Abstract
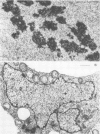
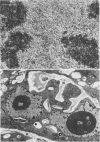
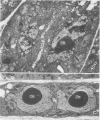
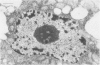
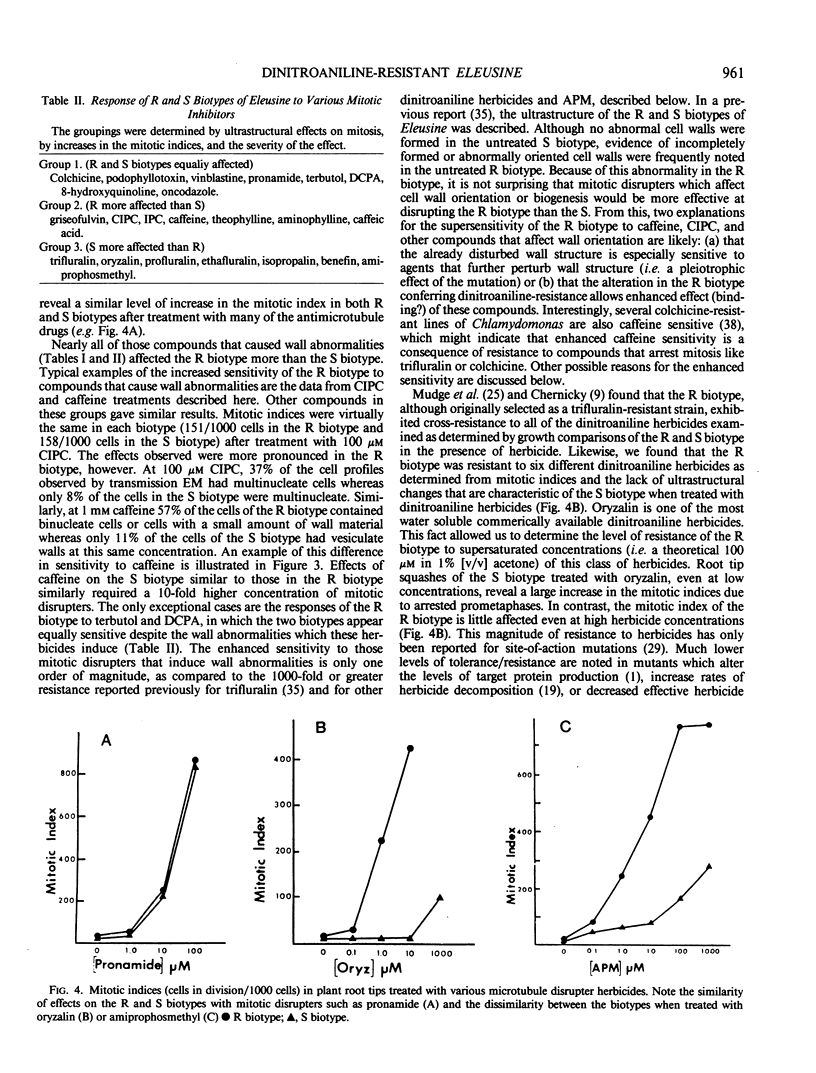
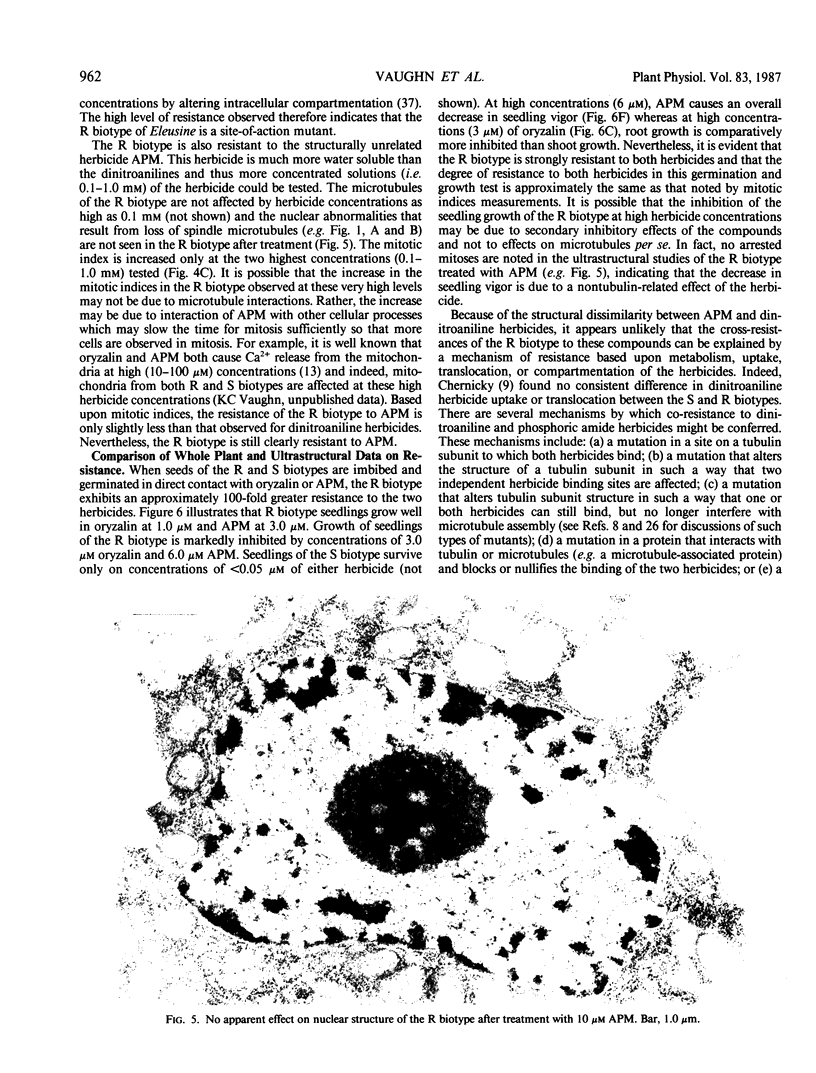
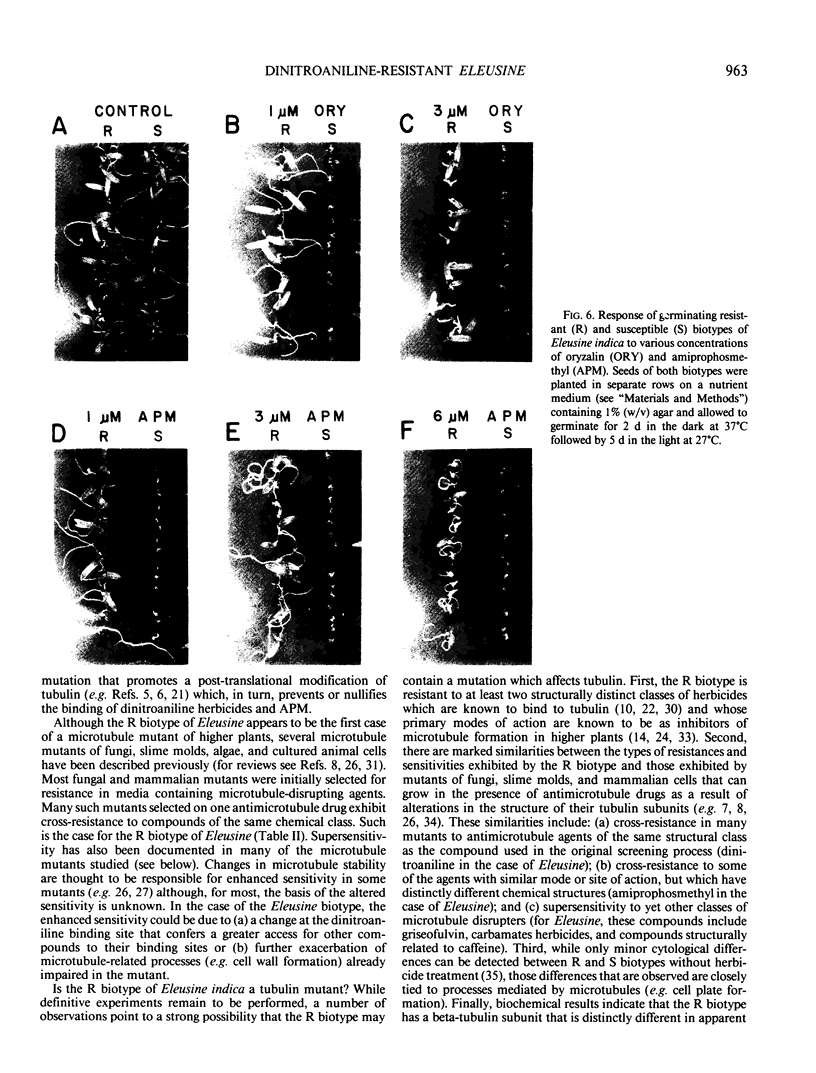
A dinitroaniline-resistant (R) biotype of Eleusine indica (L.) Gaertner. (goosegrass) is demonstrated to be cross-resistant to a structurally non-related herbicide, amiprophosmethyl, and supersensitive to two other classes of compounds which disrupt mitosis. These characteristics of the R biotype were discovered in a comparative test of the effects of 24 different antimitotic compounds on the R biotype and susceptible (S) wild-type Eleusine. The compounds tested could be classified into three groups based upon their effects on mitosis in root tips of the susceptible (S) biotype. Class I compounds induced effects like the well known mitotic disrupter colchicine: absence of cortical and spindle microtubules, mitosis arrested at prometaphase, and the formation of polymorphic nuclei after arrested mitosis. The R biotype was resistant to treatment with some class I inhibitors (all dinitroaniline herbicides and amiprophosmethyl) but not all (e.g. colchicine, podophyllotoxin, vinblastine, and pronamide). Roots of the R biotype, when treated with either dinitroaniline herbicides or amiprophosmethyl, exhibited no or only small increases in the mitotic index nor were the spindle and cortical microtubules affected. Compounds of class II (carbamate herbicides and griseofulvin) cause misorientation of microtubules which results in multinucleated cells. Compounds of class III (caffeine and structually related alkaloids) cause imcomplete cell walls to form at telophase. Each of these last two classes of compounds affected the R biotype more than the S biotype (supersensitivity). The cross-resistance and high levels of resistance of the R biotype of Eleusine to the dinitroaniline herbicides and the structurally distinct herbicide, amiprophosmethyl, indicate that a mechanism of resistance based upon metabolic modification, translocation, or compartmentation of the herbicides is probably not operative.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunke K. J., Collis P. S., Weeks D. P. Post-translational modification of tubulin dependent on organelle assembly. Nature. 1982 Jun 10;297(5866):516–518. doi: 10.1038/297516a0. [DOI] [PubMed] [Google Scholar]
- Burland T. G., Schedl T., Gull K., Dove W. F. Genetic analysis of resistance to benzimidazoles in Physarum: differential expression of beta-tubulin genes. Genetics. 1984 Sep;108(1):123–141. doi: 10.1093/genetics/108.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collis P. S., Weeks D. P. Selective inhibition of tubulin synthesis by amiprophos methyl during flagellar regeneration in Chlamydomonas reinhardi. Science. 1978 Oct 27;202(4366):440–442. doi: 10.1126/science.568309. [DOI] [PubMed] [Google Scholar]
- Coss R. A., Pickett-Heaps J. D. The effects of isopropyl N-phenyl carbamate on the green alga Oedogonium cardiacum. I. Cell division. J Cell Biol. 1974 Oct;63(1):84–98. doi: 10.1083/jcb.63.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess D., Bayer D. The effect of trifluralin on the ultrastructure of dividing cells of the root meristem of cotton (Gossypium hirsutum L. "Acala 4-42'). J Cell Sci. 1974 Jul;15(2):429–441. doi: 10.1242/jcs.15.2.429. [DOI] [PubMed] [Google Scholar]
- L'Hernault S. W., Rosenbaum J. L. Chlamydomonas alpha-tubulin is posttranslationally modified in the flagella during flagellar assembly. J Cell Biol. 1983 Jul;97(1):258–263. doi: 10.1083/jcb.97.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morejohn L. C., Bureau T. E., Tocchi L. P., Fosket D. E. Tubulins from different higher plant species are immunologically nonidentical and bind colchicine differentially. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1440–1444. doi: 10.1073/pnas.81.5.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morejohn L. C., Fosket D. E. Inhibition of Plant Microtubule Polymerization in vitro by the Phosphoric Amide Herbicide Amiprophos-Methyl. Science. 1984 May 25;224(4651):874–876. doi: 10.1126/science.224.4651.874. [DOI] [PubMed] [Google Scholar]
- Oakley B. R. Microtubule mutants. Can J Biochem Cell Biol. 1985 Jun;63(6):479–488. doi: 10.1139/o85-067. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Morris N. R. Nuclear movement is beta--tubulin-dependent in Aspergillus nidulans. Cell. 1980 Jan;19(1):255–262. doi: 10.1016/0092-8674(80)90407-9. [DOI] [PubMed] [Google Scholar]
- Paul D. C., Goff C. W. Comparative effects of caffeine, its analogues and calcium deficiency on cytokinesis. Exp Cell Res. 1973 Apr;78(2):399–413. doi: 10.1016/0014-4827(73)90085-2. [DOI] [PubMed] [Google Scholar]
- Pfister K., Steinback K. E., Gardner G., Arntzen C. J. Photoaffinity labeling of an herbicide receptor protein in chloroplast membranes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):981–985. doi: 10.1073/pnas.78.2.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quader H., Filner P. The action of antimitotic herbicides on flagellar regeneration in Chlamydomonas reinhardtii: a comparison with the action of colchicine. Eur J Cell Biol. 1980 Aug;21(3):301–304. [PubMed] [Google Scholar]
- Sheir-Neiss G., Lai M. H., Morris N. R. Identification of a gene for beta-tubulin in Aspergillus nidulans. Cell. 1978 Oct;15(2):639–647. doi: 10.1016/0092-8674(78)90032-6. [DOI] [PubMed] [Google Scholar]
- Umesono K., Toda T., Hayashi S., Yanagida M. Cell division cycle genes nda2 and nda3 of the fission yeast Schizosaccharomyces pombe control microtubular organization and sensitivity to anti-mitotic benzimidazole compounds. J Mol Biol. 1983 Aug 5;168(2):271–284. doi: 10.1016/s0022-2836(83)80018-7. [DOI] [PubMed] [Google Scholar]
- Warr J. R., Flanagan D., Quinn D. Mutants of Chlamydomonas reinhardii with altered sensitivity to antimicrotubular agents. Exp Cell Res. 1978 Jan;111(1):37–46. doi: 10.1016/0014-4827(78)90234-3. [DOI] [PubMed] [Google Scholar]