are the four major classes of plant secondary metabolites, which have been categorized based on their biosynthetic pathways (Erb and Kliebenstein 2020; Garagounis et al. 2021; Jan et al. 2021; Twaij and Hasan 2022; Wang et al. 2019) (Fig. 1). The shikimate pathway produces phenolics, which are divided into different classes based on the number of aromatic rings, carbon atoms, and hydroxyl groups. These classes include simple phenolics, coumarins, lignans, flavonoids, isoflavonoids, and tannins. The mevalonic acid pathway is used to synthesize terpenoids from isoprenoid units. According to the number of isoprene units present, they are divided into monoterpenes, sesquiterpenes, diterpenes, sesterterpenes, and triterpenes, among others. Alkaloids are classified into three groups: phenylethylamine alkaloids, pyrrolizidine alkaloids, and terpenoid indole alkaloids. Most alkaloids are formed from amino acids. Glucosinolates and phytoalexins contain sulfur and are generated from amino acids and glucose (Venditti and Bianco 2020). Secondary metabolites are used in medications, food additives, flavoring agents, scents, colors, and natural pesticides because of their valuable biological properties.
Fig. 1.
Classification and types of plant secondary metabolites
Naturally growing plants contain extremely low concentrations of secondary metabolites (less than 1%). Moreover, age, seasonality, environmental factors, and genotype-specific factors affect the accumulation of secondary metabolites in plants (Li et al. 2020; Sun et al. 2022). In addition, owing to the overharvesting of plants for their secondary metabolites in the wild, certain plant species have become rare and endangered (Nosov 2012). Therefore, it is desirable to apply biotechnology to produce useful secondary metabolites from adventitious roots, hairy roots, and cells (Chandran et al. 2020; Gutierrez-Valdes et al. 2020; Kreis 2019; Marchev et al. 2020; Mohaddab et al. 2022; Murthy et al. 2014, 2016, 2022, 2023a, b, c; Wawrosch and Zotchev 2021). Somatic embryogenesis is the mode of regeneration in several aromatic and therapeutic plants. Somatic embryos are organized bipolar structures that develop from a single or group of somatic cells in vitro and eventually develop into plantlets upon germination. In contrast, hairy roots are genetically transformed roots derived from the infection of Rhizobacterium rhizogenes and are thin hairy structures. Adventitious roots are unipolar structures that grow from any location other than the embryonic root (radicle) in vitro or in vivo. In contrast, calli are growing masses of unorganized plant parenchyma cells that can regenerate into organs or somatic embryos under the influence of plant growth regulators in vitro. Protocorm-like bodies (PLBs) are developed from the seeds or other organ cultures of orchids in vitro. The early stages of protocorm-like bodies in orchids have morphological and histological characteristics similar to those of the zygotic embryos (Lee et al. 2013). Hence, the protocorm-like bodies are regarded as orchid somatic embryos (Lee et al. 2013). Somatic embryos can be induced in a variety of plants and cultured in suspension cultures, and important secondary metabolites can be extracted from the embryogenic biomass. The use of somatic embryogenesis for the production of highly valuable secondary metabolites is summarized in this article. In addition, several variables affecting the accumulation of biomass and secondary chemicals as well as somatic embryo growth in bioreactors are explored.
Production of secondary metabolites from somatic embryogenic cultures
Somatic embryogenesis is the process by which somatic cells undergo reorganization to produce embryogenic cells. The formation of a somatic embryo capable of regenerating plants follows a sequence of morphological and biochemical changes in these cells (Yang and Zhang 2010). Somatic embryos can form naturally in numerous plant species or during in vitro cell and tissue culture. The developmental stages of somatic embryogenesis are comparable to those of zygotic embryos, which normally go through the globular, torpedo, and cotyledonary stages in dicots or the globular, scutellar, and coleoptile stages in monocots. Conifers undergo globular, early cotyledonary, and late cotyledonary stages (Yang and Zhang 2010). Furthermore, in vitro somatic embryogenesis can involve either callus-mediated or direct embryo regeneration. Specific plant growth regulators regulate the induction, differentiation, maturation, and germination of somatic embryos (Sugimoto et al. 2019; Tang et al. 2020; Wojcik et al. 2020). Somatic embryogenesis is frequently used for plant genetic improvement, mass propagation, and genetic transformation (Corredoira et al. 2019; Murthy et al. 2023b; Tian et al. 2020). Several plant species produce and store secondary metabolites in their zygotic seeds. According to a literature review by De-la-Cruz Chacón et al. (2012), most secondary metabolites are biosynthesized in developing embryos, whereas the remainder are partially or entirely acquired from the mother plant. Moreover, they showed that several phenolics, alkaloids, and terpenoids are synthesized in the early stages of plant development. Several studies have reported that the quantity and diversity of secondary metabolites increase and/or decrease throughout embryo development and germination. For example, the seeds of Coffea arabica contain 2% and 1% phenolic acids (chlorogenic acid) and alkaloids (caffeine and trigonelline), respectively. However, caffeine content reportedly surges by a factor of 2.5 during germination compared to earlier stages (Aerts and Baumann 1994). In contrast, phenolics are significantly decreased in Lens culinaris (lentil) (Bartolomé et al. 1997). Early embryo development is characterized by secondary metabolite variation, which is related to the interactions between developing plants and phytopathogens, insects, and allelopathic agents (Yamaji and Ichihara 2012). These examples indicate that the embryogenic system is highly active in secondary metabolism and can be used to produce secondary metabolites in vitro.
Suspension culture of embryos: optimization of parameters
Plant tissue culture depends on various factors, including the nutrients supplied for plant growth, to produce secondary metabolites. The explant response in terms of morphogenetic events, such as organogenesis or somatic embryogenesis, depends critically on the optimal nutritional content (Murthy et al. 2023b). The key elements that must be established in every culture include the type of culture medium used, the salt content of the medium used (including nitrogen and phosphorus), the type and quantity of growth regulators utilized, and the type and quantity of sugars (Murthy et al. 2014, 2022, 2023c). The physical factors that affect the growth of cultured cells and organs in vitro and that aid in the formation of secondary metabolites include temperature, lighting, light quality, medium pH, agitation, and aeration (Murthy et al. 2014, 2023a, b). Somatic embryogenesis has been used to produce biomass and various secondary metabolites in several medicinal plants. The data are shown in Tables 1 and 2, and the components affecting the production of biomass and secondary compounds are described in detail.
Table 1.
Some examples of the examples of in vitro culture of somatic embryos for the production of specialized metabolites
| Species | Specialized metabolite | Main use | Culture conditions | References | ||
|---|---|---|---|---|---|---|
| Nutrient medium | Culture type | Culture conditions (Temperature, light, photoperiod) | ||||
| Aralia elata | Triterpene glycosides: saponins | Cosmetic ingredients, antidiabetic and hepatitis drug |
Callus induction: Murashige and Skoog medium supplemented with 2,4-D (1 mg L−1) + 3% sucrose Somatic embryo initiation and proliferation: MS medium supplemented with IAA (3.0 mg L−1) + BAP (0.5 mg L−1) + 3% sucrose |
Callus cultures | 25 ± 2 °C, 12 h light and dark photoperiod, fluorescent light, 27 µmol m−2 s−1 | Cheng et al. (2021) |
| Catharanthus roseus | Alkaloid: Vinblastine | Anticancer |
Callus induction: Murashige and Skoog medium supplemented with 2,4-D (1 mg L−1) + 3% sucrose Somatic embryo initiation and proliferation: MS medium supplemented with NAA (1.0 mg L−1) + BAP (0.5 mg L−1) + 3% sucrose Somatic embryo maturation and germination: MS medium GA3 (1 mg L−1) + 3% maltose |
Callus cultures | 25 °C day/20 °C dark, 16 h photoperiod, fluorescent light at PPFD of 100 µmol m−2 s−1 | Aslam et al. (2010) |
| Citrus pardisi | Flavone glycosides: Naringin | Sweeteners | Induction embryogenic callus: Murashige and Tucker medium supplemented with 280 mM glycerol or 79 mM sucrose | Callus cultures | 26 ± 1 °C, 16 h photoperiod, fluorescent light, 14 µmol m−2 s−1 | Gavish et al. (1989) |
| Eleutherococcus chiisanensis |
Triterpenoid glycosides: Eleutheroside A or Phenylpropanoid glycosides: Eleutheroside B, Eleutheroside E Coumarin glycoside: Eleutheroside E1 |
Immunostimulants, anti-inflammatory and antiviral |
Induction of somatic embryos: Murashige and Skoog medium supplemented with 2,4-D (1 mg L−1) + 3% sucrose Somatic embryo suspension cultures in Erlenmeyer flasks/bioreactors: Murashige and Skoog medium + 3%. Embryo germination: Murashige and Skoog medium + 3% sucrose and 20 µM GA3. |
Shake flask cultures: 250 mL Erlenmeyer flasks containing 50 mL of medium were maintained on a gyratory shaker at 110 rpm. and 10 L bioreactors Balloon-type bubble bioreactors containing 5 L medium were aerated with 0.1 vvm sterile air |
24 ± 1 °C, 16 h photoperiod, fluorescent light, 40 µmol m−2 s−1 | Jeong et al. (2005) |
| Eleutherococcus koreanum | Phenylpropanoid glycosides: Eleutheroside B, Eleutheroside E | Immunostimulants, anti-inflammatory and antiviral |
Induction of somatic embryos: Murashige and Skoog medium of 1/3, 1/2, full and double strength supplemented with 2,4-D (0, 0.5, 1 and 2 mg L−1), TDZ (0, 0.01, 0.1, 1.0 mg L−1) or without growth regulators and 30 g L−1 sucrose were tested. In another set of experiments effect of 1/2 strength Murashige and Skoog medium supplemented with 15, 30, 60, and 90 g L−1 sucrose was tested on embryo induction. Somatic embryo suspension cultures in Erlenmeyer flasks/bioreactors: 1/3 strength Murashige and Skoog medium + 6% sucrose |
Shake flask culture: 300 mL Erlenmeyer flasks containing 100 mL of medium were maintained on a gyratory shaker at 110 rpm Bioreactor cultures: 20 L capacity balloon-type bubble/airlift bioreactors containing 18 L of 1/3 MS medium supplemented with 60 g L−1 sucrose were used. Bioreactor cultures were aerated at 0.1 vvm |
Cultures maintained at 23 °C under continuous dark conditions | Park et al. (2005) |
| Eleutherococcus senticosus |
Phenylpropanoid glycosides: Eleutheroside B, Eleutheroside E Coumarin glycoside: Eleutheroside E1 |
Immunostimulants, anti-inflammatory and antiviral |
Induction of somatic embryos: Murashige and Skoog medium supplemented with 2,4-D (1 mg L−1) + 3% sucrose Somatic embryo suspension cultures in Erlenmeyer flasks/bioreactors: Murashige and Skoog medium + 3% sucrose |
Bioreactors: 3 L capacity balloon-type bubble bioreactors containing 2 L medium and cultures were aerated with 0.1 vvm sterile air |
During induction of embryos: 24 ± 1 °C, 16 h photoperiod, fluorescent light, 24 µmol m−2 s−1 Bioreactor cultures: Maintained at 12, 18, 24, and 30 °C in the dark |
Shohael et al. (2006b) |
| Eleutherococcus senticosus |
Phenylpropanoid glycosides: Eleutheroside B, Eleutheroside E Coumarin glycoside: Eleutheroside E1 |
Immunostimulants, anti-inflammatory and antiviral |
Induction of somatic embryos: Murashige and Skoog medium supplemented with 2,4-D (1 mg L−1) + 3% sucrose Somatic embryo suspension cultures in bioreactors: Murashige and Skoog medium + 3% sucrose |
Bioreactors: 3 L capacity balloon-type bubble bioreactors containing 2 L medium and cultures were aerated with 0.1 vvm sterile air | 24 ± 1 °C, 16 h photoperiod, 50 µmol m−2 s−1, and radiations were tested dark (control), fluorescent, monochromatic red LED (peak emission 660 nm), monochromatic blue LED (peak emission 470 nm), blue plus far-red LED (1:1) | Shohael et al. (2006a) |
| Eleutherococcus senticosus |
Phenylpropanoid glycosides: Eleutheroside B, Eleutheroside E Coumarin glycoside: Eleutheroside E1 |
Immunostimulants, anti-inflammatory and antiviral |
Induction of somatic embryos: Murashige and Skoog medium supplemented with 2,4-D (1 mg L−1) + 3% sucrose Somatic embryo suspension cultures bioreactors: Murashige and Skoog medium + 3% sucrose |
Bioreactors: 3 L capacity balloon-type bubble bioreactors containing 2 L medium and cultures were aerated with 0.1 vvm sterile air | Bioreactor cultures: 25 °C 16 h photoperiod, fluorescent light, 35 µmol m−2 s−1; The cultures were elicited at 0, 50, 100, 150, 200, 300 and 400 µM methyl jasmonate | Shohael et al. (2007) |
| Eleutherococcus senticosus |
Phenylpropanoid glycosides: Eleutheroside B, Eleutheroside E Coumarin glycoside: Eleutheroside E1 |
Immunostimulants, anti-inflammatory and antiviral |
Induction of somatic embryos: Murashige and Skoog medium supplemented with 2,4-D (1 mg L−1) + 3% sucrose Somatic embryo suspension cultures in Erlenmeyer flasks/bioreactors: Murashige and Skoog medium + 3% sucrose |
Bioreactors: 3 L capacity balloon-type bubble bioreactors containing 2 L medium and cultures were aerated with 0.1 vvm sterile air |
During induction of embryos: 24 ± 1 °C, 16 h photoperiod, fluorescent light, 24 µmol m−2 s−1 Bioreactor cultures: 24 ± 1 oC and dark incubation. Effect of inoculum density (1,3, 5, 7, 10 g cells L−1), aeration volume (0.05, 0.1, 0.2, and 0.3 vvm) Pilot scale bioreactor cultures: 500 L capacity balloon and drum type bioreactors |
Shohael et al. (2014a, b) |
| Eleutherococcus sessiliflorus |
Phenylpropanoid glycosides: Eleutheroside B, Eleutheroside E Coumarin glycoside: Eleutheroside E1 |
Immunostimulants, anti-inflammatory and antiviral |
Induction of somatic embryos: Murashige and Skoog medium supplemented with 2,4-D (1 mg L−1) + 3% sucrose Somatic embryo suspension cultures in Erlenmeyer flasks/bioreactors: Murashige and Skoog medium + 3% sucrose + 4 mg L−1 GA3. |
Bioreactors: 3 L capacity balloon-type bubble bioreactors containing 2 L medium and cultures were aerated with 0.1 vvm sterile air; Temporary immersion and continuous immersion methods were tested. |
During induction of embryos: 24 ± 1 °C, 16 h photoperiod, fluorescent light, 24 µmol m−2 s−1 Bioreactor cultures: 25 °C 16 h photoperiod, fluorescent light, 35 µmol m−2 s−1 |
Shohael et al. (2005) |
| Eleutherococcus sessiliflorus |
Phenylpropanoid glycosides: Eleutheroside B, Eleutheroside E Coumarin glycoside: Eleutheroside E1 |
Immunostimulants, anti-inflammatory and antiviral |
Induction of somatic embryos: Murashige and Skoog medium supplemented with 2,4-D (1 mg L−1) + 3% sucrose Somatic embryo suspension cultures in bioreactors: Murashige and Skoog medium + 3% sucrose + 4 mg L−1 GA3. |
Bioreactors: 3 L capacity balloon-type bubble bioreactors containing 2 L medium and cultures were aerated with 0.1 vvm sterile air | Bioreactor cultures: 25 °C 16 h photoperiod, fluorescent light, 35 µmol m−2 s−1; The cultures were elicited at 0, 50, 100, 150, 200, 300 and 400 µM methyl jasmonate | Shohael et al. (2008) |
| Peucedanum japonicum | Phenolics: Chlorogenic acid | Antioxidant, food additive, and nutraceutical agent |
Callus induction: Murashige and Skoog medium supplemented with 2,4-D (0.1-5.0 mg L−1) + 3% sucrose Somatic embryo initiation and proliferation: MS medium supplemented with ABA (1.0 mg L−1) + 3% sucrose |
Callus cultures |
Callus induction: 25 ± 2 °C and dark condition; Induction of somatic embryos: The effect of blue (450 nm), green (525 nm), red (660 nm), and far-red (730 nm) LED in different combinations (at 54–64 µmol m−2 s−1) have been studied. |
Chen et al. (2016) |
| Plumbago rosea | Naphthoquinone: Plumbagin | Anticoagulant and antineoplastic agent |
Somatic embryo suspension cultures in Erlenmeyer flasks: Murashige and Skoog medium + 3% sucrose + 5.06 µM IAA + 2.68 µM NAA + 1.33 µM BA. Effect of different concentrations of NH+ 4 (1–15 µM) and KNO3 (1–15 µM); acetylsalicylic acid (ASA, 0, 2.77, 5.54, 8.32, 11.09 µM) in combination with IAA (2.53, 5.06 µM) was tested. |
Shake flask cultures: 250 mL Erlenmeyer flasks containing 60 mL medium on a gyratory shaker at 100 rpm. | 25 ± 2 °C, 12 h light and dark photoperiod, fluorescent light, 30 µmol m−2 s−1 | Komaraiah et al. (2004) |
| Psoralea corylifolia | Furanocoumarins: Psoralen | Anti-vitiligo and anti-psoriasis agent |
Induction of somatic embryos: Murashige and Skoog’s medium supplemented with 6 µM NAA and 30 µM glutamine + 3% sucrose Somatic embryo suspension cultures in Erlenmeyer flasks: Murashige and Skoog medium + 3% sucrose + 1 µM NAA + 4 µM BA. Studied the effect of 10–50 g L−1 glucose or fructose or sucrose or maltose; or 1 µM NAA + 4 µM BA, 30 g L−1 sucrose, and 5–25 µM glutamic acid alone and 1–5 µM ABA alone or combination of glutamine plus ABA |
Shake flask cultures: 250 mL Erlenmeyer flasks containing 50 mL medium on a gyratory shaker at 100–110 rpm. | 25 ± 2 °C, 16 h light and dark photoperiod, fluorescent light, 12 µmol m−2 s−1 | Baskaran and Jayabalan (2009) |
| Rosa rugosa | Phenolics and Flavonoids | Antioxidants and cosmetic ingredient |
Induction of somatic embryos: Murashige and Skoog’s medium supplemented with 45.0 µM 2,4-D + 3% sucrose Somatic embryo suspension cultures in Erlenmeyer flasks: Murashige and Skoog medium + 3% sucrose. |
Shake flask cultures: 250 mL Erlenmeyer flasks containing 100 mL medium on a gyratory shaker at 100 rpm. | 24 ± 2 °C, continuous fluorescent light, 40 µmol m−2 s−1 | Jang et al. (2016) |
| Santalum album | Sesquiterpenoids: α-santalol and β-santalol | Natural medicine, Perfume; Anticancer agent |
Induction of somatic embryos: Woody plant medium supplemented with 4.52 µM 2,4-D + 100 mg L−1 myoinositol + 3% sucrose Somatic embryo suspension cultures in Bioreactors: WPM supplemented with 100 mg L−1 myoinositol + 0.5 100 mg L−1 IAA + 0.5 100 mg L−1 BA + 3% sucrose |
Bioreactors: 2 L capacity airlift bioreactors and 10 L Nelagene culture vessel cultures were aerated with 4 L min−1 airflow | 25 ± 2 °C, 16 h photoperiod, fluorescent light, 40 µmol m−2 s−1 | Misra and Dey (2013) |
| Vitis amurensis | Stilbene: Resveratrol | Dietary supplement |
Induction of somatic embryos: Murashige and Skoog medium supplemented with 2,4-D (1 mg L−1) + BA (2 mg L−1) + 3% sucrose Somatic embryo suspension cultures in bioreactors: B5 medium + 0.3 mg L−1 + 0.01 mg L−1 NAA and 3% sucrose. |
Bioreactors: 3 L capacity balloon-type bubble bioreactors containing 2 L medium and cultures were aerated with 0.2 vvm sterile air |
During induction of embryos: 25 °C, 16 h photoperiod, fluorescent light, 30 µmol m−2 s−1 Bioreactor cultures: 25 °C in the dark |
Sun et al. (2016) |
ABA Abscisic acid; 2,4-D: 2,4-dichlorophenoxyacetic acid; BA N6-benzyladenine; BAP N6-beznylaminopurine; GA3 Gibberellic acid; IAA Indole-3-acetic acid; IBA Indole-3-butyric acid; LED Light emitting diodes; MS Murashige and Skoog (1962) medium; MT Murashige and Tucker (1969) medium; NAA α-naphthalene acetic acid; PPFD Photosynthetic photon flux density; TDZ Thidiazuron; vvm air volume/medium volume/minute; WPM Woody plant medium (Lloyd and McCown 1981)
Table 2.
Some examples of the examples of in vitro culture of protocorm-like bodies for the production of specialized metabolites
| Species | Specialized metabolite | Main use | Culture conditions | References | ||
|---|---|---|---|---|---|---|
| Nutrient medium | Culture type | Culture conditions (Temperature, light, photoperiod) | ||||
| Anoectochilus formosanus | Phenolics, flavonoids, and polysaccharides | Antioxidants and immunostimulants |
Protocorm induction: MS medium supplemented with 0.2 mg L−1 TDZ + 0.5 mg L−1 NAA + 3% sucrose PLB suspension culture: MS liquid medium containing 3% sucrose. Effect of cytokinin BA (0–7 mg L−1) and KN (0–7 mg L−1); Ammonia/nitrate (NH4 Cl/KNO3), NH4+:NO3− at 0:60, 10:50 20:40, 30:30, 40:30, 50:10 and 60: 0 mM; sucrose, glucose, and fructose (3% each) and sucrose: glucose (1:1), glucose and fructose (1:1), fructose and sucrose (1:1) were tested |
PLB culture in shake flasks | Cultures were maintained under a 10 h day and 14 h dark photoperiod and a PPFD of 20 µmol m−2 s−1 | Zhi-Wei et al. (2012) |
| Dendrobium candidum | Phenolics, flavonoids, coumarins, and polysaccharides | Antioxidants and immunostimulants |
Protocorm induction: MS medium supplemented with 0.2 mg L−1 NAA + 0.5 mg L−1 BA + 3% sucrose PLB suspension culture in shake flasks: MS liquid medium containing 3% sucrose. PLB suspension culture in bioreactors: PLB suspension cultures in 3 L balloon-type bubble bioreactors containing 2 L MS medium supplemented with 0.5 mg L−1 NAA + 2.5% sucrose + 150 mg L−1 NaH2PO4 + 1% banana homogenate. Effect of inoculum density (10, 30, 50, 70, and 90 g L−1), aeration volumes (0.05, 0.1, 0.2, and 0.3 vvm), and culture methods (raft, immersion, and Ebb and flood) were tested |
PLB culture in shake flasks and bioreactors | Cultures were maintained at 25 ± 1 °C and under a 16 h day and 8 h dark photoperiod and a PPFD of 40 µmol m−2 s−1 | Cui et al. (2014a, b, 2015) |
| Dendrobium candidum | Alkaloids and polysaccharides | Antioxidants and immunostimulants |
Protocorm induction: MS medium supplemented with 0.5 mg L−1 NAA + 2.0 mg L−1 BA + 10% coconut water + 3% sucrose PLB suspension culture in bioreactors: PLB suspension cultures in 3 L balloon-type bubble bioreactors containing 2 L MS medium supplemented with 0.5 mg L−1 NAA + 2.0 mg L−1 BA + 10% coconut water + 3% sucrose. Effect of carbon source (30 g L−1 sucrose, glucose, and fructose), salt strength of MS medium (1/4x, 1/2x, 3/4x, and 1x), phosphate concentration (0, 0.6, 1.2, 1.8, 2.4, and 3 mM) and NH4+/NO3− ratio (0/30/, 10/20, 15/15, 20/10. And 30/0 mM using NH4Cl and KNO3 using a constant nitrogen source of 30 mM) were tested |
PLB culture in bioreactors | The bioreactors were aerated at 0.1 vvm and kept at 25 ± 1 °C and 16 h photoperiod and a PPFD of 40 µmol m−2 s−1 | Yang et al. (2015) |
| Dendrobium candidum | Alkaloids, phenolics, flavonoids, and polysaccharides | Antioxidants and immunostimulants |
PLB suspension culture in shake flasks: 3 g of PLBs were inoculated in 25 mL MS medium taken with 50 mL of Erlenmeyer flasks. The cultures were elicited with 25, 50, 75, 100, and 125 µM MeJA. Another set of cultures was treated with 75 µM MeJA or 75 µM SA or 75 µM MeJA + 75 µM SA or 37.5 µM MeJA + µM SA. PLB suspension culture in bioreactors: 20 g PLB suspension cultures in 3 L balloon-type bubble bioreactors containing 2 L MS medium and treated 75 µM MeJA after 30 days of culture, and treated for 2, 4, 6, 8, or 10 days of elicitation. |
PLB culture in shake flasks and bioreactors | Both flask and bioreactor cultures were kept at 25 ± 1 °C and 16 h photoperiod and a PPFD of 40 µmol m−2 s−1. Bioreactors were aerated with 0.1 vvm. | Wang et al. (2016) |
| Dendrobium Enopi x Dendrobium Pink Lady | Phenolics, and flavonoids | Antioxidants | PLB proliferation in Petri plates: PLBs were cultured with half-strength MS medium 2% sucrose without growth regulators. | PLB culture |
Cultures were maintained at 24 ± 2 °C and 16 h photoperiod. Effect of LED lights such as white light (400–700 nm), far-red (730 nm), green (530 nm), blue (440 nm), red (660 nm), and blue-red (440 and 660 nm) tested. In another experiment effect of LED light intensities such as white (4.63, 5.18, 17.0 µmol m−2 s−1), far-red (1.11, 9.12, 20.8 µmol m−2 s−1), green (0.77, 6.15, 16.9 µmol m−2 s−1), blue (0.91, 6.72, 15.7 µmol m−2 s−1), red (1.26, 15.40, 29.30 µmol m−2 s−1) and blue-red (2.01, 20.30 and 44.80 µmol m−2 s−1) were tested |
Yeow et al. (2020) |
| Dendrobium Sabin Blue | Anthocyanin: Petunidin | Food coloring agent |
PLB proliferation in Petri plates: PLBs were cultured with half-strength MS medium 2% sucrose + 1 mg L−1 BA. Effect of elicitors such as MeJA (25–200 µM), SA (25–200 µM), and melatonin (0.1–25 µM) |
PLB culture | Cultures were maintained at 25 ± 2 °C and 16 h light and 8 h dark photoperiod | Malik et al. (2021) |
| Dendrobium Sabin Blue |
Alkaloid: Dendrobine and Anthocyanin: Petunidin |
Dendrobine is used as analgesic and antipyretic. Petunidin is used food coloring agent |
PLB proliferation in Petri plates: PLBs were cultured with half-strength MS medium 2% sucrose + 1 mg L−1 BA. Effect of elicitors such as Yeast extract (50–200 mg L−1), chitosan (0.5–10 mg L−1), salicylic acid (5-200 mg L−1), silver nitrate (0.1–20 mg L−1) and glutamine (50–200 mg L−1) were tested |
PLB culture | Cultures were maintained at 25 ± 2 °C and 16 h light and 8 h dark photoperiod | Chin et al. (2021) |
| Dendrobium officinale | Alkaloids, flavonoids, and polysaccharides | Dendrobium alkaloids are useful in Alzheimer’s treatment, and flavonoids and polysaccharides are immunostimulants | PLB regeneration and proliferation: PLBs were regenerated on half MS medium + 0.5 mg L−1 NAA + 1.0 mg L−1 BA + 2.0 mg L−1 2,4-D and 100 g L−1 potato homogenate | PLB culture | Cultures were maintained at 25 ± 2 °C and 16 h light and 8 h dark photoperiod | Wang et al. (2021) |
2,4-D: 2,4-dichlorophenoxyacetic acid; BA: N6-benzyladenine; LED: Light emitting diodes; MeJA; Methyl jasmonate; MS: Murashige and Skoog (1962) medium; NAA: α-naphthalene acetic acid; PPFD: Photosynthetic photon flux density; PLB: Protocorm like bodies; TDZ: Thidiazuron; vvm: air volume/medium volume/minute
Impact of the medium parameters
An appropriate medium must be selected to establish cell and organ cultures (Espinosa-Leal et al. 2018; Murthy et al. 2021, 2022, 2023a, b, c). The MS (Murashige and Skoog 1962), B5 (Gamborg et al. 1968), and woody plant media (Lloyd and McCown 1981) are three of the most commonly used culture media for the induction and proliferation of somatic embryos and their suspension cultures (Tables 1 and 2). The highest levels of total salts and nitrogen are found in the MS basal medium, and it has been demonstrated that the MS medium has an impact on the growth of several species (Espinosa-Leal et al. 2018). Several plant species were found to induce and develop embryogenesis in MS media (Tables 1 and 2). For instance, an MS medium has been used in Eleutherococcus sessiliflorus (Shohael et al. 2005), E. chiisanensis (Jeong et al. 2005), E. senticosus (Shohael et al. 2014b), Plumbago rosea (Komaraiah et al. 2004), and Rosa rugosa (Jang et al. 2016) for induction, differentiation, and maturation of somatic embryos. The MS medium is also frequently used to establish embryogenic suspension cultures and produce embryogenic biomass and secondary metabolites (Tables 1 and 2). However, in Citrus paradisi (Gavish et al. 1989) the MT medium (Murashige and Tucker 1969) was found to be suitable for the induction of embryogenesis and establishment of embryogenic callus cultures. In Santalum album (Misra and Dey 2013), the woody plant medium was identified as being effective for somatic embryogenesis and the production of sesquiterpenes. In contrast, the MS medium has been used to induce somatic embryos in Vitis amurensis (Sun et al. 2016). However, the B5 medium has been found to be excellent for the establishment of embryogenic suspension cultures and the accumulation of stilbenes in embryogenic cultures. The induction, maturation, and cultivation of somatic embryos in suspension media all depend on the salt content of the medium. For instance, Park et al. (2005) investigated the effect of the MS medium at 1/3, 1/2, full, and double strengths on the regeneration of somatic embryos from Eleutherococcus koreanum root cultures. According to their findings, 1/3 strength MS medium was effective in inducing the highest number of somatic embryos per explant, making it appropriate for embryogenesis. These results support earlier findings that embryo development from the root segment of Spinach oleracea is facilitated by MS media with lower concentrations of mineral ions (Komai et al. 1996).
To produce biomass and secondary metabolites, PLB cultures have been established in several orchid species, and choosing the correct media was crucial. To produce PLB biomass in Dendrobium candidum, Cui et al. (2015) tested the effects of MS, B5, Knudson C (KC, Knudson 1951), Vacin and Went (VC, Vacin and Went 1949), White (White 1963), Schenk and Hildebrandt (SH, Schenk and Hildebrandt 1972), and Chu (N6, Chu 1978) media. They found that the N6 medium was superior for biomass accumulation, but secondary compound accumulation was low in this medium. In contrast, the MS medium was more effective for accumulating biomass and producing phenolic and flavonoid compounds. According to Wang et al. (2021), D. officinale accumulates PLB biomass on a half-strength MS medium. According to these studies, different plant species have distinct nutritional requirements. Therefore, in plant cell and organ cultures, one approach to produce biomass and secondary metabolites is to select an appropriate medium composition and salt strength (Murthy et al. 2014).
Impact of growth regulators
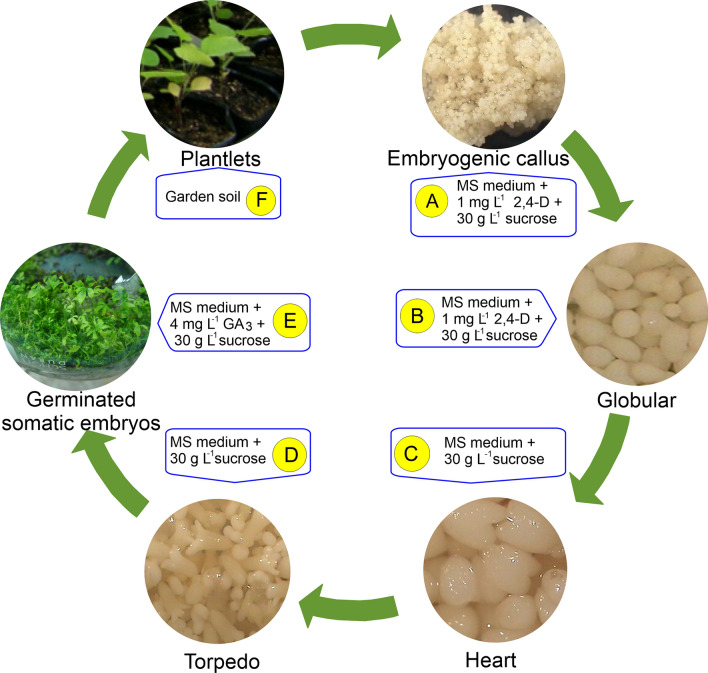
Growth regulators are typically added exogenously to cell and organ cultures to promote growth and accumulation of biomass and secondary metabolites (Murthy et al. 2014, 2023a, b). Auxins, 2,4-dichloro-phenoxy acetic acid (2,4-D), and naphthalene acetic acid (NAA) have been used to induce somatic embryogenesis; however, cytokinins, particularly benzyl adenine (BA) and kinetin, are responsible for regulating the growth and proliferation of somatic embryos. However, in some plant species, somatic embryonic development occurs in hormone-free environments (Sugimoto et al. 2019). Abscisic acid supplementation is occasionally necessary for embryo maturation, whereas gibberellic acid (GA) promotes embryo germination (Sugimoto et al. 2019). Consequently, by adjusting the type and concentration of growth regulators, the proliferation and accumulation of somatic embryo biomass in suspension cultures can be managed. For example, in an MS medium supplemented with 1 mg L−1 2,4-D, E. sessiliflorus leaf segments exhibit embryogenic callus formation (Fig. 2A) and embryo differentiation (Shohael et al. 2005; Fig. 2B). Shohael et al. (2005) developed embryogenic cell suspension cultures in an MS liquid medium and reported that an increase in 2,4-D concentration (> 2 mg L−1) in the culture medium resulted in the development of non-embryogenic calli, whereas further somatic embryo development was reported in the MS medium without growth regulators (globular, heart, and cotyledonary; Fig. 2C, D). Somatic embryos germinate in a medium enriched with 4 mg L−1 GA3 (Fig. 2E) and converted into plantlets (Fig. 2F), according to Shohael et al. (2005). In contrast, after the development of somatic embryos from cotyledon explants on an MS medium supplemented with 6 mM NAA and 30 mM glutamine, Psoralea corylifolia requires abscisic acid treatment for embryo maturation and GA3 treatment for embryo germination in subsequent establishing suspension cultures (Baskaran and Jayabalan 2009). Eleutherosides (B, E, and E1), chlorogenic acid, phenolics, and flavonoids accumulate in E. sessiliflorus during embryonic development (globular > heart > torpedo > cotyledonary), with the highest concentrations observed in germinated embryos (Shohael et al. 2005). Eleutherococcus chiisanensis (Jeong et al. 2005), E. koreanum (Park et al. 2005), E. senticosus (Shohael et al. 2014a, b), and Catharanthus roseus (Aslam et al. 2010) have demonstrated identical incidences of secondary metabolite accumulation.
Fig. 2.
Somatic embryogenesis of Eleutherococcus sessiliflorus: A Embryogenic callus developed from leaf explants on MS medium with 1 mg L−1 2,4-D and 30 g L−1 sucrose after 6 weeks of culture; B globular embryos developed from an embryogenic callus on MS medium with 1 mg L−1 2,4-D and 30 g L−1 sucrose after 8 weeks of culture; C heart-staged and D torpedo-staged embryos developed from an embryogenic callus upon transfer to an MS medium with 30 g L−1 sucrose after 10 and 12 weeks of culture, respectively; E germinated embryos upon transfer to an MS medium supplemented with 30 g L−1 sucrose and 4 mg L−1 GA3; and F plantlets growing in the greenhouse 2 months after transfer to garden soil (Cocopeat 51%, Peat moss 10%, Vermiculite 13%, Humic acid 0.1%, Perlite 15%, Zeolite 10%, Fertilizer 0.4%; Shinsung Mineral Co., Ltd., Dunchon-aero, Republic of Korea)
Protocorms have been induced from the nodal segment in D. candidum on an MS medium supplemented with 0.1 mg L−1 NAA, 2 mg L−1 BA, and 0.1 mg L−1 kinetin in research by Cui et al. (2015). The proliferation of protocorms, accumulation of biomass, and production of bioactive compounds was subsequently tested in MS media containing 0.05, 0.1, 0.5, 1.0, and 2.0 mg L−1 NAA or indole butyric acid. According to Cui et al. (2015), a medium supplemented with 0.5 mg L−1 NAA promotes protocorm proliferation, the largest biomass accumulation, and accumulation of phenolics (4.26 mg g−1 dry weight (DW) and flavonoids (1.23 mg g−1 DW). Therefore, when controlling the embryogenic phases in suspension cultures, the use of particular types and combinations of growth regulators is greatly beneficial.
Impact of sugars in the medium
Plant cell and organ cultures are usually grown using a single simple sugar or a combination of simple sugars such as glucose, fructose, maltose, and sucrose. The sugars in the medium act as energy sources and supply inorganic nutrients. The supplemental concentration of carbohydrates in the medium greatly affects biomass and metabolite production. For example, Nagella et al. (2011) verified the effects of 10, 20, 30, 40, 50, 60, 70, and 80 g L−1 sucrose on cell cultures of Gymnema sylvestre; 30 g L−1 sucrose favored increased accumulation of biomass, whereas 40 g L−1 sucrose was responsible for the highest accumulation of gymnemic acid. They also examined several sugars, such as glucose, fructose, maltose, and sucrose, in cell suspension cultures of G. sylvestre and found that sucrose was an ideal carbohydrate source for both biomass and gymnemic acid production. Therefore, suitable carbohydrate sources and concentrations should be identified for the production of secondary metabolites in cell and organ cultures. To verify the role of sucrose concentration in embryogenesis, Park et al. (2005) cultured the root segments of E. koreanum in an MS liquid medium supplemented with 15, 30, 60, and 90 g L−1 sucrose. Higher sucrose concentrations were found to be beneficial for promoting embryogenesis, and in the medium supplemented with 60 g L−1 sucrose, they reported optimum embryo regeneration, which was responsible for the increase in embryo biomass when compared with the medium supplemented with 30 g L−1 sucrose (Table 1). Sun et al. (2016) examined the effects of sugars, such as glucose, fructose, lactose, sucrose, and sorbitol, at a concentration of 30 g L−1 on somatic embryo cultures of V. amurensis to produce biomass and resveratrol. They found that the maximum biomass produced was 329.45 g L−1 and the maximum amount of resveratrol produced was 42.88 mg L−1. Sucrose supplementation had a stronger effect on biomass accumulation and metabolite production than did glucose, fructose, lactose, or sorbitol supplementation.
Cui et al. (2015) cultured D. candidum PLBs in an MS medium supplemented with 10, 15, 20, 25, 30, or 50 g L−1 sucrose concentrations to increase protocorm proliferation and metabolite production. They found that 50 g L−1 sucrose was more suitable for biomass accumulation, whereas the highest production of phenolics and flavonoids was recorded in a medium supplemented with 25 g L−1 sucrose. Zhi-wei et al. (2012) found that Anoectochilus formosanus accumulated maximum PLB biomass and phenolic compounds in an MS medium supplemented with 30 g L−1 sucrose. Osmotic pressure and nutrient uptake are significantly affected by sucrose concentration; for PLB proliferation and growth in a variety of orchid species, 30 g L−1 sucrose is typically used (Yang et al. 2015).
Impact of nitrogen concentration
The amount of nitrogen in the nutritional medium has a significant effect on the amount of biomass and metabolites accumulated in plant cells and organ cultures (Murthy et al. 2014, 2022, 2023a, b, c). An MS medium without ammonium ions shows the lowest biomass production, whereas the highest somatic embryo production occurs at 15 mM NH4+, according to Komaraiah et al. (2004), who verified the effect of ammonium (0, 5, 10, 15, and 20 mM NH4+) concentrations on the somatic embryogenesis of P. rosea.
Yang et al. (2015) tested how the NH4+:NO3− ratio affected the accumulation of bioactive and PLB biomass in D. candidum PLBs. They used half-strength MS media at 30 mmol levels with various NH4+:NO3− ratios (0:30, 10:20, 15:15, 20:10, and 30:0) and discovered that NO3− in the culture medium caused increased amounts of PLB biomass (190.1 g fresh and 21.1 g dry biomass). According to Zhi-wei et al. (2012), when the concentration of the NH4+:NO3− ratio is at 60:0 mmol in the MS medium, A. formosanus PLB cultures accumulate flavonoids, phenolics, and polysaccharides at the highest levels (5.43 mg g−1 DW, 2.87 mg g−1 DW, and 243.23 mg g−1 DW, respectively). Correspondingly, they discovered that when NO3− is utilized as the only nitrogen source, PLBs accumulate the most polysaccharides (545.36 g g−1 dry weight) and alkaloids (302.51 g g−1 DW).
Impact of the concentration of phosphate
The production of secondary metabolites is significantly influenced by the phosphate content of plant cells and the tissue culture medium. In cell cultures of Panax ginseng and P. quinquefolius, a higher concentration of saponins is caused by an increase in phosphate levels (Liu and Zhong 1998). Yang et al. (2015) investigated the effects of MS medium phosphate concentrations (0, 0.6, 1.2, 1.8, 2.4, and 3.0 mmol) on D. candidum PLB biomass and metabolite accumulation. When the phosphate concentration was at the 1.8 mmol level, they recorded the maximum biomass accumulation (159.2 g−1 fresh and 15.7 g−1 dry biomass) and the polysaccharide (296.41 mg g−1 DW) and alkaloid (336.12 g g−1 DW) production.
Somatic embryo suspension culture in bioreactors
Manual handling of different stages of in vitro culture might be eliminated and production costs could be reduced by using a liquid medium in the bioreactor growth of plant cells and organs (Murthy et al. 2023a, b). Because bioreactors can control several variables, including aeration, gas levels such as those of oxygen, carbon dioxide, and ethylene, and hydrogen ion concentrations, the bioreactor culture system has more advantages than the conventional tissue culture approach. Nutrient absorption can be boosted by continuous agitation of the medium. Increasing the cell proliferation and regeneration rates can also accelerate production and improve product quality (Murthy et al. 2014, 2022, 2023a, b). Stirred tank, bubble column, airlift, and wave-mixed bioreactors are the most popular bioreactor designs used for biomass and secondary metabolite production in plants (Murthy et al. 2023a, b). The most crucial decision in choosing a bioreactor design is the type of culture that will be used. Because stirred tank bioreactors typically have mechanical components, such as impellers, that could harm organized structures, they are not suitable for culturing somatic embryos. However, pneumatically driven, bubble column, and airlift bioreactors are often employed. These bioreactors also facilitate an efficient mixing process and have a high oxygen capacity. To cultivate somatic embryos and protocorm-like bodies, modified airlift bioreactors such as balloon-type bubble bioreactors have been studied and used for biomass and secondary metabolite production (Tables 1 and 2).
Eleutherococcus sessiliflorus (Shohael et al. 2005), E. senticosus (Shohael et al. 2014a), E. koreanum (Park et al. 2005) and E. chiisanensis (Jeong et al. 2005) are among the species whose somatic embryos have been cultured in bubble or airlift bioreactors to produce secondary metabolites. Misra and Dey (2013) have used both airlift bioreactors and Nalgene culture vessels to cultivate S. album somatic embryos. Similarly, PLB cultures of D. candidum have been established in modified bubble or airlift bioreactors (Cui et al. 2014a, b; Wang et al. 2016; Yang et al. 2015), and various parameters affecting biomass have been determined, including inoculum density, aeration volume, mode or method of culture, light quality, temperature, and elicitation. The following sections list several variables that affect the generation of secondary metabolites and embryogenic biomass in bioreactor cultures.
The choice of bioreactors and the factors that affect culturing techniques
An appropriate bioreactor configuration must be selected to promote the accumulation of biomass and secondary metabolites (Murthy et al. 2023a, b; Thanh et al. 2006). Shohael et al. (2014a) used balloon-, bulb-, cone-, and cylinder-type bubble bioreactors for culturing E. senticosus somatic embryos and reported the highest accumulation of biomass (102.3 g L−1 fresh and 11.3 g L−1 dry biomass), eleutheroside B (20.0 µg g−1 DW), eleutheroside E (47.2 µg g−1 DW), eleutheroside E1 (34.4 µg g−1 DW), and chlorogenic acid (1.1 mg g−1 DW) in balloon-type bubble bioreactors. These findings were related to the volumetric oxygen transfer coefficient (kLa), which was best in the balloon-type bubble bioreactors. Eleutherococcus sessiliflorus embryo cultures have been tested using a variety of culture methods, including continuous and temporary immersion, using the ebb and flood approach described by Shohael et al. (2005). The continuous immersion system produces the maximum biomass (239.65 g−1 FW and 25.53 g−1 DW). Furthermore, Dendrobium candidum PLB cultures have been established by Cui et al. (2014a) in balloon-type bubble bioreactors using continuous immersion, raft, and ebb and flood techniques. In the immersion cultures, fresh and dry biomass accumulation reached optimal levels of 323.33 g L−1 and 16.13 g L−1, respectively. Immersion cultures were also discovered to have the highest levels of polysaccharides (404.48 mg g−1 DW), coumarins (18.36 mg g−1 DW), polyphenolics (13.33 mg g−1 DW), and flavonoids (3.97 mg g−1 DW).
Inoculum density effects
The ideal inoculum density is one of the elements that influence the biomass and metabolites in bioreactor cultures (Jeong et al. 2009; Murthy et al. 2023a, b). In somatic embryo cultures of E. senticosus, Shohael et al. (2014a) similarly showed the significant impact of inoculum on the accumulation of biomass and the generation of metabolites. They used balloon-type bubble bioreactors with a 3 L capacity and 2 L of MS medium to examine 1, 3, 5, 7, and 9 g L−1 inoculum. They obtained maximum biomass (1037 g L−1 FW and 11.5 g L−1 DW) and optimal productivity of eleutherosides (21.2 µg g−1 DW of eleutheroside B, 49.9 µg g−1 DW of eleutheroside E, 28.9 µg g−1 DW of eleutheroside E1) and chlorogenic acid (1.2 mg g−1 DW) at an inoculum density of 5 g L−1.
In 3 L balloon-type bubble bioreactors using 2 L of MS media, Cui et al. (2014a) generated PLB cultures of D. candidum and examined the effects of inoculum densities of 10, 30, 50, 70, and 90 g L−1 on the accumulation of biomass and secondary metabolites. They observed an increase in biomass with increasing inoculum density, but at an inoculum density of 50.0 g L−1, they observed the best growth rates and the highest concentrations of polysaccharides (399.65 mg g−1 DW), coumarins (19.64 mg g−1 DW), phenolics (14.63 mg g−1 DW), and flavonoids (4.75 mg g−1 DW). These findings highlight the effect of inoculum density on the buildup of biomass and metabolites in bioreactor cultures.
Impact of aeration volume
Because aeration is responsible for mixing biomass with a liquid medium, providing crucial nutrients, and supplying crucial gaseous substances such as oxygen and carbon dioxide, it is a key element that regulates the accumulation of biomass and the generation of metabolites in bioreactors (Jeong et al. 2006; Murthy et al. 2023a, b; Thanh et al. 2006, 2014). Shohael et al. (2014a) evaluated E. senticosus somatic embryo cultures with both a constant air supply of 0.05, 0.1, 0.2, and 0.3 vvm (air volume/medium volume/min) and incremental air supply of 0.05, 0.1, 0.2, and 0.3 vvm (air supply was adjusted once per week over the culture period). They observed the highest accumulation of fresh and dry biomass (99.2 g L−1 FW and 11.3 g L−1 DW), as well as the formation of eleutherosides and chlorogenic acid with variable air volume over the culture period.
In 3 L capacity balloon-type bubble bioreactors for D. candidum PLB cultures, Cui et al. (2014a) investigated the effects of aeration volumes of 0.05, 0.1, 0.2, and 0.3 vvm constant air supply and 0.05–0.3 vvm incremental air supply (increments of 0.5 vvm per week). They reported that a 0.3 vvm continuous air supply was appropriate for biomass growth and metabolite accumulation (416.53 mg g−1 DW of polysaccharides, 20.77 mg g−1 DW of coumarins, 12.01 mg g−1 DW of phenolics, and 5.00 mg g−1 DW of flavonoids). According to Cui et al. (2014a), increasing the aeration volume helps ensure appropriate agitation and prevents embryogenic biomass from settling at the bottom of the bioreactors.
Influence of light quality
Light intensity and quality are essential abiotic factors required by plants for photosynthesis, growth, and secondary metabolite accumulation (Tang et al. 2022). For example, the yield of essential cyclic monoterpenes increases in response to light intensity and quality (Ueda et al. 2021). The light quality (red, far-red, and blue) provided by light-emitting diodes also influences the accumulation of secondary metabolites in plants (Alrifai et al. 2019; Yeow et al. 2020). Light intensity and quality also control growth, biomass accumulation, and secondary metabolite production in cultured cells and organs (Murthy et al. 2014, 2023a, b). Shohael et al. (2006a) tested how fluorescent, blue, red, and blue plus far-red lights affected E. senticosus somatic embryo suspension culture development and metabolite buildup. Although the effect of light on biomass accumulation was negligible, their experimental results demonstrated a stimulatory effect on secondary metabolite accumulation. Eleutheroside E (54.5 g g-1 DW) and eleutheroside E1 (50.4 g g−1 DW) accumulation was stimulated in cultures exposed to red light, whereas eleutheroside B (27.9 g g−1 DW) accumulation was stimulated in cultures exposed to blue light. These findings imply that altering the light quality can be used to manipulate secondary metabolites.
The effect of temperature
Suspension cultures need to be subjected to the best temperature treatment for the accumulation of biomass and secondary metabolites (Murthy et al. 2014). Shohael et al. (2006b) investigated the effects of several temperature regimes, namely 12, 18, 24, and 30 °C, on biomass and metabolite accumulation in E. senticosus somatic embryo bioreactor cultures. They claimed that cultures incubated at 24 °C produced the highest accumulation levels of biomass (102.1 g L−1 fresh and 11.10 g L−1 dry biomass) and metabolites (21.2 g g−1 DW of eleutheroside B, 39.6 g g−1 DW of eleutheroside E1, and 1.0 mg g−1 DW of chlorogenic acid). However, they noted that cultures incubated at a temperature of 12 °C showed the greatest accumulation of eleutheroside E (43.1 g g−1 DW). Incubation temperature can therefore be adjusted to control the accumulation of specific metabolites in suspension cultures.
Elicitation
Plants produce secondary chemicals to defend themselves against pathogen attacks and combat the effects of abiotic stressors. As a result, the use of biotic and abiotic elicitors causes the buildup of secondary chemicals in in vitro cultures (Chen et al. 2019; Ho et al. 2020). In cell and organ cultures, the accumulation of secondary chemicals has been successfully induced by methyl jasmonate (MJ), jasmonic acid, and salicylic acid (Gai et al. 2019; Malik et al. 2021; Murthy et al. 2014). Shohael et al. (2007, 2008) carried out multiple tests and confirmed the impact of MJ (50, 100, 150, 200, 300, and 400 µM) on Eleutherococcus species somatic embryo suspension cultures. They claimed that the MJ treatment affected the growth of E. senticosus embryogenic cultures. Additionally, the amount of total eleutherosides and chlorogenic acid increased with increasing MJ concentration and peaked at 200 µM MJ, indicating 7.3-fold (649.9 g g−1 DW) and 3.9-fold (4.4 g g−1 DW) increases, respectively, in comparison to the control treatments. Moreover, they discovered 1.4-, 3.4-, and 14.9-fold increases in the levels of eleutherosides B, E, and E1, respectively.
Wang et al. (2016) researched the impact of MJ at concentrations of 25, 50, 75, 100, and 125 µM on D. candidum PLB cultures. Their early studies on PLB suspension cultures in Erlenmeyer flasks showed that MJ inhibits PLB growth. To address this issue, they established PLB cultures in bioreactors and maintained the cultures for 30 days without elicitor treatments. After 30 days of cultivation, the cultures were treated with 75 µM MJ and maintained for an additional 6 days. The highest levels of alkaloids (269.5 mg g−1 DW) and polysaccharides were observed in the MJ-treated cells (386.7 mg g− 1 DW). According to these findings, elicitation is a helpful strategy for the hyperaccumulation of secondary metabolites in bioreactor cultures when used with the appropriate elicitor, concentration, and period of treatment.
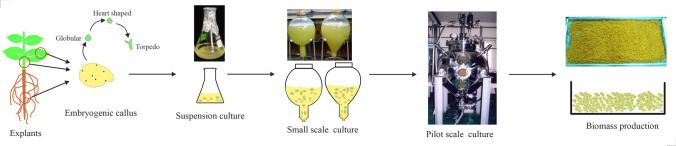
Scale-up process
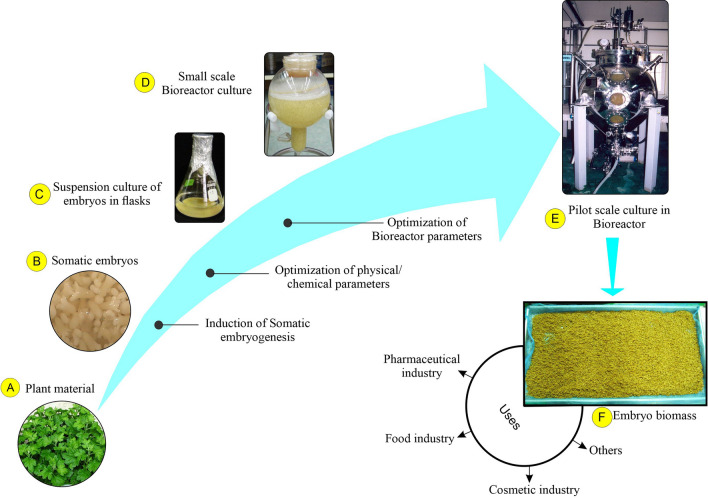
Somatic embryos have been cultured in large-scale airlift bioreactors to produce phenylpropanoid glycosides and coumarin glycosides (Fig. 3). Somatic embryos were induced by using leaf explants (Fig. 3A, B), following systematic testing in small-scale cultures (Fig. 3C), Shohael et al. (2014b) cultivated somatic embryos of E. senticosus using MS media with 3% (w/v) sucrose without growth regulators in 20 L (Fig. 3D) and 500 L balloon-type (Fig. 3E) bioreactors. A 3 g L−1 inoculum of somatic embryos was used to establish the large-scale bioreactors. The bioreactors were then aerated with 0.1 vvm, and the cultures were maintained for 30 days. They obtained 5.7 kg of dried embryo biomass and 63 kg of fresh biomass using the 500 L balloon-type bioreactors (Fig. 3F). They also reported eleutherosides B, E, and E1 concentrations of 220, 413, and 262 g L−1, respectively, with the embryogenic biomass. This study illustrates that the large-scale generation of secondary metabolites can be accomplished using embryogenic suspension cultures and embryo biomass produced by bioreactor cultures is utilized by pharmaceutical, food, cosmetic, and other industries.
Fig. 3.
Suspension cultures of Eleutherococcus senticosus somatic embryos: A Leaf explants were used for callus and somatic embryo induction, B somatic embryos in MS medium supplemented with 30 g L−1 sucrose and 1.0 mg L−1 2,4-D, C embryogenic suspension in flasks containing MS liquid medium supplemented with 30 g L−1 sucrose and 1.0 mg L−1 2,4-D, D embryogenic suspension in 20 L capacity balloon-type bubble bioreactors containing MS medium with 30 g L−1 sucrose, E embryogenic suspension in a 500 L balloon-type bubble bioreactor containing MS medium with 30 g L−1 sucrose, and F somatic embryo biomass harvested from 500 L bioreactors
Conclusions and future perspectives
The pharmaceutical, food, and cosmetic industries currently use cell, adventitious root, and hairy root cultures as in vitro production platforms for secondary metabolites. Somatic embryonic development occurs in some plant species as a result of in vitro cultivation. Somatic embryos cultivated in vitro are capable of secondary metabolism, biomass increase, and multiplication. To date, many plant species have been used to establish embryogenic suspension cultures and different aspects of biomass and metabolite accumulation have been studied. In bioreactor systems, somatic embryos have also been raised to produce secondary metabolites. Other strategies that can be used with embryogenic cultures include the selection of high-performing embryogenic lines, culture optimization, permeabilization, and biotransformation. The use of elicitation and scaled-up techniques can improve the acquisition of secondary products. It is desirable to improve bioprocess procedures to allow for the continual accumulation and release of metabolites. Heterologous gene cloning is also possible if somatic embryos are reliably produced. The somatic embryogenic system may be a dependable system for the production of bioactive substances, heterologous proteins or enzymes, and valuable biomolecules using various molecular, genomic, and proteomic approaches.
Acknowledgements
Hosakatte Niranjana Murthy is thankful to the National Foundation of Korea for the award of Brain Pool Fellowship (No. 2022H1D3A2A02056665).
Author contribution
HNM: conceptualization; HNM, KSJ, JEH, SHL, KYP: investigation, data curation, formalization; SYP: resources, validation; HNM, KSJ, KYP, SYP: writing, review, and editing. All the authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) Grant (MSIT) (No. NRF-2020R1A2C2102401) and the Korea and Technology Innovation Program (Grant Number P0018148) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea).
Data Availability
This review was based on an analysis of prior studies and therefore did not generate a dataset.
Declarations
Conflict of interests
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
The authors did not perform studies with human participants or animals for this review.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hosakatte Niranjana Murthy, Email: hnmurthy60@gmail.com.
So Young Park, Email: soypark7@cbnu.ac.kr.
References
- Aerts RJ, Baumann TW. Distribution and utilization of chlorogenic acid in Coffea seedlings. J Exp Bot. 1994;45:497–503. doi: 10.1093/jxb/45.4.497. [DOI] [Google Scholar]
- Alrifai O, Hao X, Marcone MF, Tsao R. Current review of the modulatory effects of LED lights on photosynthesis of secondary metabolites and future perspectives of microgreen vegetables. J Agric Food Chem. 2019;67:6075–6090. doi: 10.1021/acs.jafc.9b00819. [DOI] [PubMed] [Google Scholar]
- Aslam J, Mujib A, Fatima Z, Sharma MP. Variations in vinblastine production at different stages of somatic embryogenesis, embryo, and field-grown plantlets of Catharanthus roseus L. (G) Don, as revealed by HPLC. In Vitro Cell Dev Biol Plant. 2010;46:348–353. doi: 10.1007/s11627-010-9290-y. [DOI] [Google Scholar]
- Bartolomé B, Estrella I, Hernández T. Changes in phenolic compounds in lentils (Lens culinaris) during germination and fermentation. Z Lebensm Unters Forsch. 1997;205:290–294. doi: 10.1007/s002170050167. [DOI] [Google Scholar]
- Baskaran P, Jayabalan N. In vitro propagation of Psoralea corylifolia L. by somatic embryogenesis in cell suspension culture. Acta Physiol Plant. 2009;31:1119–1127. doi: 10.1007/s11738-009-0330-3. [DOI] [Google Scholar]
- Chandran H, Meena M, Barupal T, Sharma K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol Rep (Amst) 2020;26:e00450. doi: 10.1016/j.btre.2020.e00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Agarwal DC, Lee MR, Lee RJ, Kuo CL, Wu CR, Tsay HS, Chang HC (2016) Influence of LED light spectra on in vitro somatic embryogenesis and LC-MS analysis of chlorogenic acid and rutin in Peucedanum japoanicum Thumb.: a medicinal herb. Bot Stud 57:9. 10.1186/s40529-016-0124-z [DOI] [PMC free article] [PubMed]
- Chen X, Wang DD, Fang XY, Chen XY, Mao YB. Plant specialized metabolism regulated by jasmonate signaling. Plant Cell Physiol. 2019;60:2638–2647. doi: 10.1093/pcp/pcz161. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Liu H, Tong X, Liu Z, Zhang X, Jiang X, Yu X (2021) Somatic embryogenesis and tritepenoid saponin production in Aralia elata (Miq.) Seem. Sci Hortic 285: 110162. 10.1016/j.scienta.2021.110162
- Chin CK, Stanly C, Muniandy A, Ramanathan S, Murugaiyah V, Chew BL, Subramaniam S. Protocorm-like bodies (PLBs) of Dendrobium Sabin blue: a novel source for in vitro production of dendrobine and anthocyanin. In Vitro Cell Dev Biol Plant. 2021;57:874–882. doi: 10.1007/s11627-021-10159-3. [DOI] [Google Scholar]
- Chu CC. Proc Symp Plant tissue culture. Beijing: Science Press; 1978. The N6 medium and its applications to anther culture of cereal crops; pp. 43–50. [Google Scholar]
- Corredoira E, Merkle SA, Martínez MT, Toribio M, Canhoto JM, Correia SI, Ballester A, Vieitez AM. Non-zygotic embryogenesis in hardwood species. Crit Rev Plant Sci. 2019;38:29–97. doi: 10.1080/07352689.2018.1551122. [DOI] [Google Scholar]
- Cui HY, Murthy HN, Moh SH, Cui YY, Lee EJ, Paek KY. Production of biomass and bioactive compounds in protocorm cultures of Dendrobium candidum Wall ex Lindl. Using balloon type bubble bioreactors. Ind Crops Prod. 2014;53:28–33. doi: 10.1016/j.indcrop.2013.11.049. [DOI] [Google Scholar]
- Cui HY, Murthy HN, Moh SH, Cui Y, Lee EJ, Paek KY. Protocorm culture of Dendrobium candidum in balloon type bubble bioreactors. Biochem Eng J. 2014;88:26–29. doi: 10.1016/j.bej.2014.04.003. [DOI] [Google Scholar]
- Cui HY, Murthy HN, Moh SH, Cui YY, Paek KY. Establishment of protocorm suspension cultures of Dendrobium candidum for the production of bioactive compounds. Hortic Environ Biotechnol. 2015;56:114–122. doi: 10.1007/s13580-015-0082-5. [DOI] [Google Scholar]
- De-la-Cruz Chacón ID, Riley-Saldaña CA, González-Esquinca AR. Secondary metabolites during early development in plants. Phytochem Rev. 2012;12:47–64. doi: 10.1007/s11101-012-9250-8. [DOI] [Google Scholar]
- Erb M, Kliebenstein DJ. Plant secondary metabolites as defenses, regulators, and primary metabolites: the blurred functional trichotomy. Plant Physiol. 2020;184:39–52. doi: 10.1104/pp.20.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa-Leal CA, Puente-Garza CA, García-Lara S. In vitro plant tissue culture: means for production of biological active compounds. Planta. 2018;248:1–18. doi: 10.1007/s00425-018-2910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang C, Fernie AR, Luo J. Exploring the diversity of plant metabolism. Trends Plant Sci. 2019;24:83–98. doi: 10.1016/j.tplants.2018.09.006. [DOI] [PubMed] [Google Scholar]
- Gai QY, Jiao J, Wang X, Zang YP, Niu LL, Fu YJ. Elicitation of Isatis tinctoria L. hairy root cultures by salicylic acid and methyl jasmonate for the enhanced production of pharmacologically active alkaloids and flavonoids. Plant Cell Tissue Organ Cult. 2019;137:77–86. doi: 10.1007/s11240-018-01553-8. [DOI] [Google Scholar]
- Gamborg OL, Miller RA, Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968;50:151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Garagounis C, Delkis N, Papadopoulou KK. Unraveling the roles of plant specialized metabolites: using synthetic biology to design molecular biosensors. New Phytol. 2021;231:1338–1352. doi: 10.1111/nph.17470. [DOI] [PubMed] [Google Scholar]
- Gavish H, Lewinsohn E, Vardi A, Fluhr R. Production of flavanone-neohesperidosides in Citrus embryos. Plant Cell Rep. 1989;8:391–394. doi: 10.1007/BF00270076. [DOI] [PubMed] [Google Scholar]
- Guerrieri A, Dong L, Bouwmeester HJ. Role and exploitation of underground chemical signaling in plants. Pest Manag Sci. 2019;75:2455–2463. doi: 10.1002/ps.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Valdes N, Häkkinen ST, Lemasson C, Guillet M, Oksman-Caldentey KM, Ritala A, Cardon F. Hairy root cultures: a versatile tool with multiple applications. Front Plant Sci. 2020;11:33. doi: 10.3389/fpls.2020.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TT, Murthy HN, Park SY. Methyl jasmonate induced oxidative stress and accumulation of secondary metabolites in plant cell and organ cultures. Int J Mol Sci. 2020;21:716. doi: 10.3390/ijms21030716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan R, Asaf S, Numan M, Lubna KKM, Kim K. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy. 2021;11:968. doi: 10.3390/agronomy11050968. [DOI] [Google Scholar]
- Jang HR, Lee HJ, Park BJ, Pee OJ, Paek KY, Park SY. Establishment of embryogenic cultures and determination of their bioactive properties in Rosa rugosa. Hortic Environ Biotechnol. 2016;57:291–298. doi: 10.1007/s13580-016-0012-1. [DOI] [Google Scholar]
- Jeong CS, Chakrabarty D, Hahn EJ, Lee HY, Paek KY. Effects of oxygen, carbon dioxide and ethylene on growth and bioactive compound production in bioreactor culture of ginseng adventitious roots. Biochem Eng J. 2006;27:252–263. doi: 10.1016/j.bej.2005.08.025. [DOI] [Google Scholar]
- Jeong JH, Jung SJ, Murthy HN, Yu KW, Paek KY, Moon HK, Choi YE. Production of eleutherosides in in vitro regenerated embryos and plantlets of Eleutherococcus chiisanensis. Biotechnol Lett. 2005;27:701–704. doi: 10.1007/s10529-005-4693-2. [DOI] [PubMed] [Google Scholar]
- Jeong CS, Murthy HN, Hahn EJ, Lee HL, Paek KY. Inoculum size and auxin concentration influence the growth of adventitious roots and accumulation of ginsenosides in suspension cultures of ginseng (Panax ginseng C.A. Meyer) Acta Physiol Plant. 2009;31:219–222. doi: 10.1007/s11738-008-0206-y. [DOI] [Google Scholar]
- Knudson L. Nutrient solutions for orchids. Bot Gaz. 1951;112:528–532. doi: 10.1086/335687. [DOI] [Google Scholar]
- Komai F, Okuse I, Harada T. Somatic embryogenesis and plant regeneration in culture of root segments of spinach (Spinacia oleracea L) Plant Sci. 1996;113:203–208. doi: 10.1016/0168-9452(95)04285-7. [DOI] [Google Scholar]
- Komaraiah P, Jogeswar C, Ramakrishna SV, Kavi Kishor PB. Acetylsalicylic acid and ammonium-induced somatic embryogenesis and enhanced plumbagin production in suspension cultures of Plumbago rosea L. Vitro Cell Dev Biol Plant. 2004;40:230–234. doi: 10.1079/IVP2003502. [DOI] [Google Scholar]
- Kreis W. Exploring plant cell culture for natural product formation. J Appl Bot Food Qual. 2019;92:216–225. doi: 10.5073/JABFQ.2019.092.030. [DOI] [Google Scholar]
- Lee YI, Hsu ST, Yeung EC. Orchid protocorm-like bodies are somatic embryos. Am J Bot. 2013;100:2121–2131. doi: 10.3732/ajb.1300193. [DOI] [PubMed] [Google Scholar]
- Li Y, Kong D, Fu Y, Sussman MR, Wu H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol Biochem. 2020;148:80–89. doi: 10.1016/j.plaphy.2020.01.006. [DOI] [PubMed] [Google Scholar]
- Liu S, Zhong JJ. Phosphate effect on production of ginseng saponin and polysaccharide by cell suspension cultures of Panax ginseng and Panax quinquefolium. Process Biochem. 1998;33:69–74. doi: 10.1016/S0032-9592(97)00064-2. [DOI] [Google Scholar]
- Lloyd G, McCown BH. Commercially feasible micropropagation of mountain laurel, Kalmia latifolia by shoot tip culture. Proc Int Plant Prop Soc. 1981;30:421–427. [Google Scholar]
- Malik ANA, Uddain J, Chin CK, Chew BL, Subramaniam S. Elicitation of protocorm-like bodies (PLBs) of Dendrobium ‘Sabin blue’ using methyl jasmonate, salicylic acid and melatonin for in vitro production of anthocyanin. Phytochem Lett. 2021;43:60–64. doi: 10.1016/j.phytol.2021.03.008. [DOI] [Google Scholar]
- Marchev AS, Yordanova ZP, Georgiev MI. Green (cell) factories for advanced production of plant secondary metabolites. Crit Rev Biotechnol. 2020;40:443–458. doi: 10.1080/07388551.2020.1731414. [DOI] [PubMed] [Google Scholar]
- Misra BB, Dey S. Culture of East Indian sandalwood tree somatic embryos in air-lift bioreactors for production of santalols, phenolics and arabinogalactan proteins. AoB Plants. 2013;5:plt025. doi: 10.1093/aobpla/plt025. [DOI] [Google Scholar]
- Mohaddab M, El Goumi Y, Gallo M, Montesano D, Zengin G, Bouyahya A, Fakiri M. Biotechnology and in vitro culture as an alternative system for secondary metabolite production. Molecules. 2022;27:8093. doi: 10.3390/molecules27228093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Murashige T, Tucker DPH (1969) Growth factor requirements of Citrus tissue culture. In: Proceedings of the first international citrus symposium, vol 3, pp 1155–1161
- Murthy HN, Lee EJ, Paek KY. Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass and metabolite accumulation. Plant Cell Tissue Organ Cult. 2014;118:1–16. doi: 10.1007/s11240-014-0467-7. [DOI] [Google Scholar]
- Murthy HN, Dandin VS, Paek KY. Tools for biotechnological production of useful phytochemicals from adventitious root cultures. Phytochem Rev. 2016;15:129–145. doi: 10.1007/s11101-014-9391-z. [DOI] [Google Scholar]
- Murthy HN, Dalawai D, Bhat MA, Dandin VS, Paek KY, Park SY. Biotechnological production of useful phytochemicals from adventitious root cultures. In: Ramawat KGA, Ekiert HM, Goyal S, editors. Plant Cell and tissue differentiation and secondary metabolites. Switzerland: Springer; 2021. pp. 469–485. [Google Scholar]
- Murthy HN, Joseph KS, Paek KY, Park SY. Anthraquinone production from cell and organ cultures of Rubia species: an overview. Metabolites. 2022;13:39. doi: 10.3390/metabo13010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy HN, Joseph KS, Paek KY, Park SY. Bioreactor configurations for adventitious root culture: recent advances toward the commercial production of specialized metabolites. Crit Rev Biotechnol. 2023 doi: 10.1080/07388551.2023.2233690. [DOI] [PubMed] [Google Scholar]
- Murthy HN, Joseph KS, Paek KY, Park SY. Bioreactor systems for micropropagation of plants: present scenario and future prospects. Front Plant Sci. 2023;14:1159588. doi: 10.3389/fpls.2023.1159588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy HN, Joseph KS, Paek KY, Park SY. Production of anthraquinones from cell and organ cultures of Morinda species. Appl Microbiol Biotechnol. 2023;107:2061–2071. doi: 10.1007/s00253-023-12440-4. [DOI] [PubMed] [Google Scholar]
- Nagella P, Murthy HN, Chung IM. In vitro production of gymnemic acid from cell suspension cultures of Gymnema sylvestre. R Br Eng Life Sci. 2011;11:537–540. doi: 10.1002/elsc.201000167. [DOI] [PubMed] [Google Scholar]
- Nosov AM. Application of cell technologies for production of plant-derived bioactive substances of plant origin. Appl Biochem Microbiol. 2012;48:609–624. doi: 10.1134/S000368381107009X. [DOI] [Google Scholar]
- Pang Z, Chen J, Wang T, Gao C, Li Z, Guo L, Xu J, Cheng Y. Linking plant secondary metabolites and plant microbiomes: a review. Front Plant Sci. 2021;12:621276. doi: 10.3389/fpls.2021.621276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Ahn JW, Lee WY, Murthy HN, Paek KY. Mass production of Eleutherococcus koreanum plantlets via somatic embryogenesis from root cultures and accumulation of eleutherosides in regenerants. Plant Sci. 2005;168:1221–1225. doi: 10.1016/j.plantsci.2004.12.023. [DOI] [Google Scholar]
- Schenk RU, Hildebrandt AC. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can J Bot. 1972;50:199–204. doi: 10.1139/b72-026. [DOI] [Google Scholar]
- Shohael AM, Ali MB, Yu KW, Hahn EJ, Islam R, Paek KY. Effect of light on oxidative stress, secondary metabolites and induction of antioxidant enzymes in Eleutherococcus senticosus somatic embryos in bioreactor. Process Biochem. 2006;41:1179–1185. doi: 10.1016/j.procbio.2005.12.015. [DOI] [Google Scholar]
- Shohael AM, Ali MB, Yu KW, Hahn EJ, Paek KY. Effect of temperature on secondary metabolites production and antioxidant enzyme activities in Eleutherococcus senticosus somatic embryos. Plant Cell Tissue Organ Cult. 2006;85:219–228. doi: 10.1007/s11240-005-9075-x. [DOI] [Google Scholar]
- Shohael AM, Chakrabarty D, Yu KW, Hahn EJ, Paek KY. Application of bioreactor system for large-scale production of Eleutherococcus sessiliflorus somatic embryos in an air-lift bioreactor and production of eleutherosides. J Biotechnol. 2005;120:228–236. doi: 10.1016/j.jbiotec.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Shohael AM, Murthy HN, Hahn EJ, Lee HL, Paek KY. Increased eleutheroside production in Eleutherococcus sessiliflorus embryogenic suspension cultures with methyl jasmonate treatment. Biochem Eng J. 2008;38:270–273. doi: 10.1016/j.bej.2007.07.010. [DOI] [Google Scholar]
- Shohael AM, Murthy HN, Hahn EJ, Paek KY. Methyl jasmonate induced overproduction of eleutherosides in somatic embryos of Eleutherococcus senticosus cultured in bioreactors. Electron J Biotechnol. 2007;10:533–637. doi: 10.2225/vol10-issue4-fulltext-13. [DOI] [Google Scholar]
- Shohael AM, Khatun SM, Murthy HN, Paek KY. Production of bioactive compounds from somatic embryo suspension cultures of siberian ginseng in bioreactors. In: Paek KY, Murthy HN, Zhong JJ, editors. Production of biomass and bioactive compounds using bioreactor technology. Dordrecht: Springer; 2014. pp. 317–335. [Google Scholar]
- Shohael AM, Murthy HN, Paek KY. Pilot-scale culture of somatic embryos of Eleutherococcus senticosus in airlift bioreactors for the production of eleutherosides. Biotechnol Lett. 2014;36:1727–1733. doi: 10.1007/s10529-014-1534-1. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Temman H, Kadokura S, Matsunaga S. To regenerate or not to regenerate: factors that drive plant regeneration. Curr Opin Plant Biol. 2019;47:138–150. doi: 10.1016/j.pbi.2018.12.002. [DOI] [PubMed] [Google Scholar]
- Sun D, Li C, Qin H, Zhang Q, Yang Y, Ai J. Somatic embryos cultures of Vitis amurensis Rupr. In air-lift bioreactors for the production of biomass and resveratrol. J Plant Biol. 2016;59:427–434. doi: 10.1007/s12374-016-0022-7. [DOI] [Google Scholar]
- Sun C, Shang X, Fang S, Yang W, Cao Y, Ding H, Li X. Association analysis between genotype and environment: differentiation between Cyclocarya paliurus resources that accumulate triterpenoids. Front Plant Sci. 2022;13:945897. doi: 10.3389/fpls.2022.945897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Guo H, Baskin CC, Xiong W, Yang C, Li Z, Song H, Wang T, Yin J, Wu X, Miao F, Zhong S, Tao Q, Zhao Y, Sun J. Effect of light intensity on morphology, photosynthesis and carbon metabolism of alfalfa (Medicago sativa) seedlings. Plants (Basel) 2022;11:1688. doi: 10.3390/plants11131688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang LP, Zhang XS, Su YH. Regulation of cell reprogramming by auxin during somatic embryogenesis. Abiotech. 2020;1:185–193. doi: 10.1007/s42994-020-00029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanh NT, Murthy HN, Paek KY. Optimization of ginseng cell culture in airlift bioreactors and developing the large-scale production system. Ind Crops Prod. 2014;60:343–348. doi: 10.1016/j.indcrop.2014.06.036. [DOI] [Google Scholar]
- Thanh NT, Murthy HN, Yu KW, Seung Jeong C, Hahn EJ, Paek KY. Effect of oxygen supply on cell growth and saponin production in bioreactor cultures of Panax ginseng. J Plant Physiol. 2006;163:1337–1341. doi: 10.1016/j.jplph.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Tian R, Paul P, Joshi S, Perry SE. Genetic activity during early plant embryogenesis. Biochem J. 2020;477:3743–3767. doi: 10.1042/BCJ20190161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twaij BM, Hasan MN. Bioactive secondary metabolites from plant sources: types, synthesis, and their therapeutic uses. Int J Plant Biol. 2022;13:4–14. doi: 10.3390/ijpb13010003. [DOI] [Google Scholar]
- Ueda T, Murata M, Yokawa K. Single wavelengths of LED light supplement promote the biosynthesis of major cyclic monoterpenes in Japanese mint. Plants (Basel) 2021;10:1420. doi: 10.3390/plants10071420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacin EF, Went FW. Some pH changes in nutrient solutions. Bot Gaz. 1949;110:605–613. doi: 10.1086/335561. [DOI] [Google Scholar]
- Venditti A, Bianco A. Sulfur-containing secondary metabolites as neuroprotective agents. Curr Med Chem. 2020;27:4421–4436. doi: 10.2174/0929867325666180912105036. [DOI] [PubMed] [Google Scholar]
- Wang S, Alseekh S, Fernie AR, Luo J. The structure and function of major plant metabolite modifications. Mol Plant. 2019;12:899–919. doi: 10.1016/j.molp.2019.06.001. [DOI] [PubMed] [Google Scholar]
- Wang Z, Jiang W, Liu Y, Meng X, Su X, Cao M, Wu L, Yu N, Xing S, Peng D. Putative genes in alkaloid biosynthesis identified in Dendrobium officinale by correlating the contents of major bioactive metabolites with genes expression between protocorm-like bodies and leaves. BMC Genomics. 2021;22:579. doi: 10.1186/s12864-021-07887-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HQ, Jin MY, Paek KY, Piao XC, Lian ML. An efficient strategy for enhancement of bioactive compounds by protocorm-like body culture of Dendrobium candidum. Ind Crops Prod. 2016;84:121–130. doi: 10.1016/j.indcrop.2016.02.001. [DOI] [Google Scholar]
- Wawrosch C, Zotchev SB. Production of bioactive plant secondary metabolites through in vitro technologies-status and outlook. Appl Microbiol Biotechnol. 2021;105:6649–6668. doi: 10.1007/s00253-021-11539-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PR. The cultivation of animal and plant cells. 2. New York: Ronald Press; 1963. [Google Scholar]
- Wojcik AM, Wojcikowska B, Gaj MD. Current perspectives on auxin-mediated genetic network that controls the induction of somatic embryogenesis in plants. Int J Mol Sci. 2020;21:1333. doi: 10.1042/BCJ20190161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji K, Ichihara Y. The role of catechin and epicatechin in chemical defense against damping-off fungi of current-year Fagus crenata seedlings in natural forest. For Pathol. 2012;42:1–7. doi: 10.1111/j.1439-0329.2010.00709.x. [DOI] [Google Scholar]
- Yang X, Zhang X. Regulation of somatic embryogenesis in higher plants. Crit Rev Plant Sci. 2010;29:36–57. doi: 10.1080/07352680903436291. [DOI] [Google Scholar]
- Yang F, Wei NN, Gao R, Piao XC, Lian ML. Effect of several medium factors on polysaccharide and alkaloid accumulation in protocorm-like bodies of Dendrobium candidum during bioreactor culture. Acta Physiol Plant. 2015;37:94. doi: 10.1007/s11738-015-1843-6. [DOI] [Google Scholar]
- Yeow LC, Chew BL, Sreeramanan S. Elevation of secondary metabolites production through light-emitting diodes (LEDs) illumination in protocorm-like bodies (PLBs) of Dendrobium hybrid orchid rich in phytochemicals with therapeutic effects. Biotechnol Rep (Amst) 2020;27:e00497. doi: 10.1016/j.btre.2020.e00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi-wei LV, Wen-Ping Wu L-WBI, Xing-Xing J, Wa-ying W. Establishment of protocorm-like bodies of Anoectochilus formosanus Hayata in suspension culture for the production of secondary metabolites. China Biotechnol. 2012;32:43–50. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This review was based on an analysis of prior studies and therefore did not generate a dataset.