Abstract
A major obstacle to agricultural production and yield quality is heavy metal contamination of the soil and water, which leads to lower productivity and quality of crops. The situation has significantly worsened as a result of the growing population and subsequent rise in food consumption. The growth of nutrient-rich plants is hampered by lead (Pb) toxicity in the soil. Brassica oleracea L. (broccoli) is a prominent vegetable crop in the Brassicaceae family subjected to a number of biotic and abiotic stresses that dramatically lower crop yields. Seed priming is a novel, practicable, and cost-effective method that can improve various abiotic stress tolerances. Many plant metabolic activities depend on the antioxidant enzyme glutathione (GSH), which also chelates heavy metals. Keeping in view the stress mitigation potential of GSH, current research work was designed to inspect the beneficial role of seed priming with GSH on the growth, morphological and gas exchange attributes of broccoli seedlings under Pb stress. For this purpose, broccoli seeds were primed with 25, 50, and 75 µM L−1 GSH. Plant growth and photosynthetic activity were adversely affected by Pb stress. Furthermore, Pb stress enhanced proline levels along with reduced protein and phenol content. The application of GSH improved growth traits, total soluble proteins, chlorophyll content, mineral content, and gas exchange parameters. The involvement of GSH in reducing Pb concentrations was demonstrated by an improved metal tolerance index and lower Pb levels in broccoli plants. The results of the current study suggest that GSH can be used as a strategy to increase broccoli tolerance to Pb by enhancing nutrient uptake, growth and proline.
Keywords: Lead, Glutathione, Proline, Seed priming, Growth
Introduction
The rapid growth in population led to environmental pollution and chemical toxicity problems in agricultural soil (Yan et al. 2020). Heavy metals, acids, and pesticides have internalized in air, soil, and water resources as a result of rapid industrialization and urbanization and they impact the ecosystem, growth of plants and animals (Gill et al. 2021; Moussa et al. 2023). Heavy metals cause agro-biological system damage and lower profitability by altering their physiology functions. Lead (Pb) is known as the second-worst heavy metal for all living things. Automobile exhaust and Pb-containing fertilizers has become a major issue for Pb pollution worldwide (Öğütücü et al. 2021; Salavati et al. 2021). Agricultural sites become contaminated with Pb because of the continuous irrigation of agricultural fields with soil drained water from industrial effluents. According to WHO, the safe Pb levels for plants and soil are 2 ppm and 85 ppm, respectively (Öğütücü et al. 2021). The majority of plants exhibit hazardous symptoms when Pb concentration in the soil exceed 200 mg kg−1 (Yasseen and Al-Thani 2013). In addition, animals are more exposed to metals when they eat food cultivated on sites with metal contamination (Khan et al. 2019). Zinc (Zn), copper (Cu), manganese (Mn), and iron (Fe) uptake and distribution are impeded by metal stress (González et al. 2016). The family Brassicaceae includes some of the most popular vegetables, including broccoli, which is widely grown throughout the world. In comparison to 15.00 million tons in 1999, around 26.92 million tons of broccoli and cauliflower were produced globally in 2019 (FAOSTAT 2021). Due to the presence of nutrients like vitamins, minerals, dietary fibers, etc. and phytochemicals like phenolic compounds, glucosinolates, etc., it is recommended as healthy food and nutrient rich vegetable (Chen et al. 2022; Li et al. 2022). Numerous reports have revealed the health benefits of broccoli, including its anti-inflammatory, anti-microbial, anti-cancerous, neuroprotection, ability to regulate metabolic disorders, and renoprotective qualities (Gigliotti et al. 2020; Lee et al. 2019; Chillón et al. 2019; Nadeem et al. 2020). The consequences of growing broccoli in soil contaminated with Pb were examined by Isabel et al. (2008), who came to the conclusion that the accumulation of Pb in the roots and shoots casts doubt on the healthfulness of broccoli consumption under Pb pollution.
The viability of the seed and the physiological characteristics of the plants are improved by hydration treatments like priming. It also increases the germination rate, emergence, and promoted strong root development of plants (Afzal et al. 2013). Glutathione is a beneficial metabolite that is frequently present in the reduced form of GSH where it serves as a reactive oxygen species (ROS) scavenger prior to being oxidized to produce disulfide of glutathione (Sabetta et al. 2017). Under stressful conditions, GSH biosynthesis is activated to maintain the ratio of reduced/oxidized compounds (Lovato et al. 2017). It is a crucial component of the human and plant immune system because it scavenges reactive oxygen species to help them to reduced abiotic stress (Hameed et al. 2014).
GSH serves a number of biochemical purposes in the plants reaction to stress and is a thiol component that is crucial non-protein, in plant cells (Foyer and Noctor 2005). A prominent function of GSH several cellular tasks is scavenging ROS. Glutathione increases resistance to oxidative stress by directly eliminating certain ROS and maintaining the activity of specific antioxidant components (Hasanuzzaman et al. 2012). The thiol group can form mercaptide bonds with metals because it is nucleophilic. As a result, it helps to sequester toxic metals in the vacuoles, protecting cells from the negative effects of metals (Foyer and Noctor 2005).
Glutathione integrates into primary metabolism, speeds up chlorophyll preservation, and improves photosynthetic pigment production (Khattab 2007). Exogenous GSH enhanced rice seedling development and nutrient uptake while simultaneously increasing endogenous GSH levels and lowering cysteine levels in leaf tissue under chromium stress (Qiu et al. 2013). Furthermore, extensive study has shown that extracellular GSH gives protective effects by upsurge nutrient levels and increasing the tolerance of different plant species to heavy metals (Cai et al. 2011). Additionally, GSH prevents the production of ROS and speeds up the absorption and utilization of nutrients and metals (Mostofa et al. 2014). GSH promotes development, growth, protein activation, and gene expression, in normal and stress conditions and has the ability to bind to and sequester Pb that has been immobilized on its thiol group (Noctor et al. 1998; Shao et al. 2008; Hasanuzzaman et al. 2017a, b). The fundamental mechanisms by which GSH induces stress tolerance in plants are now being investigated. It is vital to assess the impact of Pb-induced toxicity and find an effective remedy for stress relief in broccoli. However, no research has been conducted on the efficacy of GSH for mitigation of Pb stress in broccoli. The current study's objective was to investigate that seed priming with GSH can improve the physicochemical characteristics and growth of broccoli growing in Pb-polluted soil.
Materials and methods
Experiment setup and seed priming
The broccoli seeds were surface sterilized for three minutes by using sodium hypochlorite (0.5%). Glutathione (GSH) was acquired from Sigma Aldrich (Saint-Louis, MO, USA). GSH, 307.3 mg of the substance was dissolved in 1000 mL of distilled water to formulate 1 mM stock of GSH. Then, according to Hassini et al. (2017), three concentrations of GSH 25, 50, and 75 µmol L−1 were made by using this stock solution, and seeds were primed for 10 h in a controlled dim light at 28 °C. In order to facilitate water absorption and expedite germination, the remaining seeds were immersed in water for an hour and which served as control seeds (Cervantes et al. 2013).
The Botanical Garden, Quaid-e-Azam Campus, University of the Punjab was selected as the site for the pot experiment. There was 7 kg of soil in each pot. Lead acetate (Pb (C2H3O2)2 (300 mg/kg) was added as Pb source to the soil and left for homogenization for 15 days under shade prior to sowing of seeds. There were 8 treatments; control (un-contaminated), Pb (contaminated control), GSH1 (25 µmol L−1), GSH2 (50 µmol L−1), GSH3 (75 µmol L−1), GSH1 + Pb, GSH2 + Pb, and GSH3 + Pb. The pots were placed by using a randomized complete block design. Each treatment was repeated four times. Five seeds were sown per pot, and thinning was done after one week, allowing one plant to grow further. The plants were harvested 40 days after sowing.
Assessment of germination and growth
The plants were harvest and cleaned with water to get rid of any dirt that had remained. The root and shoot length was measured. The fresh weight of the root and shoot was estimated by using electrical balance. For the purpose of estimating root and shoot dry weight, plant parts were oven dried at 72 °C for two days. The formula developed by Awasthi et al. (2016) was used to compute the germination percentage.
The formula developed by Carleton and Foote (1965) was then used to calculate leaf area:
Heavy metal detection
The plant material was digested by using HNO3/HCLO4 digestion (Moseley and Jones method 1984). 5 mL of 70% HNO3 (density: 1.3 g mL−1) was added to a dry shoot sample (0.5 g). HCLO4 was also added to this solution. The solution was heated until there is no more emittance of brown fumes. 5mL HCl was diluted (1:1) (with a density of 1.18 g mL−1) after cooling. The resulting solution was then diluted by adding 25 mL of water. Atomic absorption spectrometry (XPLOR AA) was used to calculate the concentration of heavy metals in the extracts (Chapman and Dale 1976).
Estimation of accumulation coefficient (AC) and metal tolerance index (MTI)
The Al-Farraj et al. (2009) formula was used to compute the AC of Pb.
Moreover, the metal tolerance index was determined using the Balint et al. (2007) formula,
Assessment of mineral content
Dried shoot (1 g) was mixed with 20 mL of 65% (w/v) nitric acid. The volume was raised to 100 mL by adding twice as much distilled water to every digested sample. The concentrations of Na+ and K+ were recorded using a flame photometer (Model 410, Corning) (Sagner et al. 1998). Additionally, atomic absorption spectrometry (XPLOR AA-Dual) was used to detect Zn+2 and Mg+2.
Assessment of gas exchange attributes
At 9:30–10:30 A.M, the Infrared gas analyzer (IRGA) LCA-4 (ADC, Ltd) was used to observe the stomatal conductance (gs), net photosynthesis rate (A), and transpiration rate (E) from the top most plant leaf.
Assessment of photosynthetic pigments
20–40 mL of 80% acetone were used to grind 1g of finely chopped fresh leaves and following, centrifugation at 5000–10,000 rpm for 5 min. The process was repeated until the residue was colorless after the supernatant was transferred. The spectrophotometric value was recorded at 663 and 645 nm for chlorophyll a and chlorophyll b, respectively (Arnon 1949). Carotenoids content was recorded by using spectrophotometer at 480 nm (Davies 1965).
whereas OD = Optical Density, FW = Fresh Weight, A = Absorbance.
Determination of proline
After extraction with 10 mL of 0.1 M sulphosalicylic acid and 5 h of centrifugation at 5000 rpm, the 2 g plant sample was obtained. A mixture of 10 mL of acid ninhydrin (140 mM) and 10 mL of glacial acetic acid was vortexed with the supernatant (4 mL). The solution was kept in a hot water bath. When the solution was cooled, 20 mL of toluene was added and the optical density was observed at 520 nm. The proline curve was illustrated using standard L-proline and following formula was used to calculate proline content (Bates et al. 1973).
Assessment of total soluble protein
Before, 1 g of each treatment and control leaf sample was crushed in a pestle and mortar with 2 mL of 1N phosphate buffer (17 g K2HPO4 in 1000 mL of distilled water) and subjected to centrifugation at 6000 rpm for 15 min. Following centrifugation, 0.4 mL of the supernatant was mixed with 2 mL of the Folin mixture and allowed to sit for 15 min. Each sample was then given 0.5 mL of Folin’s Ciocalteau Reagent and shaken before being left at room temperature for 45 min. The optical density at 715 nm was measured with a spectrophotometer. The soluble protein content was determined using a BSA (bovine serum albumin) standard curve (Peterson 1977).
Estimation of total phenolic content
One gram of shoot samples was homogenized in 10 mL of 80% (v/v) methanol and stirred for 15 min at 70 °C (Singleton and Rossi 1965). 1 mL of the methanolic extract was mixed with 250 µL of Folin-Ciocalteu reagent (1 N) and 5 mL of distilled water. The mixture was then kept at 25 °C. The total phenol content of this blue mixture was then determined by comparing the optical density (725 nm) value to the standard curve of gallic acid.
Estimation of 2, 2-diphenyl-1-picrylhydrazyl (DPPH) activity
The antioxidant activity was estimated using the DPPH according to chen et al. (2008) with minor modifications. 1 g plant sample crushed in 20 mL methanol and from 1 mL of this extract, 5 mL of freshly prepared methanolic solution, and 1 mL of 0.1 mM DPPH were added, and the mixture was thoroughly mixed following this mixture was kept in the dark for 60 min. The optical density was determined at 517 nm and % DPPH activity was calculated by using following equation.
Statistical analysis
The average data from four replications were subjected to a one-way ANOVA and Duncan's Multiple Range Test. The values of the variables were considered significant if the P value was at least 0.05. Pearson's correlation analysis was utilized to measure the correlations between the variables under consideration. Principal component analysis (PCA) was also computed between the observed data for broccoli using the Rstudio software. For a better understanding of photosynthetic activity and gaseous exchange characteristics, a radar map was developed using Microsoft Excel.
Results
Estimation of growth attributes
The effects of Pb stress and GSH priming on germination, shoot length, plant root length, as well as fresh and dry weight of shoot and root, are shown in Table 1. The GSH2 (50 µmol L−1) primed seed showed enhanced morphological characteristics both in control and Pb stress. The seed germination was decreased (1.3 folds) in Pb-spiked soil as compared to control. Seed primed with GSH2 treatment enhanced germination by 1.1 folds compared to control seedlings. Whereas, GSH2 + Pb showed 23% increase in germination as compared to Pb-treated control (Pb-TC) condition. While, when plants treated with GSH1 showed 6% enhanced germination as compared to GSH3 under Pb stress. Seed primed with GSH1 enhanced root length by 75% compared to Pb stress. Nevertheless, GSH2 treated plants exhibited increased root length compared to GSH1 and GSH3. The Pb stress reduced the broccoli leaf area by 1.7-folds compared to control. Seed primed with GSH2 had enhanced biomass (1.2-folds) than control. Table 2 showed that application of GSH2 increased shoot fresh weight by 16% over control seedlings. The root fresh weight in the Pb-TC treatment decreased by 28% compared to control. The GSH2 primed seed enhance the total fresh weight (76%) over the Pb-TC (Table 2).
Table 1.
Effect of glutathione on broccoli seedlings under Pb stress in terms of shoot length, root length, overall length, leaf area, number of leaves, and germination %
| Treatments | Shoot length (cm) | Root length (cm) | Total length (cm) | Leaf area (cm2) | No. of leaves | Germination (%) |
|---|---|---|---|---|---|---|
| C | 8.22 ± 0.46b | 1.97 ± 0.07a | 10.15 ± 0.53b | 7.2 ± 0.73b | 5.5 ± 0.28b | 85 ± 8.4bc |
| Pb | 6.5 ± 0.28a | 1.75 ± 0.32a | 8.25 ± 0.14a | 2.93 ± 0.41a | 3.75 ± 0.47a | 65 ± 7.1a |
| GSH1 | 8.7 ± 0.10b | 4.45 ± 0.32b | 13.15 ± 0.40c | 7.76 ± 0.46b | 5 ± 0.40b | 85 ± 6.3bc |
| GSH2 | 10.8 ± 0.27c | 6.25 ± 0.25c | 17.05 ± 0.45e | 15.26 ± 0.44d | 7.75 ± 0.25c | 95 ± 8.12d |
| GSH3 | 8.97 ± 0.02b | 5.75 ± 0.47c | 14.72 ± 0.49d | 15 ± 1.01d | 6 ± 0.40b | 90 ± 7.65 cd |
| GSH1 + Pb | 8.55 ± 0.14b | 3.97 ± 0.02b | 12.52 ± 0.15c | 8.7 ± 1.0b | 5.25 ± 0.21b | 70 ± 8.12a |
| GSH2 + Pb | 8.75 ± 0.24b | 4.7 ± 0.23b | 13.42 ± 0.27c | 12.59 ± 0.98 cd | 6.25 ± 0.47b | 80 ± 7.4b |
| GSH3 + Pb | 8.7 ± 0.12b | 4.4 ± 0.54b | 13.1 ± 0.64c | 10.42 ± 1.9bc | 6 ± 0.40b | 75 ± 8.12bc |
Data display means ± SE of 4 replicates. Non-identical letters indicated significant differences between the treatments at P ≤ 0.05. C = un-contaminated control, Pb = contaminated control (300 mg/kg Pb), GSH1 = 25 μM Glutathione, GSH2 = 50 μM Glutathione, GSH3 = 75 μM Glutathione, Pb + GSH1 = 300 mg/kg Pb + 25 μM GSH, Pb + GSH2 = 300 mg/kg Pb + 50 μM GSH, Pb + GSH3 = 300 mg/kg Pb + 75 μM GSH
Table 2.
Impact of glutathione on total soluble proteins, fresh weight of roots, fresh weight of shoots, and dry mass of broccoli seedlings under Pb stress
| Treatments | Shoot fresh weight (g) |
Root fresh weight (g) |
Total fresh weight (g) |
Shoot dry weight (g) |
Root dry weight (g) |
Total dry Weight (g) |
Total soluble proteins (mg g−1 FW) |
|---|---|---|---|---|---|---|---|
| C | 5.6 ± 0.35bc | 1.95 ± 0.12ab | 7.55 ± 0.45b | 1.77 ± 0.08a | 0.27 ± 0.10b | 2.05 ± 0.15ab | 14.5 ± 0.12b |
| Pb | 3.75 ± 0.28a | 1.52 ± 0.04a | 5.25 ± 0.25a | 0.57 ± 0.27a | 0.12 ± 0.02a | 1.7 ± 0.29a | 9.42 ± 0.41a |
| GSH1 | 5.75 ± 0.45bc | 1.75 ± 0.23a | 7.51 ± 0.44b | 1.97 ± 0.34ab | 0.25 ± 0.02ab | 2.22 ± 0.33abc | 17.65 ± 0.34c |
| GSH2 | 6.52 ± 0.34c | 2.75 ± 0.27 cd | 9.27 ± 0.26c | 3.12 ± 0.11c | 0.32 ± 0.02b | 3.45 ± 0.11d | 22.4 ± 0.42e |
| GSH3 | 6.3 ± 0.17c | 2.4 ± 0.36bc | 8.7 ± 0.46bc | 2.55 ± 0.15bc | 0.25 ± 0.02ab | 2.8 ± 0.13bc | 20.43 ± 0.51d |
| GSH1 + Pb | 4.87 ± 0.42b | 2.67 ± 0.13 cd | 7.55 ± 0.52b | 2.57 ± 0.31bc | 0.23 ± 0.02ab | 2.8 ± 0.29bcd | 16.32 ± 0.41bc |
| GSH2 + Pb | 6.02 ± 0.37c | 3.27 ± 0.12d | 9.30 ± 0.47c | 2.75 ± 0.30bc | 0.29 ± 0.03b | 3.04 ± 0.32d | 18.44 ± 0.43cd |
| GSH3 + Pb | 5.97 ± 0.10c | 3.15 ± 0.08d | 9.12 ± 0.12c | 2.62 ± 0.25bc | 0.28 ± 0.02b | 2.91 ± 0.25cd | 17.54 ± 0.25c |
Data display means ± SE of 4 replicates. Non-identical letters indicated significant differences between the treatments at P ≤ 0.05. C = un-contaminated control, Pb = contaminated control (300 mg/kg Pb), GSH1 = 25 μM Glutathione, GSH2 = 50 μM Glutathione, GSH3 = 75 μM Glutathione, Pb + GSH1 = 300 mg/kg Pb + 25 μM GSH, Pb + GSH2 = 300 mg/kg Pb + 50 μM GSH, Pb + GSH3 = 300 mg/kg Pb + 75 μM GSH
Determination of lead content
Table 3 demonstrated that plants raised in Pb-spiked soil have elevated concentration of Pb, but GSH primed seedlings reduced Pb uptake in broccoli. Pb uptake in GSH2 plants was reduced by 52% compared to Pb-TC plants, (Table 3).
Table 3.
Effects of glutathione and Pb on Pb uptake, accumulation coefficient (AC) and metal tolerance index (MTI) in broccoli
| Treatments | Pb uptake in plant (mg/g) | AC Factor | % MTI |
|---|---|---|---|
| Control | – | – | – |
| Pb | 0.91 ± 0.02c | 3.03 ± 0.008c | 82.92 ± 3.45a |
| GSH1 | – | – | – |
| GSH2 | – | – | – |
| GSH3 | – | – | – |
| GSH1 + Pb | 0.52 ± 0.002b | 1.75 ± 0.01b | 137.07 ± 3.54b |
| GSH2 + Pb | 0.43 ± 0.001a | 1.45 ± 0.04a | 148.4 ± 4.43a |
| GSH3 + Pb | 0.53 ± 0.004b | 1.75 ± 0.01b | 141.9 ± 5.54b |
Data display means ± SE of 4 replicates. Non-identical letters indicated significant differences between the treatments at P ≤ 0.05. C = un-contaminated control, Pb = contaminated control (300 mg/kg Pb), GSH1 = 25 μM Glutathione, GSH2 = 50 μM Glutathione, GSH3 = 75 μM Glutathione, Pb + GSH1 = 300 mg/kg Pb + 25 μM GSH, Pb + GSH2 = 300 mg/kg Pb + 50 μM GSH, Pb + GSH3 = 300 mg/kg Pb + 75 μM GSH
Estimation of accumulation coefficient and metal tolerance index
The seed primed with GSH2 + Pb reduced the AC by 20% compared to GSH1 + Pb. There was a 42% decrease in the accumulation coefficient of GSH1 and GSH3 plants when compared to Pb-TC plants (Table 3). Whereas, GSH2 application decreased the accumulation factor by 52% over Pb-TC plants. The application of GSH2 improved the MTI of broccoli seedlings by 45% in Pb-TC plants. Whereas, GSH2 + Pb treated plants increased MTI by 7.4% over GSH1 + Pb (Table 3).
Assessment of mineral content
Pb toxicity reduced the uptake of mineral nutrients (Mg+2, Na+1, K+1, Zn+2). As compared to plants grown under Pb stress and control conditions, the GSH-primed plants showed enhanced nutrient value. Compared to control and Pb-stressed plants, plants that received GSH2 seed priming significantly enhanced nutrient absorption and accumulation. The magnesium ion concentration improved to 2.8-folds in GSH2 applied plants over Pb-TC plants, while under stress a 1.5-fold increase in Mg levels of GSH2 + Pb plants was recorded as compared to Pb-TC plants. There was a 59% decrease in the Zn+2 content of Pb-TC plants when compared to control. K+ was highest for GSH2 treatment under stress that was onefold higher than GSH1 and GSH3. While the Na+ concentration was 1.5-folds higher in control when compared to Pb-TC plants. While under GSH treatment, GSH2 showed a onefold increase as compared to GSH1 and 1.5 -fold over GSH3. Under Pb spiked soil GSH2 treated plants exhibited a 41% upsurge in Na+ content over Pb only plants (Table 4).
Table 4.
Effects of glutathione and Pb on nutrient content (mg g−1 DW) of broccoli
| Treatments | Mg+2 | Zn+2 | Na+ | K+ |
|---|---|---|---|---|
| Control | 0.44 ± 0.007d | 0.61 ± 0.004d | 21.43 ± 0.30e | 25.16 ± 0.07b |
| Pb | 0.28 ± 0.001a | 0.25 ± 0.006a | 13.41 ± 0.07a | 22.62 ± 0.27a |
| GSH1 | 0.67 ± 0.004f | 0.60 ± 0.006d | 27.47 ± 0.06 g | 34.16 ± 0.08e |
| GSH2 | 0.76 ± 0.004 g | 0.60 ± 0.006e | 29.27 ± 0.03 h | 35.31 ± 0.03 g |
| GSH3 | 0.64 ± 0.004e | 0.60 ± 0.009d | 25.46 ± 0.02f | 34.71 ± 0.006f |
| GSH1 + Pb | 0.38 ± 0.004c | 0.48 ± 0.004b | 16.7 ± 0.04b | 25.16 ± 0.004b |
| GSH2 + Pb | 0.43 ± 0.006d | 0.53 ± 0.004c | 18.79 ± 0.003d | 25.67 ± 0.006d |
| GSH3 + Pb | 0.34 ± 0.004b | 0.48 ± 0.04b | 17.53 ± 0.005c | 25.38 ± 0.02c |
Data display means ± SE of 4 replicates. Non-identical letters indicated significant differences between the treatments at P ≤ 0.05. C = un-polluted control, Pb = polluted control (300 mg/kg Pb), GSH1 = 25 μM Glutathione, GSH2 = 50 μM Glutathione, GSH3 = 75 μM Glutathione, Pb + GSH1 = 300 mg/kg Pb + 25 μM GSH, Pb + GSH2 = 300 mg/kg Pb + 50 μM GSH, Pb + GSH3 = 300 mg/kg Pb + 75 μM GSH
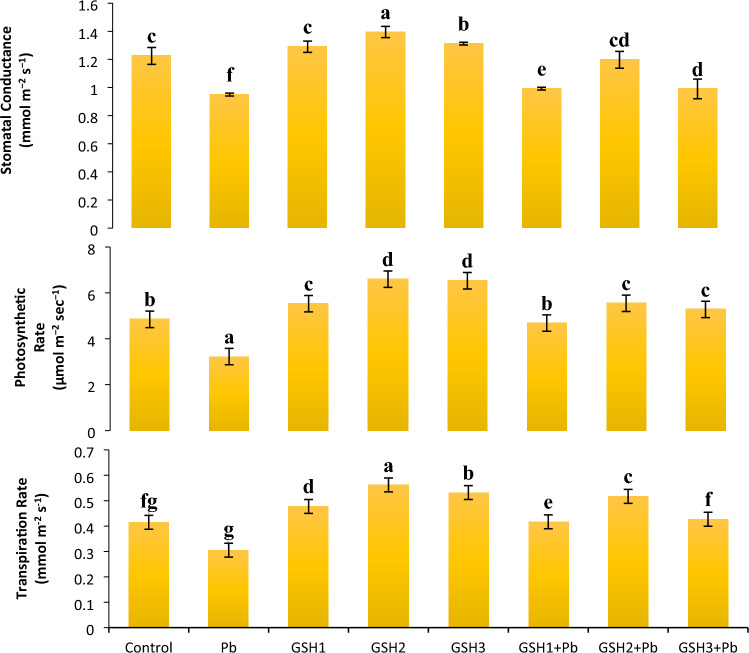
Assessment of gas exchange attributes
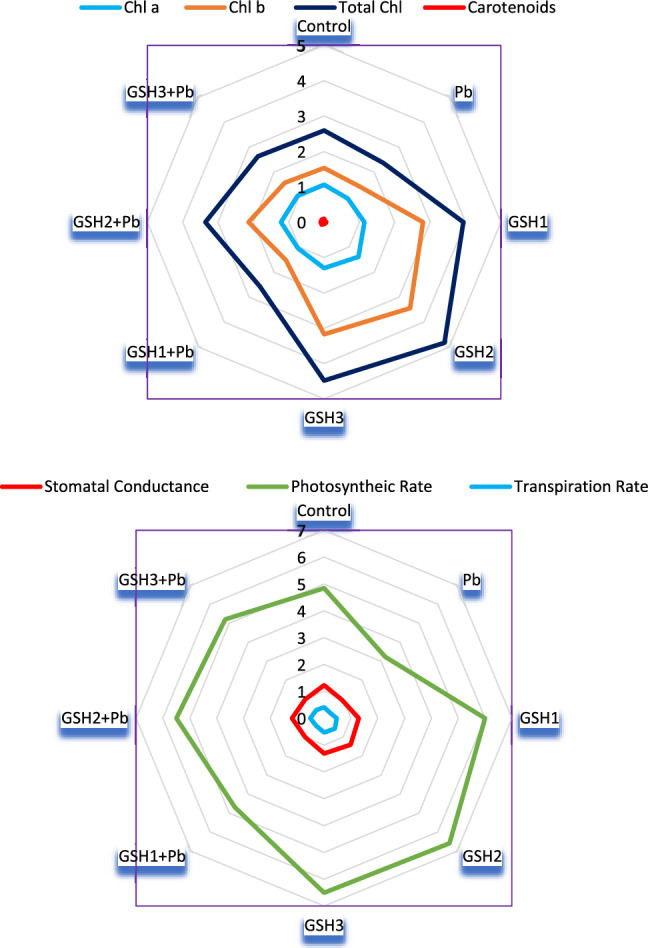
According to Fig. 1, in contrast to plants cultivated in Pb treated control circumstances, plants developed from GSH2 primed seeds showed a twofold greater photosynthetic rate (A) in Pb stress conditions. Figure 1 indicated that gas exchange characteristics were reduced under Pb stress. GSH2 priming significantly increased stomatal conductance (gs) to 13% in non-contaminated conditions compared to control. Lead stress dramatically decreased gs (1.2-folds), net photosynthetic (A) rate (1.5-folds), and transpiration rate (E) (1.3-folds) in broccoli plants compared to control. As compared to the control treatment, GSH2 treated plants exhibited an increase in E (36.5), A (36%), and gs (9.3%). When under Pb stress, the transpiration rate decreased, however, it increased in plants primed with GSH. GSH1 showed a 42% and GSH3 showed 61% increase in photosynthetic rate in Pb stress over Pb only treatment (Fig. 1). The photosynthetic rate was on a upsurge under GSH2 both under stress and non-stress conditions; similarly, the transpiration rate and stomatal conductance showed the same results in radar plot (Fig. 2). GSH priming had an overall positive role in enhancing the gas exchange parameters as compared to control and pronounced results observed against Pb stress.
Fig. 1.
Effects of glutathione on gas exchange attributes in broccoli plants under Pb stress. Data display means ± SE of 4 replicates. Different letters above the bars specify significant variations between the treatments at P ≤ 0.05. C = un-contaminated control, Pb = contaminated control (300 mg/kg Pb), GSH1 = 25 μM L−1 Glutathione, GSH2 = 50 μM L−1 Glutathione, GSH3 = 75 μM L−1 Glutathione, Pb + GSH1 = 300 mg/kg Pb + 25 μM L−1 GSH, Pb + GSH2 = 300 mg/kg Pb + 50 μM L−1 GSH, Pb + GSH3 = 300 mg/kg Pb + 75 μM L−1 GSH
Fig. 2.
Radar graph displaying the photosynthetic activity and gas exchange characteristics of GSH primed broccoli plants grown under Pb stress. At the p ≤ 0.05 level of significance, each point in the radii is the average of 4 replicate for each treatment. C = un-contaminated control, Pb = contaminated control (300 mg/kg Pb), GSH1 = 25 μM L−1 Glutathione, GSH2 = 50 μM L−1 Glutathione, GSH3 = 75 μM L−1 Glutathione, Pb + GSH1 = 300 mg/kg Pb + 25 μM L−1 GSH, Pb + GSH2 = 300 mg/kg Pb + 50 μM L−1 GSH, Pb + GSH3 = 300 mg/kg Pb + 75 μM L−1 GSH
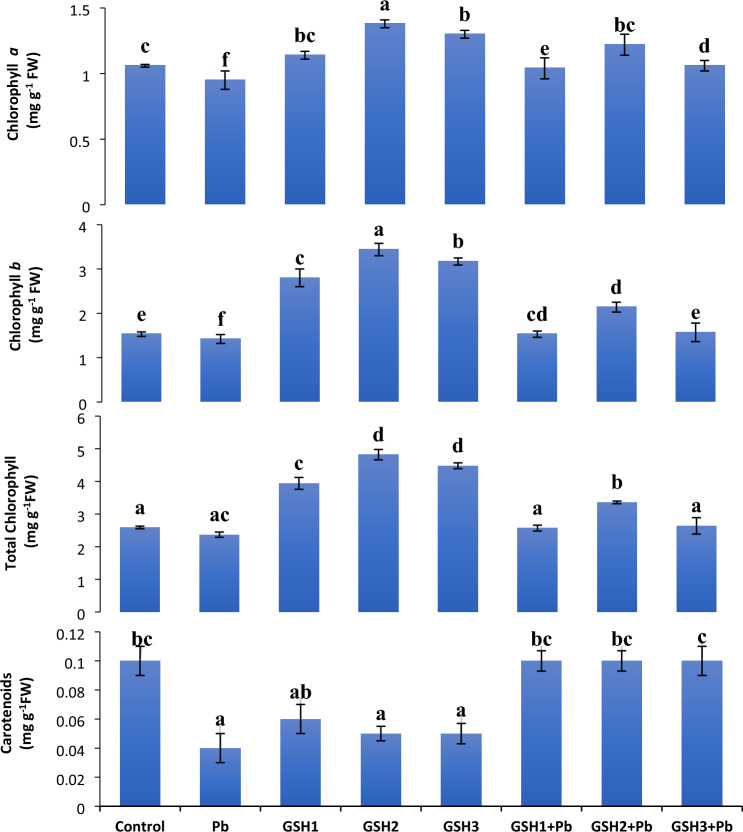
Estimation of photosynthetic pigments
Pb stress resulted in a substantial decrease in all pigment concentrations as compared to control seedligs. Lead stress declined Chl b, Chl a, and carotenoids by 7%, 10%, and 60%, respectively, compared to untreated group. Seeds treated with GSH2 significantly boosted the number of photosynthetic pigments in broccoli plants over Pb-stress seedlings. Lead stress dramatically decreased the total chlorophyll content by 8.4% of broccoli plants compared to control. Compared to control treatment, GSH2 treatment significantly boosted (86.1%) the total chlorophyll content in non-contaminated conditions (Fig. 3). Radar diagram showed that carotenoids and chl a, b were more pronounced at GSH2. While under stress, at GSH2 + Pb the photosynthetic activity was revived and improved as compared to other two conditions (Fig. 2).
Fig. 3.
The effects of glutathione on broccoli plants under Pb stress on Chl a, Chl b, Total chl, and carotenoids. Data display means ± SE of 4 replicates. Different letters above the bars specify significant differences between the treatments at P ≤ 0.05. C = un-contaminated control, Pb = contaminated control (300 mg/kg Pb), GSH1 = 25 μM L−1 Glutathione, GSH2 = 50 μM L−1 Glutathione, GSH3 = 75 μM L−1 Glutathione, Pb + GSH1 = 300 mg/kg Pb + 25 μM L−1 GSH, Pb + GSH2 = 300 mg/kg Pb + 50 μM L−1 GSH, Pb + GSH3 = 300 mg/kg Pb + 75 μM L−1 GSH
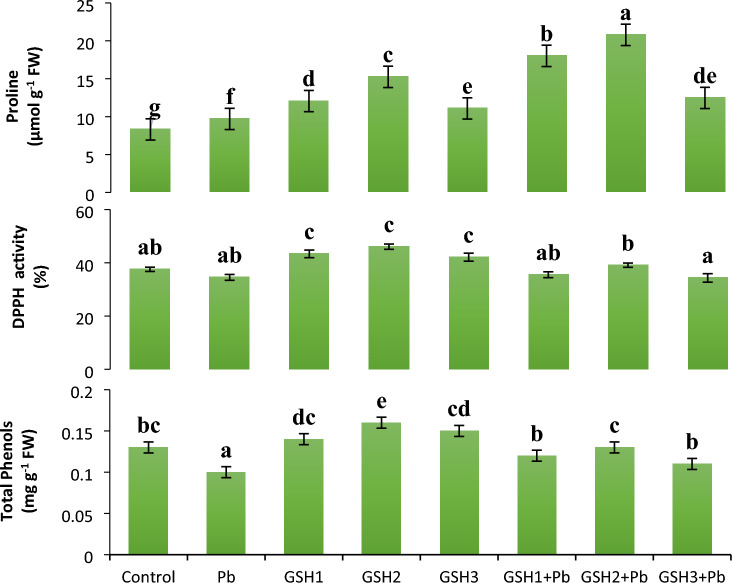
Quantification of free proline content
The results showed that proline synthesis in Pb-affected plants significantly improved by 16% compared to control plants, Additionally, proline contents were significantly enhanced in plants produced from seed primed with glutathione (Fig. 4). Pb-affected plants supplemented with GSH2 displayed the highest proline that was 2.5-folds greater than control. Compared to the plants that had only received GSH2, the Pb-influenced plants that had been primed showed a 36% increase in proline content.
Fig. 4.
The effects of glutathione on the proline, DPPH activity, and phenol content in broccoli plants under Pb stress. Data display means ± SE of 4 replicates. Different letters above the bars specify significant variations between the treatments at P ≤ 0.05. C = un-contaminated control, Pb = contaminated control (300 mg/kg Pb), GSH1 = 25 μM L−1 Glutathione, GSH2 = 50 μM L−1 Glutathione, GSH3 = 75 μM L−1 Glutathione, Pb + GSH1 = 300 mg/kg Pb + 25 μM L−1 GSH, Pb + GSH2 = 300 mg/kg Pb + 50 μM L−1 GSH, Pb + GSH3 = 300 mg/kg Pb + 75 μM L−1 GSH
Estimation of total soluble proteins
The amount of soluble proteins increased in plants that had received GSH2 treatment. Soluble protein in GSH2 plants were enhanced (8.88%) compared to control plants. The protein was enhanced (onefold) in plants treated with GSH2 + Pb over Pb only plants. Compared to the GSH + Pb and Pb-TC treatment, the protein increased by 13.82% (Table 2).
Assessment of total phenolic content
Results demonstrated that proline reduced under Pb stress in broccoli seedlings. The increased levels of phenol were recorded in plants treated with GSH2, which was 1.6-folds higher as compared to Pb-TC plants. When compared to Pb alone, the content of GSH2 phenol increased by 60%. The phenol content in GSH2 treated plants compared to control was 23% enhanced (Fig. 4).
Estimation of antioxidant activity
Increased DPPH activity significantly upsurged in control and GSH primed seedlings. Highest DPPH activity was noted to be 22% elevated in GSH2 plants in comparison to control, and 8% decreased in Pb-TC compared to control. Under GSH2 + Pb, the DPPH activity was 39.12% which was 2 folds higher than control (Fig. 4).
Pearson correlation analysis
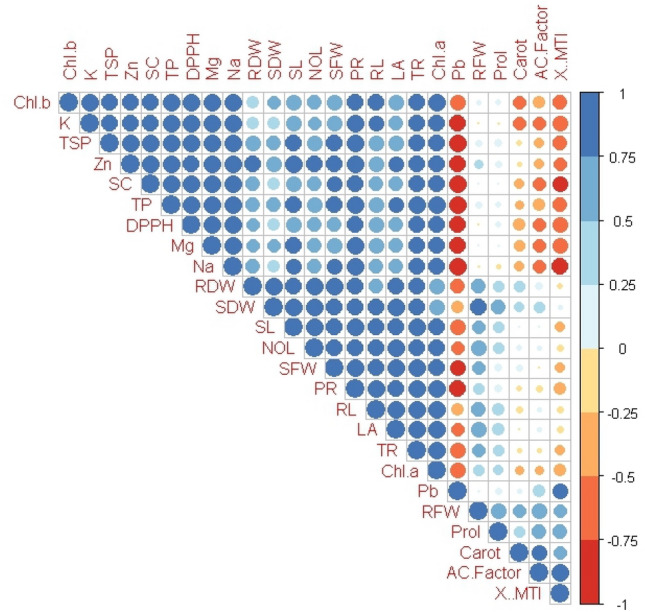
After seed priming with glutathione, the Pearson correlation analysis utilized to assess the relationship between the various studied attributes of broccoli raised in Pb-spiked soil (Fig. 5). The potassium concentration in broccoli shows a negative correlation with Pb and accumulation coefficient while it is positively correlated with shoot fresh weight and root dry weight. Pb content in the plant is positively correlated with proline and accumulation coefficient. While DPPH has a negative correlation with Pb concentration in plant (Fig. 5).
Fig. 5.
Correlation matrix of morphological, physiological, and biochemical parameters of Broccoli evaluated in this research. The abbreviation in the figure denotes: Chl. B (Chlorophyll b), K (Potassium), TSP (Total soluble protein), Zn (Zinc), SC (Stomatal conductance), TP (Total phenols), DPPH (2,2-Diphenyl-1-Picrylhydrazyl), Mg (Magnesium), Na (Sodium), RDW (Root dry weight), SDW (Shoot dry weight), SL (Shoot length), NOL (Number of leaves), SFW (Shoot fresh weight), PR (Photosynthetic rate), RL (Root length), LA (Leaf area), TR (Transpiration rate), Chl. a (Chlorophyll a), Pb (Lead), RFW (Root fresh weight), Prol (Proline), Carot (Carotenoids), AC. Factor (Accumulation factor), MTI (Metal tolerance index)
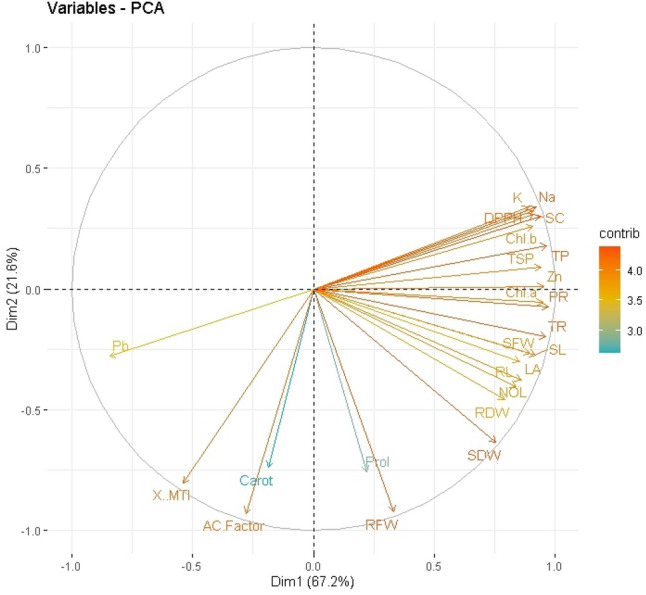
Principal component analysis
Principal Component Analysis loading plots were utilized to further highlight the association between the physiological characteristics and growth attributes of GSH primed broccoli growing in Pb-spiked soil (Fig. 6). With more than 88.8% of the whole database, the first two, Dim1 and Dim2 primary components the largest portion of all components are generated. Dim1 makes up 67.2% of the whole dataset, whereas Dim2 makes up 21.6%. The results showed that the relevant treatments were successfully distributed across the entire dataset. The distribution of all the dataset variables demonstrates clearly that the effects of Pb stress on various morphological and physio-biochemical characteristics were significant for all treatments examined in this study. While seed priming assisted in overcoming adversity. The Pb content was most distant from all other treatments in the current investigation, demonstrating that it plays a substantial detrimental influence in broccoli plant development and physiology. Potassium concentration, DPPH activity, total soluble proteins, photosynthetic rate, number of leaves, shoot length, root length, root and shoot dry weight, and proline are all positively correlated with PC1. The metal tolerance index (MTI) in the shoot, the Pb concentration in the shoot, and the AF of Pb in the shoot were all shown to be significantly inversely correlated (Fig. 6).
Fig. 6.
Principal component analysis loading plots showed a relationship between growth and physiological factors in broccoli plants growing in Pb-contaminated soil following glutathione priming. The following are some of the abbreviations used in the figure: K (Potassium), Na (Sodium), DPPH (2,2-Diphenyl-1-Picrylhydrazyl), Chl. b (Chlorophyll b), TSP (Total soluble proteins), TP (Total phenols), ZN (Zinc), Chl. a (Chlorophyl a), PR (Photosynthetic rate), TR (Transpiration rate), SFW (Shoot fresh weight), SL (Shoot length), LA (Leaf area), RL (Root length), NOL (Number of leaves), RDW (Root dry weight), SDW (Shoot dry weight), Prol (Proline), RFW (Root fresh weight), AC. Factor (Accumulation factor), Carot (Carotenoids), MTI (Metal tolerance index), Pb (Lead)
Discussion
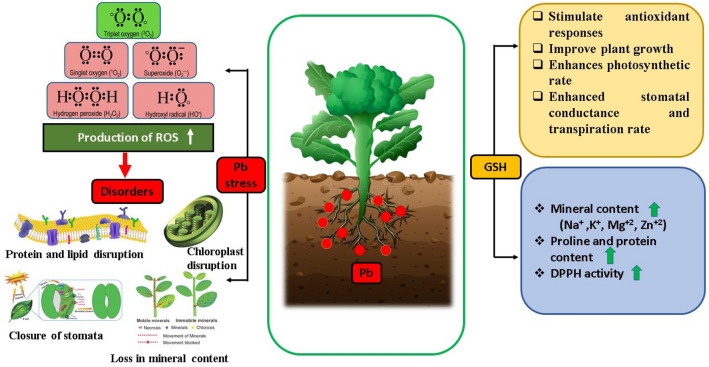
Heavy metal pollution has developed into a critical challenge worldwide in many terrestrial ecosystems. Today, excessive industrialization has caused addition of heavy metals, which has an adverse effect on soil quality and agricultural production (Shahid et al. 2015). As the findings of current investigation revealed that, Pb adversely affected the morphological traits of broccoli (Fig. 7). The Table 1 showed marked reductions in shoot and root length, leaf area and number of leaves similar to previously reported literature where it was concluded that Pb toxic effects on the growth parameters might be attributed to the blockage in the pathway of nutrient uptake in root zone of plant due to Pb pollution (Singh et al. 2016). Pb stress reduced the number of leaves and leaf area in the current study that is in agreement with the results of Marschner (2011), who stated that Pb or Cd stress caused reduction in cell expansion and cell division result in change of morphology of leaf and leaf area is disturbed. In the current study it has been observed that Pb toxicity caused significant decrease in plant biomass and overall growth attributes of plant (Table 1, 2) which is similar to the findings of Arbaoui et al. (2014) where root growth is impeded by Pb exposure because of nutrient deficiencies, an imbalance brought on by Pb accumulation, and ion absorption. According to Herlina et al. (2021) stem dry biomass is also influenced by plant diameter, which further explains the decline in stem biomass exposed to Pb as a result of plant growth suppression. However, the results showed that glutathione (25, 50 and 75 µmol GSH) primed plants exhibited substantial improvement in growth as GSH acts as a master antioxidant and helps to cope up against environmental hazards (Hasanuzzaman et al. 2018).
Fig. 7.
Diagrammatic representation of the impact of glutathione on the physiochemical and growth characteristics of broccoli under Pb stress
Seed priming enhanced the seed vigour, viability, and survival of rice seeds in both stress and non-stress conditions (Zheng et al. 2016). In addition to promoting more coordinated and healthier crop stand, priming improves seed germination (Sarkar et al. 2020). Reduced seed germination and poorer plant biomass output throughout this investigation demonstrated that Pb poisoning has a major detrimental effect on root and shoot development in broccoli (Table 1). GSH seed priming improved seed viability and accelerated early seedling emergence (Molnár et al. 2020), enabling the plants to increase Pb tolerance through improved seedling establishment. GSH priming increased Pb tolerance in the current investigation, as seen by the many physiological and biochemical characteristics recorded in broccoli plant (Table 3). GSH also helps other antioxidant components for better function under stress conditions. It is also involved in ROS detoxification and heavy metal chelation in plants. Since GSH is essential to the glyoxalase and antioxidant defense systems, it protects cells against oxidative damage brought on by heavy metals (Hasanuzzaman et al. 2017a, b).
In addition, GSH and the enzymes that metabolize it, such as glutathione peroxidase (GPX), glutathione-s-transferase (GST), glutathione reductase (GR), etc. detoxify, chelate, and absorb heavy metal, which protects against ROS (Hasanuzzaman et al. 2019). Hossain et al. (2012) reported that because a substantial GSH pool is maintained and the cell regulated redox equilibrium is maintained that leads to increased proline levels in heavy metal tolerance, similar to the results of this study (Fig. 4). In the current study, GSH 2 (50 µmol L−1) treatment reduced Pb uptake in the broccoli plant by 52%, supporting earlier research that GSH protects plants from heavy metal stress (Table 3). The application of GSH increased the Ca+2, K+1, Mg+2, and Mn+2 levels in rice plants in the Cr stress compared to the control (Qiu et al. 2013) supported our results in broccoli seedlings (Table 4). Magnesium is necessary for a number of physiological and biochemical functions, including the transfer of photoassimilates and photosynthetic activity in plants. (Tränkner et al. 2018). According to Häussling et al. (1998), Picea under Pb stress had a lower Mg+2 content. To boost activities of enzyme in the cytoplasm necessary for supporting crop growth and enhancing yield, an optimal potassium K+: Na+ ratio is required (Wakeel 2013). Pb changes various cell structures and activities that rely on the integrity of these components by enhanced K+ inflow from the cell and intake of Ca2+ and Mg2+ linked to the cell wall that caused nutrient deficiency (Ali et al. 2014). GSH has been shown to stabilize the plasma membrane during stress in previous studies (Zechmann 2014). Similar results have been observed in the current investigation, where GSH assist in mitigation of the Pb induced stress and enhanced the uptake of nutrient content (Table 4).
The findings of current study showed a loss in the photosynthetic activity under Pb stress because impairment in chlorophylls and carotenoids which was restored by GSH application (Fig. 3), augmented to the study of Khan et al. (2019). According Khan et al. (2019), upon exposure to Pb, conformational alterations in the chloroplasts were seen in the distal and central leaflets of upland cotton while GSH (50 μM) caused increase in carotenoids, chlorophyll a and b contents and increased fluorescence ratios. In addition, Mg2+ ions in chlorophyll are changed by heavy metals into heavy metal-chlorophyll complexes (Küpper et al. 2006). Additionally, because Pb2+ is similar in configuration to Mg2+, it may take the place of the latter in chlorophylls, further degrading the chloroplast ultrastructure (Gill et al. 2022). Rearrangement of the chloroplast is a defensive mechanism against Pb phytotoxicity, according to studies on Lemna trisulca L. (Samardakiewicz et al. 2015), that is consistent with present results where chl a, b was deteriorated by Pb stress. Additionally, GSH aids in the preservation of chloroplast ultrastructure by promoting glutathionylation, a mechanism of protein post—translational changes via disulphide bonds that uses H2O2 as a stimulator in chloroplasts (Zaffagnini et al. 2012). When subjected to abiotic stress, glutathione may prevent the degradation of photosynthetic pigments (Kattab 2007). In our study, GSH primed seed significantly elevated the photosynthetic activity (Fig. 1). To maintain a high amount of hydration during abiotic stress, stomata are typically closed by plants. Reduced photosynthesis and transpiration rate are brought on by stomatal closure (Fathi and Tari 2016). One of the many functions that glutathione and proline perform in plants is to regulate plant growth and developmental processes (Ogawa 2005; Lehmann et al. 2010). When a plant is subjected to metal stress, GSH-based defense mechanisms as well as an augmented accumulation of osmolytes like proline are triggered. (Noctor et al. 2012; Talukdar 2012; Talukdar and Talukdar 2014). A GSH-regulated defense mechanism is triggered in response to abiotic stress to maintain a decreased cellular redox potential by metabolizing the different ROS and related byproducts, as well as the buildup of proline that sustains turgor pressure along with cellular redox equilibrium (Lehmann et al. 2010; Kishor and Sreenivasulu, 2014). To sustain the lowered cellular redox state, the GSH-based defense mechanism is activated by metabolizing different ROS and their metabolites (Kocsy et al. 2004). Many plants have been shown to accumulate proline under heavy metal stress. Proline functions as a heavy metal chelating agent in addition to being an osmoprotectant and a ROS quencher, reduction of the hazardous effects of heavy metals (Dai et al. 2023). The findings of this study shows that GSH priming enhanced the proline level in order to combat stress (Fig. 4).
To move metals from one cellular organelle to the next and then remove it from the plants, several plant proteins function as transporters. Heavy metals and metalloids cause overexpression of certain proteins in plants at first, which in turn activates genes associated to stress. Some antioxidant enzymes found in plant proteins, such as glutathione and ascorbate peroxidase, protect plants from abiotic stress (Jain et al. 2018). Along with actively participating in the removal of ROS, GSH also keeps other antioxidants like zeaxanthin and tocopherols in their reduced state, protecting cell membranes indirectly. Additionally, under stressful circumstances, GSH prevents denaturation of proteins. It sends signals to plants to maintain cellular homeostasis, preventing stress-related cellular damage (Noctor 2006). In the current investigation, the total soluble protein was highest for plants under GSH2 treatment (Table 2).
The distribution of phenolic, which are most evident secondary metabolites in plants, may be seen throughout the whole metabolic process. These phenolic compounds, also known as polyphenols, are made up of a different substance, like simple phenolic acids, complicated flavonoids, and vibrant anthocyanins (Babbar et al. 2014). These phenolic chemicals are often connected to plant protective mechanisms (Edreva et al. 2008). When compared to broccoli control leaves in the current study, the plant's total phenolic components have usually reduced under Pb contamination (Fig. 4). According to prior research, CdSO4 treatment had little to no effect on the total phenolic content of Erica andevalensis leaves in general (Garca et al, 2012). According to the findings of this study, the decrease in phenolic compounds should be due to heavy metal stress, which impairs the activity of specific enzymes involved in the production of phenolic compounds (Fig. 4).
Conclusions
Our findings showed that seed priming with GSH reduces the stress caused by Pb stress. Seed priming may be a useful strategy for crop development. In Pb-amended soils, seed primed with 50 µmol L−1 has demonstrated outstanding potential for generating sustainable growth by enhancing antioxidant defenses, minimizing oxidative damage, and promoting plant development. Despite significant resistance in growth from Pb toxicity, we evaluated this approach on broccoli plants and observed that GSH2 (50 µmol L−1) enhanced gas exchange parameters, altered the redox balance, and enhanced nutrition to lessen Pb toxicity. Remarkably, the accumulation factor and Pb uptake both decreased in plants, suggesting how well GSH reduces toxicity. The results of this study provide evidence for the importance of GSH in development of broccoli under Pb regimes for stimulating growth and minimizing stress. However, a thorough molecular and proteomic analysis is required to adequately explain the conclusions drawn from the results of the current research.
Author contribution
Maria Ahmad: Performed experiment, Data curation, Writing- Original draft preparation, Software. Prof. Dr. Shakil Ahmed: Conceptualization, Methodology, Supervision. Dr. Nasim Ahmad Yasin: Statistical analysis, Editing, Validation, Dr. Rehana Sardar: Conceptualization, Visualization, Investigation, Supervision, Software. Prof. Dr. Abdul Wahid: Writing- Reviewing and Editing, Validation.
Declarations
Conflict of interest
Authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Afzal I, Basra SMA, Cheema MA, Farooq M, Jafar MZ, Shahid M, Yasmeen A (2013) Seed priming: a shotgun approach for alleviation of salt stress in wheat. Int J Agric Biol 15(6)
- Al-Farraj AS, Al-Otabi TG, Al-Wabel MI. Accumulation coefficient and translocation factor of heavy metals through Ochradenus baccatus plant grown on mining area at Mahad AD'Dahab, Saudi Arabia. WIT Trans Ecol Environ. 2009;122:459–468. doi: 10.2495/ECO090421. [DOI] [Google Scholar]
- Ali B, Song WJ, Hu WZ, Luo XN, Gill RA, Wang J, Zhou WJ. Hydrogen sulfide alleviates lead-induced photosynthetic and ultrastructural changes in oilseed rape. Ecotoxicol Environ Saf. 2014;102:25–33. doi: 10.1016/j.ecoenv.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Arbaoui S, Campanella B, Rezgui S, Paul R, Bettaieb T. Bioaccumulation and photosynthetic activity response of kenaf (Hibicus cannabinus L.) to cadmium and zinc. Greener J Agri Sci. 2014;4(3):91–100. doi: 10.15580/GJAS.2014.3.1216131031. [DOI] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24(1):1. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi P, Karki H, Bargali K, Bargali SS. Germination and seedling growth of pulse crop (Vigna spp.) as affected by soil salt stress. Curr Agric Res J. 2016;4(2):159–170. doi: 10.12944/CARJ.4.2.05. [DOI] [Google Scholar]
- Babbar N, Oberoi HS, Sandhu SK, Bhargav VK. Influence of different solvents in extraction of phenolic compounds from vegetable residues and their evaluation as natural sources of antioxidants. J Food Sci Technol. 2014;51(10):2568–2575. doi: 10.1007/s13197-012-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balint AF, Röder MS, Hell R, Galiba G, Börner A. Mapping of QTLs affecting copper tolerance and the Cu, Fe, Mn and Zn contents in the shoots of wheat seedlings. Biol Plant. 2007;51(1):129–134. doi: 10.1007/s10535-007-0025-9. [DOI] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Bousquet J, Le Moing V, Blain H, Czarlewski W, Zuberbier T, de la Torre R, Anto JM, et al. Efficacy of broccoli and glucoraphanin in COVID-19: from hypothesis to proof-of-concept with three experimental clinical cases. World Allergy Org J. 2021;14(1):100498. doi: 10.1016/j.waojou.2020.100498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Cao F, Wei K, Zhang G, Wu F. Genotypic dependent effect of exogenous glutathione on Cd-induced changes in proteins, ultrastructure and antioxidant defense enzymes in rice seedlings. J Hazard Mater. 2011;192(3):1056–1066. doi: 10.1016/j.jhazmat.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Carleton AE, Foote WH. A comparison of methods for estimating total leaf area of barley plants 1. Crop Sci. 1965;5(6):602–603. doi: 10.2135/cropsci1965.0011183X000500060041x. [DOI] [Google Scholar]
- Chapman JF, Dale LS. The determination of lithium isotope abundances with a dual-beam atomic absorption spectrometer. Anal Chim Acta. 1976;87(1):91–95. doi: 10.1016/S0003-2670(01)83123-8. [DOI] [Google Scholar]
- Chen IN, Chang CC, Ng CC, Wang CY, Shyu YT, Chang TL. Antioxidant and antimicrobial activity of Zingiberaceae plants in Taiwan. Plant Foods Hum Nutr. 2008;63(1):15–20. doi: 10.1007/s11130-007-0063-7. [DOI] [PubMed] [Google Scholar]
- Chen L, Liu Q, Zhao X, Zhang H, Pang X, Yang H. Inactivation efficacies of lactic acid and mild heat treatments against Escherichia coli strains in organic broccoli sprouts. Food Control. 2022;133:108577. doi: 10.1016/j.foodcont.2021.108577. [DOI] [Google Scholar]
- Choppala G, Saifullah U, Bolan N, Bibi S, Iqbal M, Rengel Z, Ok YS, et al. Cellular mechanisms in higher plants governing tolerance to cadmium toxicity. Crit Rev Plant Sci. 2014;33(5):374–391. doi: 10.1080/07352689.2014.903747. [DOI] [Google Scholar]
- Dai ZH, Guan DX, Bundschuh J, Ma LQ. Roles of phytohormones in mitigating abiotic stress in plants induced by metal (loid) s As, Cd, Cr, Hg, and Pb. Crit Rev Environ Sci Technol. 2023;53(13):1310–1330. doi: 10.1080/10643389.2022.2134694. [DOI] [Google Scholar]
- Davies BH (1965). Analysis of carotenoid pigments. Chemistry and biochemistry of plant pigments (TW Goodwin, ed)
- Edreva A, Velikova V, Tsonev T, Dagnon S, Gürel A, Aktaş L, Gesheva E. Stress-protective role of secondary metabolites: diversity of functions and mechanisms. Gen Appl Plant Physiol. 2008;34(1–2):67–78. [Google Scholar]
- Faostat, F. A. O. fao. org. 08 August (2021).
- Fathi A, Tari DB. Effect of drought stress and its mechanism in plants. Int J Life Sci. 2016;10(1):1–6. doi: 10.3126/ijls.v10i1.14509. [DOI] [Google Scholar]
- Ferrol N, Tamayo E, Vargas P (2016) The heavy metal paradox in arbuscular mycorrhizas: from mechanisms to biotechnological applications. J Exp Bot erw403 [DOI] [PubMed]
- Foyer CH, Noctor G. Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005;28(8):1056–1071. doi: 10.1111/j.1365-3040.2005.01327.x. [DOI] [Google Scholar]
- Gigliotti JC, Tin A, Pourafshar S, Cechova S, Wang YT, Sun-sang JS, Le TH, et al. GSTM1 deletion exaggerates kidney injury in experimental mouse models and confers the protective effect of cruciferous vegetables in mice and humans. J Am Soc Nephrol. 2020;31(1):102–116. doi: 10.1681/ASN.2019050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill RA, Kanwar MK, Dos Reis AR, Ali B (2021) Heavy metal toxicity in plants: recent insights on physiological and molecular aspects. Front Plant Sci 12 [DOI] [PMC free article] [PubMed]
- González-Guerrero M, Escudero V, Saéz Á, Tejada-Jiménez M. Transition metal transport in plants and associated endosymbionts: arbuscular mycorrhizal fungi and rhizobia. Front Plant Sci. 2016;7:1088. doi: 10.3389/fpls.2016.01088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed A, Sharma I, Kumar A, Azooz MM, Lone HA, Ahmad P (2014) Glutathione metabolism in plants under environmental stress. In: Oxidative damage to plants. Academic Press, pp 183–200
- Hasanuzzaman M, Nahar K, Alam MM, Fujita M. Exogenous nitric oxide alleviates high temperature induced oxidative stress in wheat ('Triticum aestivum' L.) seedlings by modulating the antioxidant defense and glyoxalase system. Aust J Crop Sci. 2012;6(8):1314–1323. [Google Scholar]
- Hasanuzzaman M, Nahar K, Anee TI, Fujita M. Exogenous silicon attenuates cadmium-induced oxidative stress in Brassica napus L. by modulating AsA-GSH pathway and glyoxalase system. Front Plant Sci. 2017;8:1061. doi: 10.3389/fpls.2017.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanuzzaman M, Nahar K, Anee TI, Fujita M. Glutathione in plants: biosynthesis and physiological role in environmental stress tolerance. Physiol Mol Biol Plants. 2017;23(2):249–268. doi: 10.1007/s12298-017-0422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanuzzaman M, Nahar K, Rahman A, Mahmud JA, Alharby HF, Fujita M. Exogenous glutathione attenuates lead-induced oxidative stress in wheat by improving antioxidant defense and physiological mechanisms. J Plant Interact. 2018;13(1):203–212. doi: 10.1080/17429145.2018.1458913. [DOI] [Google Scholar]
- Hasanuzzaman M, Bhuyan MB, Anee TI, Parvin K, Nahar K, Mahmud JA, Fujita M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants. 2019;8(9):384. doi: 10.3390/antiox8090384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassini I, Martinez-Ballesta MC, Boughanmi N, Moreno DA, Carvajal M. Improvement of broccoli sprouts (Brassica oleracea L. var. italica) growth and quality by KCl seed priming and methyl jasmonate under salinity stress. Sci Hortic. 2017;226:141–151. doi: 10.1016/j.scienta.2017.08.030. [DOI] [Google Scholar]
- Häussling M, Jorns CA, Lehmbecker G, Hecht-Buchholz C, Marschner H. Ion and water uptake in relation to root development in Norway spruce (Picea abies (L.) Karst.) J Plant Physiol. 1988;133(4):486–491. doi: 10.1016/S0176-1617(88)80042-7. [DOI] [Google Scholar]
- Herlina L, Widianarko B. Sunoko HR (2021) Effect of lead on growth and physiological responses of Hanjuang plant (Cordyline fruicosa) J Phys: Conf Ser. 1918;5:052034. [Google Scholar]
- Hossain MA, Piyatida P, da Silva JAT, Fujita M (2012) Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J Bot
- Isabel de Haro-Bravo M, del Rio-Celestino M, de Haro-Bailon A. Uptake and accumulation of lead by broccoli plants grown in contaminated soils. Fresenius Environ Bull. 2008;17(10A):1640–1643. [Google Scholar]
- Jain S, Muneer S, Guerriero G, Liu S, Vishwakarma K, Chauhan DK, Sharma S, et al. Tracing the role of plant proteins in the response to metal toxicity: a comprehensive review. Plant Signal Behav. 2018;13(9):e1507401. doi: 10.1080/15592324.2018.1507401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavi Kishor PB, Sreenivasulu NESE. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014;37(2):300–311. doi: 10.1111/pce.12157. [DOI] [PubMed] [Google Scholar]
- Khan MA, Asaf S, Khan AL, Ullah I, Ali S, Kang SM, Lee IJ. Alleviation of salt stress response in soybean plants with the endophytic bacterial isolate Curtobacterium sp. SAK1. Ann Microbiol. 2019;69(8):797–808. doi: 10.1007/s13213-019-01470-x. [DOI] [Google Scholar]
- Khattab H. Role of glutathione and polyadenylic acid on the oxidative defense systems of two different cultivars of canola seedlings grown under saline conditions. Aust J Basic Appl Sci. 2007;1(3):323–334. [Google Scholar]
- Kocsy G, Szalai G, Galiba G. Effect of osmotic stress on glutathione and hydroxy methyl glutathione accumulation in wheat. J Plant Physiol. 2004;161(7):785–794. doi: 10.1016/j.jplph.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Küpper H, Küpper FC, Spiller M (2006) [Heavy metal]-chlorophylls formed in vivo during heavy metal stress and degradation products formed during digestion, extraction and storage of plant material. In: Chlorophylls and bacteriochlorophylls. Springer, Dordrecht, pp 67–77
- Lee YR, Chen M, Lee JD, Zhang J, Lin SY, Fu TM, Pandolfi PP, et al. Reactivation of PTEN tumor suppressor for cancer treatment through inhibition of a MYC-WWP1 inhibitory pathway. Science. 2019;364(6441):eaau0159. doi: 10.1126/science.aau0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann S, Funck D, Szabados L, Rentsch D. Proline metabolism and transport in plant development. Amino Acids. 2010;39(4):949–962. doi: 10.1007/s00726-010-0525-3. [DOI] [PubMed] [Google Scholar]
- Li Z, Song L, Liu Y, Han F, Liu W. Electrophysiological, morphologic, and transcriptomic profiling of the ogura-CMS, DGMS and Maintainer Broccoli Lines. Plants. 2022;11(4):561. doi: 10.3390/plants11040561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Cervantes J, Tirado-Noriega LG, Sánchez-Machado DI, Campas-Baypoli ON, Cantú-Soto EU, Núñez-Gastélum JA. Biochemical composition of broccoli seeds and sprouts at different stages of seedling development. Int J Food Sci Technol. 2013;48(11):2267–2275. doi: 10.1111/ijfs.12213. [DOI] [Google Scholar]
- López-Chillón MT, Carazo-Díaz C, Prieto-Merino D, Zafrilla P, Moreno DA, Villaño D. Effects of long-term consumption of broccoli sprouts on inflammatory markers in overweight subjects. Clin Nutr. 2019;38(2):745–752. doi: 10.1016/j.clnu.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Lovato FL, Teixeira da Rocha JB, Dalla Corte CL. Diphenyl diselenide protects against methylmercury-induced toxicity in Saccharomyces cerevisiae via the Yap1 transcription factor. Chem Res Toxicol. 2017;30(5):1134–1144. doi: 10.1021/acs.chemrestox.6b00449. [DOI] [PubMed] [Google Scholar]
- Márquez-García B, Fernández-Recamales M, Córdoba F (2012) Effects of cadmium on phenolic composition and antioxidant activities of Erica andevalensis. J Bot
- Marschner H, editor. Marschner's mineral nutrition of higher plants. London: Academic Press; 2011. [Google Scholar]
- Molnár K, Biró-Janka B, Nyárádi II, Fodorpataki L, Varga BE, Bálint J, Duda MM. Effects of priming with ascorbic acid, L-cystein and triacontanol on germination of rapeseed (L.) Acta Biologica Marisiensis. 2020;3(2):48–55. doi: 10.2478/abmj-2020-0010. [DOI] [Google Scholar]
- Moseley G, Jones JR. The physical digestion of perennial ryegrass (Lolium perenne) and white clover (Trifolium repens) in the foregut of sheep. Br J Nutr. 1984;52(2):381–390. doi: 10.1079/BJN19840104. [DOI] [PubMed] [Google Scholar]
- Mostofa MG, Seraj ZI, Fujita M. Exogenous sodium nitroprusside and glutathione alleviate copper toxicity by reducing copper uptake and oxidative damage in rice (Oryza sativa L.) seedlings. Protoplasma. 2014;251(6):1373–1386. doi: 10.1007/s00709-014-0639-7. [DOI] [PubMed] [Google Scholar]
- Moussa HR, Taha MA, Dessoky ES, Selem E (2023) Exploring the perspectives of irradiated sodium alginate on molecular and physiological parameters of heavy metal stressed Vigna radiata L. plants. Physiol Mol Biol Plants 1–12 [DOI] [PMC free article] [PubMed]
- Nadeem A, Ahmad SF, Al-Ayadhi LY, Attia SM, Al-Harbi NO, Alzahrani KS, Bakheet SA. Differential regulation of Nrf2 is linked to elevated inflammation and nitrative stress in monocytes of children with autism. Psychoneuroendocrinology. 2020;113:104554. doi: 10.1016/j.psyneuen.2019.104554. [DOI] [PubMed] [Google Scholar]
- Noctor G. Metabolic signalling in defence and stress: the central roles of soluble redox couples. Plant Cell Environ. 2006;29(3):409–425. doi: 10.1111/j.1365-3040.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- Noctor G, Arisi ACM, Jouanin L, Kunert KJ, Rennenberg H, Foyer CH. Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J Exp Bot. 1998;49(321):623–647. [Google Scholar]
- Noctor G, Mhamdi A, Chaouch S, Han YI, Neukermans J, Marquez-Garcia BELEN, Foyer CH, et al. Glutathione in plants: an integrated overview. Plant Cell Environ. 2012;35(2):454–484. doi: 10.1111/j.1365-3040.2011.02400.x. [DOI] [PubMed] [Google Scholar]
- Ogawa KI. Glutathione-associated regulation of plant growth and stress responses. Antioxid Redox Signal. 2005;7(7–8):973–981. doi: 10.1089/ars.2005.7.973. [DOI] [PubMed] [Google Scholar]
- Öğütücü G, Özdemir G, Acararicin Z, Aydin A. Trend analysis of lead content in roadside plant and soil samples in Turkey. Turk J Pharm Sci. 2021;18(5):581. doi: 10.4274/tjps.galenos.2021.45389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Qiu B, Zeng F, Cai S, Wu X, Haider SI, Wu F, Zhang G. Alleviation of chromium toxicity in rice seedlings by applying exogenous glutathione. J Plant Physiol. 2013;170(8):772–779. doi: 10.1016/j.jplph.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Sabetta W, Paradiso A, Paciolla C, Pinto MCD (2017) Chemistry, biosynthesis, and antioxidative function of glutathione in plants. In: Glutathione in plant growth, development, and stress tolerance. Springer, Cham, pp 1–27
- Sagner S, Kneer R, Wanner G, Cosson JP, Deus-Neumann B, Zenk MH. Hyperaccumulation, complexation and distribution of nickel in Sebertia acuminata. Phytochemistry. 1998;47(3):339–347. doi: 10.1016/S0031-9422(97)00593-1. [DOI] [PubMed] [Google Scholar]
- Salavati J, Fallah H, Niknejad Y, Barari Tari D. Methyl jasmonate ameliorates lead toxicity in Oryza sativa by modulating chlorophyll metabolism, antioxidative capacity and metal translocation. Physiol Mol Biol Plants. 2021;27:1089–1104. doi: 10.1007/s12298-021-00993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samardakiewicz S, Krzeszowiec-Jeleń W, Bednarski W, Jankowski A, Suski S, Gabryś H, Woźny A. Pb-induced avoidance-like chloroplast movements in fronds of Lemna trisulca L. PLoS ONE. 2015;10(2):e0116757. doi: 10.1371/journal.pone.0116757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A, Sharangi AB, Soujannya S, Datta A. Seed yield and quality of coriander (Coriandrum sativum L.) as influenced by seed priming. J Crop Weed. 2020;16(1):51–55. doi: 10.22271/09746315.2020.v16.i1.1271. [DOI] [Google Scholar]
- Shahid M, Khalid S, Abbas G, Shahid N, Nadeem M, Sabir M, Dumat C, et al (2015) Heavy metal stress and crop productivity. In: Crop production and global environmental issues. Springer, Cham, pp 1–25
- Shao L, Cui J, Young LT, Wang JF. The effect of mood stabilizer lithium on expression and activity of glutathione s-transferase isoenzymes. Neuroscience. 2008;151(2):518–524. doi: 10.1016/j.neuroscience.2007.10.041. [DOI] [PubMed] [Google Scholar]
- Singh S, Parihar P, Singh R, Singh VP, Prasad SM. Heavy metal tolerance in plants: role of transcriptomics, proteomics, metabolomics, and ionomics. Front Plant Sci. 2016;6:1143. doi: 10.3389/fpls.2015.01143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16(3):144–158. doi: 10.5344/ajev.1965.16.3.144. [DOI] [Google Scholar]
- USDA Broccoli, raw (2018) from https://fdc.nal.usda.gov/fdc-app.html/food-details/170379/nutrients. Accessed 4th Apr 2021 USDA, 2018
- Talukdar D. An induced glutathione-deficient mutant in grass pea (Lathyrus sativus L.): modifications in plant morphology, alteration in antioxidant activities and increased sensitivity to cadmium. Biorem Biodiv Bioavail. 2012;6:75–86. [Google Scholar]
- Talukdar D, Talukdar T. RETRACTED ARTICLE: Coordinated response of sulfate transport, cysteine biosynthesis, and glutathione-mediated antioxidant defense in lentil (Lens culinaris Medik.) genotypes exposed to arsenic. Protoplasma. 2014;251(4):839–855. doi: 10.1007/s00709-013-0586-8. [DOI] [PubMed] [Google Scholar]
- Tränkner M, Tavakol E, Jákli B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol Plant. 2018;163(3):414–431. doi: 10.1111/ppl.12747. [DOI] [PubMed] [Google Scholar]
- Wakeel A. Potassium–sodium interactions in soil and plant under saline-sodic conditions. J Plant Nutr Soil Sci. 2013;176(3):344–354. doi: 10.1002/jpln.201200417. [DOI] [Google Scholar]
- Yan A, Wang Y, Tan SN, Mohd Yusof ML, Ghosh S, Chen Z. Phytoremediation: a promising approach for revegetation of heavy metal-polluted land. Front Plant Sci. 2020;11:359. doi: 10.3389/fpls.2020.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasseen BT, Al-Thani RF (2013) Ecophysiology of wild plants and conservation perspectives in the State of Qatar. Agric Chem 37
- Yuan G, Wang X, Guo R, Wang Q. Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem. 2010;121(4):1014–1019. doi: 10.1016/j.foodchem.2010.01.040. [DOI] [Google Scholar]
- Yuan-Yuan SUN, Yong-Jian SUN, Ming-Tian WANG, Xu-Yi LI, Xiang GUO, Rong HU, Jun MA. Effects of seed priming on germination and seedling growth under water stress in rice. Acta Agron Sin. 2010;36(11):1931–1940. doi: 10.1016/S1875-2780(09)60085-7. [DOI] [Google Scholar]
- Zechmann B. Compartment-specific importance of glutathione during abiotic and biotic stress. Front Plant Sci. 2014;5:566. doi: 10.3389/fpls.2014.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Tao Y, Hussain S, Jiang Q, Peng S, Huang J, Nie L, et al. Seed priming in dry direct-seeded rice: consequences for emergence, seedling growth and associated metabolic events under drought stress. Plant Growth Regul. 2016;78(2):167–178. doi: 10.1007/s10725-015-0083-5. [DOI] [Google Scholar]