Abstract
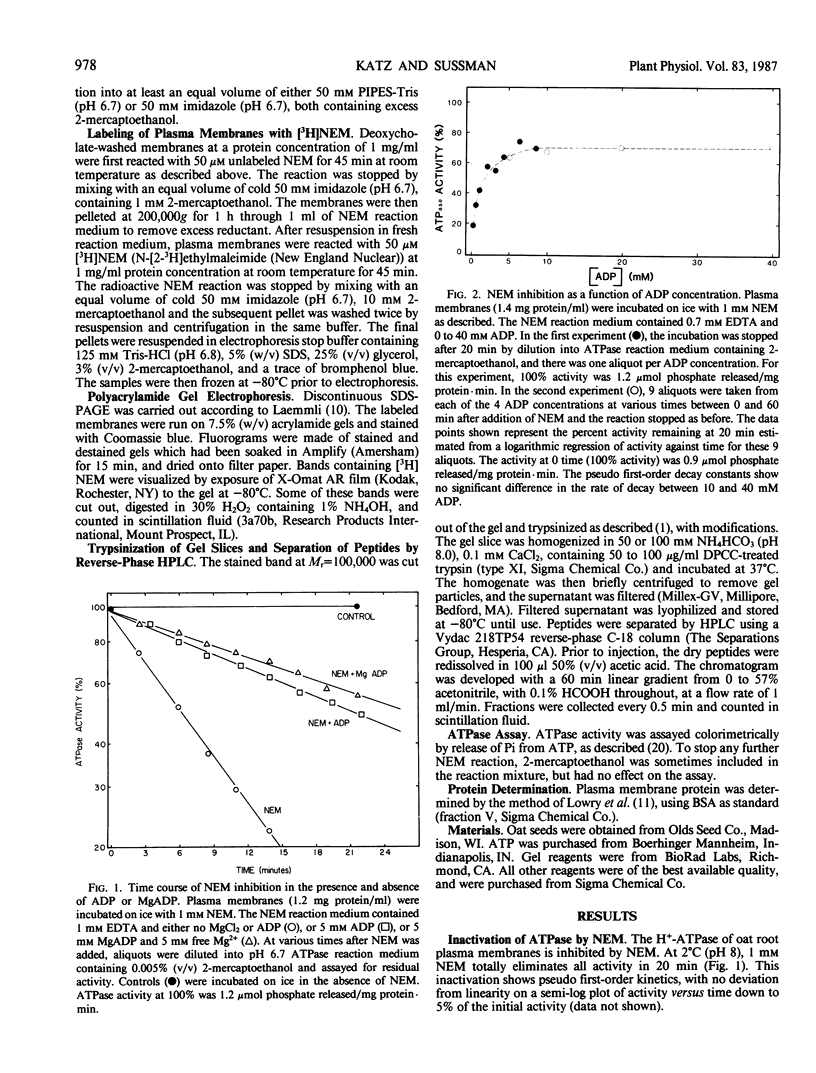
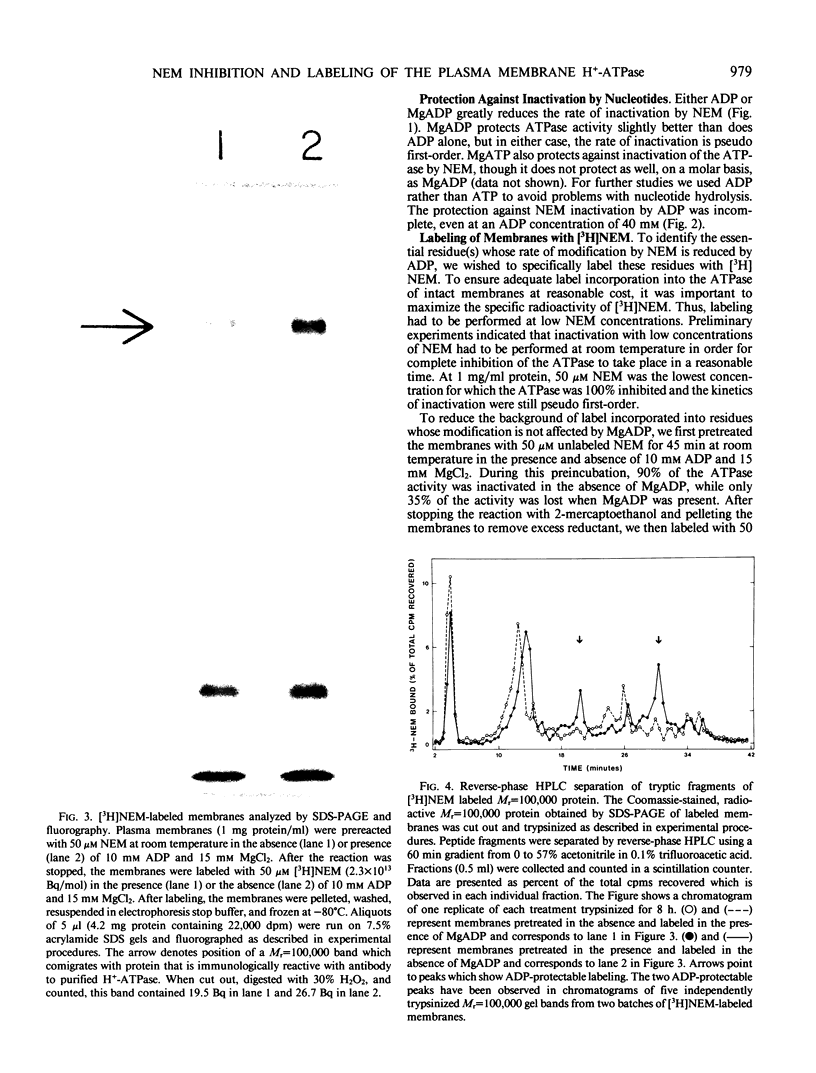
H+-ATPase activity in plasma membranes isolated from Avena sativa root cells is inhibited by N-ethylmaleimide, a covalent modifier of protein sulfhydryl groups. The rate of inhibition is reduced by ADP, MgADP, and MgATP, but even at 40 millimolar ADP the enzyme is only partially protected against inactivation. When plasma membranes are treated wth N-[2-3H]ethylmaleimide and analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis, prominent radioactive bands appear at Mr=100,000 and several other positions. However, only radioactivity in the Mr=100,000 protein is reduced by the presence of MgADP. These results provide independent evidence that the Mr=100,000 polypeptide which is observed in purified preparations of the enzyme is the catalytic subunit of the H+-ATPase. When tryptic peptides are produced from N-[2-3H]ethylmaleimide labeled Mr=100,000 protein and separated by reverse phase high performance liquid chromatography, two radioactive peaks are observed for which N-[2-3H]ethylmaleimide incorporation is reduced in the presence of MgADP.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper S. L., Palfrey H. C., DeRiemer S. A., Greengard P. Hormonal control of protein phosphorylation in turkey erythrocytes. Phosphorylation by cAMP-dependent and Ca2+-dependent protein kinases of distinct sites in goblin, a high molecular weight protein of the plasma membrane. J Biol Chem. 1980 Nov 25;255(22):11029–11039. [PubMed] [Google Scholar]
- Briskin D. P., Poole R. J. Plasma membrane ATPase of red beet forms a phosphorylated intermediate. Plant Physiol. 1983 Mar;71(3):507–512. doi: 10.1104/pp.71.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker R. J., Slayman C. W. Inhibition of the plasma membrane [H+]-ATPase of Neurospora crassa by N-ethylmaleimide. Protection by nucleotides. J Biol Chem. 1982 Oct 25;257(20):12051–12055. [PubMed] [Google Scholar]
- Brooker R. J., Slayman C. W. [14C]N-ethylmaleimide labeling of the plasma membrane [H+]-ATPase of Neurospora crassa. J Biol Chem. 1983 Jan 10;258(1):222–226. [PubMed] [Google Scholar]
- Goffeau A., Slayman C. W. The proton-translocating ATPase of the fungal plasma membrane. Biochim Biophys Acta. 1981 Dec 30;639(3-4):197–223. doi: 10.1016/0304-4173(81)90010-0. [DOI] [PubMed] [Google Scholar]
- Hart W. M., Jr, Titus E. O. Isolation of a protein component of sodium-potassium transport adenosine triphosphatase containing ligand-protected sulfhydryl groups. J Biol Chem. 1973 Feb 25;248(4):1365–1371. [PubMed] [Google Scholar]
- Hesse J. E., Wieczorek L., Altendorf K., Reicin A. S., Dorus E., Epstein W. Sequence homology between two membrane transport ATPases, the Kdp-ATPase of Escherichia coli and the Ca2+-ATPase of sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4746–4750. doi: 10.1073/pnas.81.15.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook J. J., Jeckel R. A peptide containing a reactive lysyl group from ox liver glutamate dehydrogenase. Biochem J. 1969 Mar;111(5):689–694. doi: 10.1042/bj1110689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H., Brandl C. J., Korczak B., Green N. M. Amino-acid sequence of a Ca2+ + Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature. 1985 Aug 22;316(6030):696–700. doi: 10.1038/316696a0. [DOI] [PubMed] [Google Scholar]
- Panet R., Selinger Z. Specific alkylation of the sarcoplasmic reticulum ATPase by N-ethyl-[1-14C]maleimide and identification of the labeled protein in acrylamide gel-electrophoresis. Eur J Biochem. 1970 Jul;14(3):440–444. doi: 10.1111/j.1432-1033.1970.tb00308.x. [DOI] [PubMed] [Google Scholar]
- RAY W. J., Jr, KOSHLAND D. E., Jr A method for characterizing the type and numbers of groups involved in enzyme action. J Biol Chem. 1961 Jul;236:1973–1979. [PubMed] [Google Scholar]
- Serrano R., Kielland-Brandt M. C., Fink G. R. Yeast plasma membrane ATPase is essential for growth and has homology with (Na+ + K+), K+- and Ca2+-ATPases. Nature. 1986 Feb 20;319(6055):689–693. doi: 10.1038/319689a0. [DOI] [PubMed] [Google Scholar]
- Serrano R. Plasma membrane ATPase of fungi and plants as a novel type of proton pump. Curr Top Cell Regul. 1984;23:87–126. doi: 10.1016/b978-0-12-152823-2.50007-6. [DOI] [PubMed] [Google Scholar]
- Serrano R. Purification of the proton pumping ATPase from plant plasma membranes. Biochem Biophys Res Commun. 1984 Jun 15;121(2):735–740. doi: 10.1016/0006-291x(84)90243-2. [DOI] [PubMed] [Google Scholar]
- Shull G. E., Schwartz A., Lingrel J. B. Amino-acid sequence of the catalytic subunit of the (Na+ + K+)ATPase deduced from a complementary DNA. Nature. 1985 Aug 22;316(6030):691–695. doi: 10.1038/316691a0. [DOI] [PubMed] [Google Scholar]
- Smyth D. G., Blumenfeld O. O., Konigsberg W. Reactions of N-ethylmaleimide with peptides and amino acids. Biochem J. 1964 Jun;91(3):589–595. doi: 10.1042/bj0910589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vara F., Serrano R. Partial purification and properties of the proton-translocating ATPase of plant plasma membranes. J Biol Chem. 1982 Nov 10;257(21):12826–12830. [PubMed] [Google Scholar]
- Vara F., Serrano R. Phosphorylated intermediate of the ATPase of plant plasma membranes. J Biol Chem. 1983 May 10;258(9):5334–5336. [PubMed] [Google Scholar]
- Walderhaug M. O., Post R. L., Saccomani G., Leonard R. T., Briskin D. P. Structural relatedness of three ion-transport adenosine triphosphatases around their active sites of phosphorylation. J Biol Chem. 1985 Mar 25;260(6):3852–3859. [PubMed] [Google Scholar]
- Winslow J. W. The reaction of sulfhydryl groups of sodium and potassium ion-activated adenosine triphosphatase with N-ethylmaleimide. The relationship between ligand-dependent alterations of nucleophilicity and enzymatic conformational states. J Biol Chem. 1981 Sep 25;256(18):9522–9531. [PubMed] [Google Scholar]
- Wolf H. U., Adolph L. Untersuchungen über eine Mg2plus-(Ca2+)-aktivierte ATPase aus Rinderhirnmikrosomen. Eur J Biochem. 1969 Mar;8(1):68–74. doi: 10.1111/j.1432-1033.1969.tb00496.x. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Tonomura Y. Chemical modification of the Ca2+-dependent ATPase of sarcoplasmic reticulum from skeletal muscle. I. Binding of N-ethylmaleimide to sarcoplasmic reticulum: evidence for sulfhydryl groups in the active site of ATPase and for conformational changes induced by adenosine tri- and diphosphate. J Biochem. 1976 Mar;79(3):649–654. doi: 10.1093/oxfordjournals.jbchem.a131109. [DOI] [PubMed] [Google Scholar]