Abstract
Background/Objectives
Ocular syphilis is a vision-threatening disease that can lead to permanent blindness if left untreated. The global re-emergence of syphilis warrants greater investigations into the visual prognosis of eyes affected by this potentially devastating disease. This systematic review investigates the impact of HIV on visual acuity (VA) outcomes in ocular syphilis.
Methods
A literature search of Medline, PubMed, Embase, Clinicaltrials.gov and Cochrane Reviews was conducted for studies published between 01 January 2011 and 19 March 2022, reporting non-aggregate initial and post-treatment VA data of eyes with ocular syphilis and corresponding HIV status in patients ≥ 18 years.
Results
A total of 95 studies, including 364 patients and 568 eyes, were evaluated. Among people living with HIV with a diagnosis of ocular syphilis, affected eyes were more likely to have optic nerve involvement and panuveitis. However, HIV status, CD4 cell count, and HIV viral load were not predictive of VA outcomes of treated ocular syphilis. Prognostic factors of final VA worse than 1.00 logMAR were female sex, the presence of macular edema, and VA ≥ 1.00 at presentation. The strongest predictor of a worse final VA was VA ≥ 1.00 at presentation.
Conclusions
This systematic review demonstrates that HIV status, CD4 cell count, and HIV viral load are not significant factors impacting VA outcomes of eyes with ocular syphilis. While visual prognosis is generally good, poor visual outcome is most strongly predicted by poor VA at presentation. This underscores the importance of early recognition and treatment prior to permanent vision loss.
Subject terms: HIV infections, Eye diseases, Epidemiology
Introduction
The incidence of syphilis has increased at an alarming rate over the last two decades, becoming an ever-present threat to global health [1–4]. Caused by the spirochete bacterium, Treponema pallidum subspecies pallidum, the estimated global incidence of syphilis in 2016 was approximately 6 million cases per year [3], disproportionately affecting high-risk populations such as men who have sex with men (MSM), sex workers, and people living with HIV [5–7].
Ocular syphilis is relatively uncommon, with a prevalence of 2–3% among patients with syphilis [8, 9]. It may occur at any stage of the disease, affecting various structures within the eye and causing a wide range of symptoms, including permanent vision loss [10, 11]. Syphilis and HIV are common co-infections [6, 12] and are known to facilitate the pathogenesis of one another [13]. With appropriate treatment, the visual outcome of ocular syphilis is believed to be in the range of logMAR −0.08 to 0.50 [6, 14–17]. What remains unclear, however, is the effect of HIV on visual acuity in patients with ocular syphilis.
The primary objective of this systematic review is to elucidate the effect of HIV status and related prognostic factors, such as reported CD4 counts and HIV viral load, on the post-treatment visual acuity (VA) of eyes affected by ocular syphilis. Additional objectives include identifying prognostic factors for visual outcomes in ocular syphilis and identifying between-group differences of those with a diagnosis of HIV and those without.
Methods
A literature search was conducted on 19 March 2022 following a search strategy and methodology developed with guidance from a medical librarian. The search strategy was applied to MEDLINE, PubMed, Embase, ClinicalTrials.gov, and Cochrane Library databases for English language articles published from 01 January 2011 to 19 March 2022, as described in Supplementary Table 1. Manual and computer-assisted reference mining was conducted to ensure completeness [18]. Non-peer reviewed publications, including conference presentations, were excluded. Findings were reported according to MOOSE guidelines [19]. Institutional review board approval was waived by the University of Saskatchewan Research Ethics Board for this systematic review. This systematic review protocol has been registered with PROSPERO (CRD42022304536).
Outcomes
The primary outcome was final VA following treatment for ocular syphilis. Secondary outcomes included pre-treatment VA, laterality of disease, ocular symptoms, associated ocular diagnoses, and all documented treatment.
Eligibility criteria
We included studies reporting non-aggregate data for at least one patient diagnosed with ocular syphilis and known HIV status with quantitative VA before and after ocular syphilis treatment. Only patients ≥18 years of age were eligible for inclusion. Additionally, those with a secondary ocular pathology known to significantly impact VA were evaluated by a board-certified ophthalmologist (LB) on a case-by-case basis for exclusion. Patients with congenital syphilis were excluded.
Study selection and data extraction
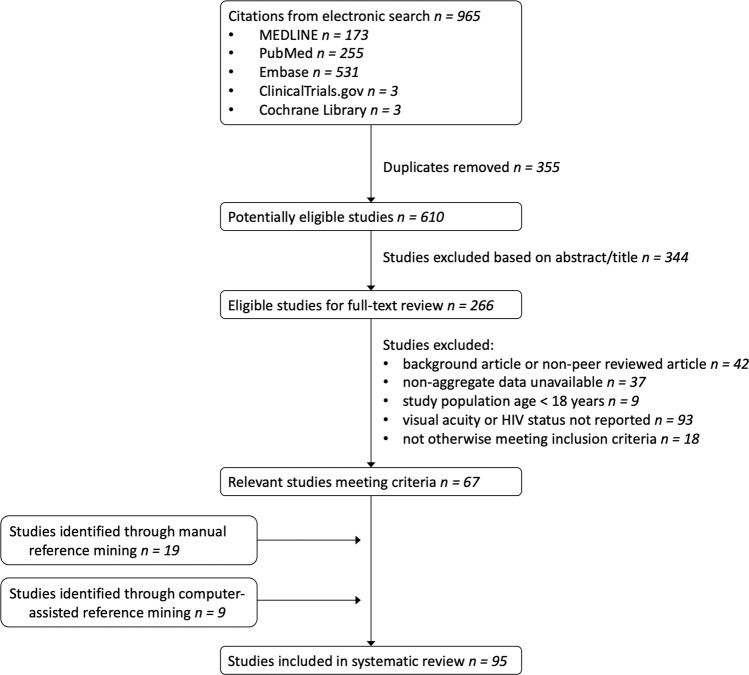
Two investigators (LZW and TMO) were independently responsible for literature screening, full-text review of screened articles, data extraction, and risk of bias assessment. Literature screening was conducted via Rayyan software [20], and any disagreements were advanced to full-text review until consensus was reached. All eligible articles underwent full-text review for inclusion eligibility. See Fig. 1 for study selection details.
Fig. 1. PRISMA style flow diagram showing selection of eligible studies.
Of the 95 studies included in the final analysis, 67 were identified through an electronic database search, 19 through manual reference mining, and 9 through computer-assisted reference mining.
Data extraction was undertaken by reviewers and data for each affected eye was input into a Microsoft Excel file designed to collect outcomes specified by the study protocol. Risk of bias was assessed using the Joanna Briggs Institute critical appraisal tools for case reports, case studies, and cohort studies, as appropriate (Supplementary Table 2) [21–23].
Data elements included study design, patient demographics, HIV status, VA, symptomatology, and laboratory findings. In cases where multiple VAs were reported, only the most recent pre- and post-treatment VA were collected and analysed. Uveitis was classified according to the Standardization of Uveitis Nomenclature (SUN) criteria [24]. Visual acuities measured in Snellen and decimal notation were converted to logMAR format for uniformity. Non-numerical visual acuities were reported according to generally accepted standards [25–28]. Post-treatment VA change was defined as a VA increase or decrease of ≥0.20 logMAR. Post-treatment VA changes <0.20 logMAR were considered stable for the purposes of this study.
Statistical analysis was conducted using SPSS version 28 (IBM SPSS Statistics; Armonk, NY) and SAS software, version 9.4 (SAS Institute Inc. Cary, NC). Patient and eye characteristics were summarized as counts (percent) for categorical data and means (standard deviation (SD)) for continuous data. We investigated between-group differences of patients with and without HIV. Correlations between categorical variables were analyzed using Chi-squared tests or Fisher’s exact tests, as appropriate. We used independent t-tests and Wilcoxon non-parametric tests to compare normally and non-normally distributed continuous variables, respectively between HIV and non-HIV groups. A multivariable mixed-effects model was used to examine the post-treatment logMAR difference by HIV status. Only significant variables from the univariable analysis were included in the multivariable model. We also investigated the effect of all potential variables on VA using a univariable mixed effects model. Correlations with final VA ≥ 1.00 were investigated using a generalized estimating equation. Subgroup analysis of patients with HIV was conducted to investigate logMAR-reduction differences by CD4 categories as well as correlation between logMAR and log-adjusted HIV viral load. Cases with missing data were excluded from the denominator. A two-sided p-value < .05 was used as the threshold for significance.
Results
An electronic database search yielded 965 citations, of which we included 67 in our final analysis. Manual and computer-assisted reference mining identified a further 28 citations, totalling 95 relevant studies for inclusion [29–123]. See Fig. 1 for PRISMA style flow diagram summarizing results of the literature search. In all cases, study design was descriptive or observational in nature, including 55 case reports, 36 case series, and 4 retrospective cohort studies. Ten eyes were evaluated and excluded because of a clinically significant secondary ocular pathology, three of which were due to secondary infections, likely facilitated by HIV coinfection. Final analysis included 364 patients and 568 eyes. A summary of the characteristics of included studies and quality of evidence are shown in Table 1.
Table 1.
Characteristics of eligible studies.
| Study no. | Method of identification | Study design | Number of participants included | Quality of evidencea | Year of publication | Citation |
|---|---|---|---|---|---|---|
| 1 | MRM | CS | 3 | 4 | 2015 | Afonso et al. [29] |
| 2 | ESS | CS | 1 | 4 | 2018 | Agarwal et al. [30] |
| 3 | MRM | CS | 12 | 4 | 2017 | Agostini et al. [31] |
| 4 | MRM | CR | 1 | 5 | 2021 | Aguilar-Gonzalez et al. [32] |
| 5 | ESS | CR | 1 | 5 | 2011 | Albini et al. [33] |
| 6 | ESS | CR | 1 | 5 | 2011 | Almekhlafi et al. [34] |
| 7 | ESS | CS | 7 | 4 | 2016 | Apinyawasisuk et al. [35] |
| 8 | ESS | CR | 1 | 5 | 2014 | Aranda et al. [36] |
| 9 | MRM | RC | 10 | 3 | 2021 | Artaechevarria Artieda et al. [37] |
| 10 | ESS | CR | 1 | 5 | 2021 | Azar et al. [38] |
| 11 | ERM | CR | 1 | 5 | 2016 | Baek et al. [39] |
| 12 | ESS | CR | 1 | 5 | 2017 | Bakhsh et al. [40] |
| 13 | MRM | CR | 1 | 5 | 2019 | Balci et al. [41] |
| 14 | ESS | CS | 3 | 4 | 2014 | Burkholder et al. [42] |
| 15 | ESS | RC | 4 | 3 | 2019 | Chen et al. [43] |
| 16 | MRM | CR | 1 | 5 | 2021 | Cheng et al. [44] |
| 17 | ERM | CR | 1 | 5 | 2019 | Christakopoulos et al. [45] |
| 18 | ESS | CR | 1 | 5 | 2012 | Cillino et al. [46] |
| 19 | ERM | CS | 1 | 4 | 2015 | Curi et al. [47] |
| 20 | MRM | CR | 1 | 5 | 2019 | De Aragao et al. [48] |
| 21 | MRM | CR | 1 | 5 | 2018 | De Simone et al. [49] |
| 22 | ESS | CS | 2 | 4 | 2020 | Deibert et al. [50] |
| 23 | ESS | CS | 16 | 4 | 2021 | DeVience et al. [51] |
| 24 | MRM | CR | 1 | 5 | 2011 | Dua et al. [52] |
| 25 | ESS | CR | 1 | 5 | 2016 | Eliott et al. [53] |
| 26 | MRM | CS | 3 | 4 | 2019 | Etheridge et al. [54] |
| 27 | ESS | CR | 1 | 5 | 2016 | Fenolland et al. [55] |
| 28 | ESS | CS | 9 | 4 | 2019 | Ghanimi Zamli et al. [56] |
| 29 | ESS | CR | 1 | 5 | 2021 | Gonzalez Collazo et al. [57] |
| 30 | ESS | CR | 1 | 5 | 2018 | Hamill et al. [58] |
| 31 | ESS | CS | 2 | 4 | 2016 | Haug et al. [59] |
| 32 | ESS | CR | 1 | 5 | 2021 | Hay et al. [60] |
| 33 | ESS | CS | 3 | 4 | 2020 | Herbort et al. [61] |
| 34 | ESS | CR | 1 | 5 | 2018 | Horng et al. [62] |
| 35 | ESS | CS | 2 | 4 | 2021 | Jahnke et al. [63] |
| 36 | ESS | CS | 2 | 4 | 2015 | Ji et al. [64] |
| 37 | ESS | CR | 1 | 5 | 2017 | Kansal et al. [65] |
| 38 | ESS | CR | 1 | 5 | 2019 | Karti et al. [66] |
| 39 | MRM | CS | 7 | 4 | 2012 | Karunaratne et al. [67] |
| 40 | ERM | CR | 1 | 5 | 2020 | Khan et al. [68] |
| 41 | MRM | CR | 1 | 5 | 2019 | Kim et al. [69] |
| 42 | ESS | CS | 15 | 4 | 2019 | Klein et al. [70] |
| 43 | ESS | CR | 1 | 5 | 2021 | Kumar et al. [71] |
| 44 | ESS | CR | 1 | 5 | 2014 | Kurtz et al. [72] |
| 45 | ESS | CR | 1 | 5 | 2020 | Latif et al. [73] |
| 46 | ESS | CR | 1 | 5 | 2013 | Lee et al. [74] |
| 47 | ESS | CS | 14 | 4 | 2015 | Lee et al. [75] |
| 48 | ESS | RC | 11 | 3 | 2011 | Li et al. [76] |
| 49 | ESS | CS | 3 | 4 | 2021 | Lim et al. [77] |
| 50 | ERM | CS | 2 | 4 | 2014 | Lima et al. [78] |
| 51 | ERM | CR | 1 | 5 | 2016 | Loureiro et al. [79] |
| 52 | MRM | CR | 1 | 5 | 2021 | Mathews et al. [80] |
| 53 | ESS | CR | 1 | 5 | 2011 | Milger et al. [81] |
| 54 | ESS | CR | 1 | 5 | 2015 | Mitchell et al. [82] |
| 55 | ERM | CR | 1 | 5 | 2014 | Monica et al. [83] |
| 56 | ESS | CR | 1 | 5 | 2020 | Morris et al. [84] |
| 57 | ESS | CR | 1 | 5 | 2019 | Motlagh et al. [85] |
| 58 | ERM | CS | 3 | 4 | 2018 | Mustapha et al. [86] |
| 59 | ESS | CR | 1 | 5 | 2014 | Nguyen et al. [87] |
| 60 | ESS | CR | 1 | 5 | 2018 | Nolan et al. [88] |
| 61 | ESS | RC | 19 | 3 | 2015 | Northey et al. [89] |
| 62 | ESS | CS | 3 | 4 | 2013 | Nurfahzura et al. [90] |
| 63 | ESS | CS | 2 | 4 | 2022 | Odendaal et al. [91] |
| 64 | MRM | CR | 1 | 5 | 2019 | Ormaechea et al. [92] |
| 65 | ESS | CR | 1 | 5 | 2020 | .Parija et al. [93] |
| 66 | ESS | CR | 1 | 5 | 2015 | Patel et al. [94] |
| 67 | ESS | CS | 18 | 4 | 2014 | Pichi et al. [95] |
| 68 | ESS | CS | 1 | 4 | 2019 | Pirani et al. [96] |
| 69 | ESS | CR | 1 | 5 | 2019 | Ploysangam et al. [97] |
| 70 | ESS | CR | 1 | 5 | 2016 | Rishi et al. [98] |
| 71 | ESS | CR | 1 | 5 | 2012 | Rodrigues et al. [99] |
| 72 | ESS | CS | 12 | 4 | 2014 | Rodrigues et al. [100] |
| 73 | ESS | CR | 1 | 5 | 2013 | Rodriguez-Una et al. [101] |
| 74 | MRM | CR | 1 | 5 | 2016 | Romao et al. [102] |
| 75 | ESS | CS | 11 | 4 | 2015 | Sahin et al. [103] |
| 76 | ESS | CR | 1 | 5 | 2019 | Schlaen et al. [104] |
| 77 | MRM | CS | 4 | 4 | 2021 | Schulz et al. [105] |
| 78 | ESS | CS | 9 | 4 | 2015 | Shen et al. [106] |
| 79 | ESS | CR | 1 | 5 | 2016 | Shinha et al. [107] |
| 80 | ESS | CR | 1 | 5 | 2020 | Sidiqi et al. [108] |
| 81 | MRM | CS | 2 | 4 | 2020 | Silpa-Archa et al. [109] |
| 82 | ESS | CR | 1 | 5 | 2019 | Sood et al. [110] |
| 83 | ESS | CR | 1 | 5 | 2021 | Świerczyńska et al. [111] |
| 84 | ESS | CR | 1 | 5 | 2020 | .Teh et al. [112] |
| 85 | ESS | CR | 1 | 5 | 2021 | Tsai et al. [113] |
| 86 | ESS | CS | 5 | 4 | 2017 | Tsen et al. [114] |
| 87 | ESS | CS | 16 | 4 | 2016 | Tsuboi et al. [115] |
| 88 | ESS | CR | 1 | 5 | 2012 | Turchetti et al. [116] |
| 89 | ESS | CR | 1 | 5 | 2020 | Vidal-Villegas et al. [117] |
| 90 | ERM | CR | 1 | 5 | 2016 | Vignesh et al. [118] |
| 91 | MRM | CS | 19 | 4 | 2012 | Yang et al. [119] |
| 92 | MRM | CS | 10 | 4 | 2014 | Yap et al. [120] |
| 93 | ESS | CR | 1 | 5 | 2019 | Yosar et al. [121] |
| 94 | ESS | CS | 13 | 4 | 2016 | Zhang et al. [122] |
| 95 | ESS | CS | 28 | 4 | 2017 | Zhu et al. [123] |
ESS electronic search strategy, MRM manual reference mining, ERM electronic reference mining, CR case report, CS case series, RC retrospective cohort study.
aAs per the modified Oxford Centre for Evidence-Based Medicine where: 1 = Properly powered and conducted randomized clinical trial; systematic review with meta-analysis, 2 = Well-designed controlled trial without randomization; prospective comparative cohort trial, 3 = Case-control studies; retrospective cohort study, 4 = Case series with or without intervention; cross-sectional study, 5 = Opinion of respected authorities; case reports.
The mean age of patients was 44.9 years (range 18 to 84 years) and 81% of patients were male, of whom 48% were MSM. Median rapid plasma reagin (RPR) titre at baseline was 1:64. Forty-six percent (166/364) of patients included in this study were known to have HIV, among whom the mean HIV viral load was 234,313 copies/mL (SD 760,654) and mean CD4 cell count was 311 cells/mL (SD 226). In the 96 patients whose CD4 cell count was known, 53 (55%) had > 200 cells/mL, 29 (30%) had 101–200 cells/mL, 5 (5%) had 51–100 cells/mL, and 9 (9%) had ≤50 cells/mL. Bilateral disease occurred in 59% (213/362) of patients. In unilateral cases, the right eye was affected 56% of the time and the left eye, 44%. Mean time from ocular symptom development to presentation was 123 days (SD 281). Syphilis was detected in the cerebrospinal fluid (CSF) of 67/138 (49%) patients who had CSF data reported. See Table 2 for participant demographics.
Table 2.
Participant demographics.
| Variable | All (n = 364) | HIV− (n = 198) | HIV+ (n = 166) | P Value |
|---|---|---|---|---|
| Mean age in years (95% CI) | 44.9 (43.6–46.2) | 48.6 (46.9–50.3) | 40.5 (38.7–42.2) | <0.001 |
| Male sex | 295/363 (81.3%) | 139/197 (70.6%) | 156/166 (94.0%) | <0.001 |
| MSMa | 68/143 (47.6%) | 15/72 (20.8%) | 53/71 (74.6%) | <0.001 |
| Median RPRb | 1:64 | 1:64 | 1:256 | <0.001 |
| CSFc reactive for syphilis | 67/138 (48.6%) | 25/57 (43.9%) | 42/81 (51.9%) | 0.355 |
| Mean time to presentation in days (95% CI) | 123 (80–167) | 200 (110–290) | 63 (37–89) | <0.001 |
| Mean time to follow up in days (95% CI) | 281 (227–335) | 336 (258–413) | 206 (137–276) | <0.001 |
| Cases with delayed diagnosis | 31/101 (30.7%) | 21/59 (35.6%) | 10/42 (23.8%) | 0.206 |
| Bilateral disease | 213/362 (58.8%) | 108/197 (54.8%) | 105/165 (63.6%) | 0.090 |
aMSM men who have sex with men.
bRPR rapid plasma reagin.
cCSF cerebrospinal fluid.
The three most common ocular complaints at presentation were VA loss or blurry vision (328/362, 91%), eye pain (49/296, 17%), and red eye (47/296, 16%). Only 16/362 (4%) symptomatic eyes were not associated with any of the above symptoms. Despite being a classic feature of neurosyphilis, Argyll Robertson pupils (bilateral miosis with light-near dissociation) were not reported in any of the eyes included in this review. The most common ocular diagnoses were posterior uveitis (192/515, 37%), anterior uveitis (144/525, 27%), and panuveitis (144/525, 27%). Optic nerve involvement was seen in 197/568 (35%) of eyes. Overall mean VA at presentation was 0.893 (95%CI 0.825–0.961) while mean post-treatment VA was 0.326 (95%CI 0.280–0.373), representing a mean improvement of 0.567 (95%CI 0.510–0.624) or approximately 6 Snellen lines. Mean time to last follow-up was 281 days (SD 485). The majority of eyes, 63%, had improvement of VA after treatment while VA remained stable in 34% and declined in only 3% of eyes.
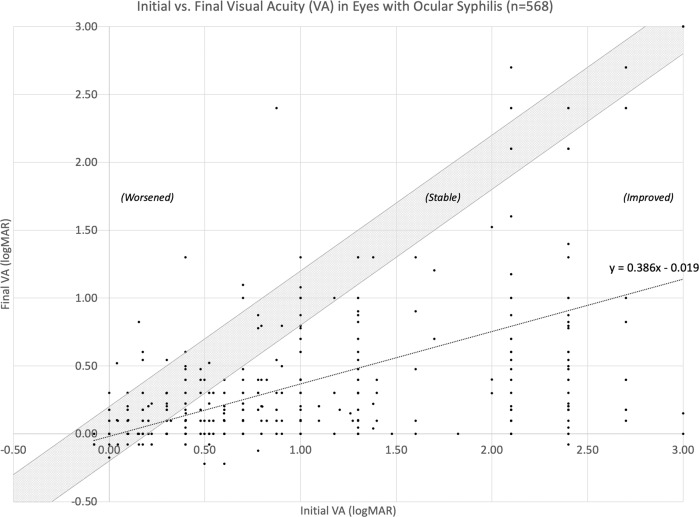
Initial VA was significantly correlated with final VA (β = 0.386, p < 0.001), as represented in Fig. 2. The overall regression model was: final VA = 0.386 (initial VA)−0.019 (R2 = 0.317, F(1566) = 262.458, p < 0.001). Prognostic factors of final VA ≥ 1.00 (worse than or equal to Snellen 20/200) included initial VA ≥ 1.00 (OR 5.48, 95%CI 4.17–7.21, p < 0.001), macular oedema (OR 4.68, 95%CI 1.54–14.21, p = 0.006), and female sex (OR 2.15, 95%CI 1.14–4.05; p = 0.017). Females experienced a significantly longer mean time to diagnosis compared to males (female 277 days vs. males 87 days; difference 190; 95%CI 84–296; p < 0.001). However, the relationship between female sex and poor final VA was found to occur independently of this delay in diagnosis. Variables significantly predicting final VA included initial VA ≥ 1.00 (mean final VA 0.647 vs. 0.128; difference 0.519, 95%CI 0.433–0.605; p < 0.001), female sex (mean final BCVA 0.463 vs. 0.296 for males; difference 0.17, 95%CI 0.046–0.288; p = 0.003), and a diagnosis of panuveitis (mean final VA 0.446 vs. 0.275 without panuveitis; difference 0.16, 95%CI 0.059–0.282; p = 0.017).
Fig. 2. Initial vs. final visual acuity (VA) in eyes with ocular syphilis.
Data points to the right of the shaded region demonstrated improved VA ≥ 0.200 logMAR after treatment. Data points falling in the shaded region demonstrated stable VA with a post-treatment change of <0.200 logMAR. Data points to the left of the shaded region demonstrated worsened VA ≥ 0.200 logMAR after treatment. Counting fingers (CF), hand movements (HM), light perception (LP), and no light perception (NLP) were reported as 2.10, 2.40, 2.70, and 3.00 logMAR, respectively.
HIV status (p = 0.289), HIV viral load (p = 0.144), and CD4 cell count ≤200 cells/mL (p = 0.962), ≤100 cells/mL (p = 0.965), and ≤50 cells/mL (p = 0.653) were not predictive of visual outcome. Final VA was not significantly associated with age (p = 0.06), RPR titre (p = 0.273), delayed diagnosis (p = 0.847), initial corticosteroid therapy (p = 0.261), bilaterality (p = 0.814), or optic nerve involvement (p = 0.537).
All 281 patients whose treatment status was described received systemic treatment for ocular syphilis: 85% received IV penicillin G, 17% IM penicillin G, and 12% IV ceftriaxone. Notably, 31% (31/101) of patients were initially misdiagnosed, and 27% (20/74) of those for whom data were available were initiated on systemic corticosteroid therapy before anti-syphilis treatment was given. In cases which were initially misdiagnosed and data were available, 80% (16/20) were treated with corticosteroids prior to antibiotic therapy. Overall, 5 cases of Jarisch-Herxheimer reaction were reported, none of which occurred in those patients who received systemic corticosteroids.
Patients with HIV were significantly more likely to be younger (mean 40.5 vs. 48.6 years; difference 8.1, 95%CI 5.72–10.57; p < 0.001), male (OR 6.51, 95%CI 3.20–13.23, p < 0.001), and have higher RPR at the time of diagnosis (median 1:256 vs. 1:64, p < 0.001) despite presenting to care earlier (mean 63 vs. 200 days; difference 137, 95%CI 53–222; p < 0.001). Amongst male patients with ocular syphilis, those with HIV were more likely to be MSM (OR 11.19, 95%CI 5.13–24.42, p < 0.001). No significant relationship was found between being infected with HIV and likelihood of positive syphilis serology in the CSF (p = 0.355), bilaterality of ocular syphilis (p = 0.090), or side of the eye affected (p = 0.688). HIV status was not significantly correlated with any presenting clinical symptom. However, infection with HIV was associated with panuveitis (OR 2.19, 95%CI 1.48–3.26, p < 0.001) and optic nerve involvement (OR 1.60, 95%CI 1.13–2.27, p = 0.009), described variably as optic neuritis, papillitis, optic nerve atrophy, and optic nerve oedema. HIV status was not significantly associated with presenting VA (p = 0.342) and there was no association with the likelihood of VA improvement (p = 0.795), stability (p = 0.722), and decline (p = 0.808), final VA (p = 0.773), or VA change from pre- to post-treatment (p = 0.499). See Table 3 for eye characteristics by HIV status.
Table 3.
Characteristics of eyes by HIV status.
| Variable | All (n = 568) | HIV− (n = 300) | HIV+ (n = 268) | P Value |
|---|---|---|---|---|
| Right eye affected | 285/568 (51.8%) | 150/294 (51.0%) | 135/256 (52.7%) | 0.688 |
| Visual acuity | ||||
| Mean initial VAa (logMAR) (95% CI) | 0.893 (0.825–0.961) | 0.837 (0.750–0.924) | 0.956 (0.849–1.062) | 0.342 |
| Mean final VAa (logMAR) (95% CI) | 0.326 (0.280–0.373) | 0.318 (0.255–0.380) | 0.336 (0.265–0.406) | 0.773 |
| Mean VAa (logMAR) change (95% CI) | 0.567 (0.510–0.624) | 0.519 (0.446–0.592) | 0.620 (0.531–0.709) | 0.499 |
| Symptoms | ||||
| Visual acuity loss or blurry vision | 328/362 (90.6%) | 178/195 (91.3%) | 150/167 (89.8%) | 0.635 |
| Eye pain | 49/296 (16.6%) | 26/148 (17.6%) | 23/148 (15.5%) | 0.639 |
| Red eye | 47/296 (15.6%) | 19/148 (12.8%) | 28/148 (18.9%) | 0.152 |
| Floaters | 46/296 (15.5%) | 19/148 (12.8%) | 27/148 (18.2%) | 0.199 |
| Scotoma | 38/306 (12.4%) | 17/148 (11.5%) | 21/158 (13.3%) | 0.632 |
| Visual field defect | 32/315 (9.4%) | 17/176 (9.7%) | 15/165 (9.1%) | 0.857 |
| Photophobia | 27/296 (9.1%) | 10/148 (6.8%) | 17/148 (11.5%) | 0.158 |
| Headache | 17/315 (5.4%) | 9/165 (5.5%) | 8/150 (5.3%) | 0.962 |
| Dyschromatopsia | 15/296 (5.1%) | 9/148 (6.1%) | 6/148 (4.1%) | 0.427 |
| Macular edema | 17/515 (3.3%) | 15/259 (5.8%) | 2/256 (0.8%) | 0.001 |
| RAPD | 16/515 (3.1%) | 9/259 (3.5%) | 7/256 (2.7%) | 0.628 |
| Photopsia | 8/296 (2.7%) | 5/148 (3.4%) | 3/148 (2.0%) | 0.473 |
| Diagnoses | ||||
| Keratitis | 88/515 (17.1%) | 45/259 (17.4%) | 43/256 (16.8%) | 0.862 |
| Scleritis | 12/515 (2.3%) | 5/259 (1.9%) | 7/256 (2.7%) | 0.545 |
| Vasculitis | 37/501 (7.4%) | 21/249 (8.4%) | 16/252 (6.3%) | 0.372 |
| Anterior uveitis | 144/525 (27.4%) | 72/259 (27.8%) | 72/266 (27.1%) | 0.851 |
| Intermediate uveitis | 126/525 (24.5%) | 62/259 (23.9%) | 64/256 (25.0%) | 0.779 |
| Posterior uveitis | 192/515 (37.3%) | 102/259 (39.4%) | 90/256 (35.2%) | 0.321 |
| Panuveitis | 144/525 (27.4%) | 51/259 (19.7%) | 93/266 (35.0%) | <0.001 |
| Optic nerve involvementb | 197/568 (34.7%) | 89/300 (29.7%) | 108/268 (40.3%) | 0.009 |
| Retinal detachment | 18/501 (3.6%) | 6/249 (2.4%) | 12/252 (4.8%) | 0.157 |
| Jarisch-Herxheimer reaction | 5/220 (2.3%) | 2/89 (2.2%) | 3/131 (2.3%) | 0.983 |
aVA visual acuity.
boptic nerve involvement includes optic neuritis, papillitis, optic nerve atrophy, and optic nerve edema.
Discussion
To our knowledge, this is the largest systematic review of visual acuity outcomes in ocular syphilis to date. Commonly known as “the great masquerader” for its many manifestations, syphilis has a unique ability to present in myriad ways. The diagnosis of syphilis involves treponemal and non-treponemal tests, which can cross-react, be serofast, or produce false positive or negative results [124, 125]. Ocular syphilis in particular, can pose a diagnostic challenge due to its variable presentation and lack of pathognomonic features [48, 56, 67, 126]. Therefore, a diagnosis of ocular syphilis is often presumptive, and a strong index of suspicion is key [126, 127]. Our systematic review reveals the typical clinical presentations of ocular syphilis to aid the clinician in their diagnostic accuracy.
The predominance of ocular syphilis in males, especially MSM, and middle-aged patient groups has been well-established [128, 129] and is consistent with the demographic findings of this study. At presentation, bilateral disease was more common than unilateral disease, and there was a similar distribution of ocular syphilis affecting the left and right eyes. VA loss or blurry vision, eye pain, and red eye were the most common ocular complaints at presentation with a combined sensitivity of 96%. The most common of these symptoms was VA loss or blurry vision, with a prevalence of 91%. Therefore, in patients with syphilis, the lack of VA loss or blurry vision reduces the likelihood of ocular involvement. The most common ocular diagnoses were posterior uveitis, panuveitis, and anterior uveitis, occurring in 38%, 27%, and 27% of patients, respectively. The morbidity of ocular syphilis stems largely from the inflammation rather than the infection itself. If a patient with an extremely suppressed immune system was unable to mount an immune response, they may have no visual symptoms or signs at all, hampering the ability to establish a diagnosis of ocular syphilis. Despite treatment guidelines by the Centers for Disease Control and Prevention and Public Health Agency of Canada for routine CSF analysis in ocular syphilis [130, 131], CSF data were only available for approximately one-third of all patients and detected syphilis in 49%. At presentation, the mean VA was 0.893 (Snellen equivalent 20/156) and median VA was 0.602 (20/80). VA improved to a mean of 0.326 (20/42) and median 0.097 (20/25) with treatment, highlighting the good prognosis of ocular syphilis with appropriate management.
Nearly one-third of patients were initially misdiagnosed, among whom 80% were initiated on systemic corticosteroid therapy. Of note, there was a large number of missing values regarding diagnostic accuracy. The role of systemic corticosteroids in ocular syphilis has been controversial, with some reporting it to cause clinical worsening while others suggest it as a valuable adjunct to antibiotic therapy [30, 132–135]. In our analysis, patients who received initial corticosteroid therapy prior to antibiotics were not found to have poorer visual outcomes, but the limited sample size limits the conclusions that can be drawn from this finding. Our study indicates the importance of future prospective work to explore and address the timing of steroids in ocular syphilis.
Visual acuity outcomes were not found to be worse in eyes affected by bilateral ocular syphilis and, contrary to previous reports [136, 137], optic nerve involvement was not a predictor of final VA in our analysis. Reporting on other measures of visual function, such as visual field defects and colour vision, was generally lacking in the studies included in this systematic review. This would be a useful outcome to investigate in future research as these are valuable measures of visual function. Prognostic factors for post-treatment VA ≥ 1.00 included VA ≥ 1.00 at presentation, female sex, and presence of macular oedema. Although females also experienced a significantly longer time to diagnosis than males, female sex was found to be a predictor of poor visual outcome independent of the delay in diagnosis. Similarly, the presence of macular oedema was significantly associated with poor visual outcome. However, we lack the long-term data to assess whether macular oedema was reversible with treatment or if these cases were associated with poor final VA even if macular oedema resolved. Of all the variables significantly associated with final VA, VA ≥ 1.00 at presentation was the strongest predictor of a worse final VA. Therefore, detection of ocular syphilis before severe VA loss is critical in achieving good visual outcomes for patients. Patients with syphilis complaining of visual acuity loss should be promptly referred to ophthalmology.
Given the relative rarity of ocular syphilis, its relationship with HIV is not well understood. This study contributes to a better understanding of the interaction between the two diseases. HIV subgroup analysis revealed that patients who were coinfected with HIV were younger, more likely to be male, and had higher RPR at diagnosis. As previously reported, MSM were at an increased risk of being coinfected with HIV and ocular syphilis [129]. Our work indicates the importance of ensuring routine syphilis testing in HIV care. Time to presentation of ocular symptoms in those patients with HIV was significantly earlier than those who tested negative for HIV. This may be, in part, due to the regular monitoring that occurs as part of routine HIV care. All patients included in this study were treated promptly with antibiotics once the diagnosis was established. Patients with HIV presented for care earlier than those without HIV, and therefore received earlier treatment, but delay in diagnosis was not found to affect visual outcome. It is possible that HIV coinfection may result in a more severe and rapid disease course, and this risk was offset by earlier treatment, but firm conclusions cannot be drawn without an untreated control arm. There could be a bias in the reported literature reflecting outcomes of patients being treated at academic centres, and rural communities may face more barriers in reaching the same level of engagement as their urban counterparts [138, 139]. While previous studies have demonstrated a propensity for bilateral disease in patients with HIV [14, 140], our systematic review did not confirm this relationship. Among those with unilateral ocular syphilis, we found a similar distribution between left and right eyes, contradicting a previously reported increased risk of left eye disease [128]. Although HIV status was not associated with any presenting symptom, patients with HIV were more likely to be diagnosed with panuveitis and demonstrate optic nerve involvement. Our study agrees with previous literature that HIV positive and negative patients appear to present with different forms of ocular inflammation (anterior, posterior, optic nerve, panuveitis), but this was not found to be a significant predictor of final visual acuity [141].
Ultimately, HIV status, CD4 cell count, and HIV viral load was not found to impact the visual prognosis of eyes with ocular syphilis. Subgroup analysis of patients with CD4 cell counts ≤200 cells/mL, ≤100 cells/mL, and ≤50 cells/mL also did not suggest a relationship to visual outcome. It has been hypothesized that immunosuppression by HIV puts patients with syphilis at risk for developing ocular involvement [142, 143]. However, HIV has never been implicated in the visual prognosis of patients with ocular syphilis [14, 128]. Our analysis relies on the data made available through the global literature, among which there are limited reports of severe immunosuppression available for analysis. While all 364 patients in our systematic review had known HIV status, CD4 cell count was reported in only 96 and HIV viral load was reported in 59. Furthermore, there were few cases of low CD4 cell count ≤100 cells/mL available for analysis, possibly due to the widespread prevalence of antiretroviral therapy for HIV in the modern era. The limited number of patients with severe immunosuppression limits our ability to draw definitive conclusions in these individuals. Future studies evaluating patients with ocular syphilis and HIV should be designed to include important variables that reflect disease activity and immune status, such as viral load and CD4 + counts.
Data used in the composition of this systematic review were primarily sourced from case reports and series. There exists a possible sampling bias as typically only unique or extraordinary cases are published. We are unable to verify or standardize the methods and techniques used to measure outcomes in each study, including VA, due to the retrospective nature of a systematic review. Finally, as discussed above, the diagnosis of ocular syphilis can be challenging and requires interpretation within a clinical context and, thus, we rely on the judgement of the studies’ authors to confirm the diagnosis in each eye analysed.
In this systematic review, post-treatment visual acuity in eyes affected by ocular syphilis was not impacted by HIV status, CD4 cell count, or HIV viral load. While visual prognosis was generally good, factors significantly associated with final VA ≥ 1.00 included initial VA ≥ 1.00, female sex, and presence of macular oedema. VA ≥ 1.00 at presentation was the strongest predictor of worse final VA. This finding highlights the importance of prompt referral of ocular syphilis to allow early diagnosis and management in preserving visual acuity.
Summary
What was known before
Ocular syphilis is a rare but increasingly prevalent vision-threatening disease, often occurring in patients with HIV.
Symptoms typically improve with appropriate antibiotic treatment, but no prognostic factors have been identified in determining the visual outcome of ocular syphilis.
What this study adds
HIV status, CD4 cell count, and HIV viral load were not found to impact the post-treatment visual acuity (VA) of eyes with ocular syphilis.
Factors significantly correlated with post-treatment VA worse than 1.00 logMAR included female sex, presence of macular edema, and VA worse than 1.00 logMAR at presentation.
Visual prognosis for eyes with ocular syphilis is generally good with antibiotic treatment, particularly if treatment is initiated before significant loss of visual acuity occurs.
Supplementary information
Supplemental Table 1 - Risk of bias assessment summary
Supplemental Table 2 - Full electronic search strategy of databases
Acknowledgements
We thank Brianna Howell-Spooner with the Saskatchewan Health Authority library for conducting a preliminary literature review on this topic of study and Erin Watson with the University of Saskatchewan Library for her guidance in developing our literature search strategy.
Author contributions
LZW was responsible for study concept and design, study design registration with PROSPERO, article screening and full-text review, risk of bias assessment, acquisition and interpretation of data, statistical analysis, manuscript drafting, updating reference lists, and creating tables and figures. TMO was responsible for article screening and full-text review, risk of bias assessment, acquisition and interpretation of data, manuscript drafting, updating reference lists. MK was responsible for analysis and interpretation of data, manuscript drafting, technical/administrative support. SL was responsible for analysis and interpretation of data, manuscript revision, technical/administrative support, and study supervision. PM was responsible for statistical analysis, analysis and interpretation of data, technical/administrative support, and manuscript revision. SK was responsible for analysis and interpretation of data, technical/administrative support, manuscript revision, and study supervision. LB was responsible for analysis and interpretation of data, technical/administrative support, manuscript drafting and revision, and study supervision.
Data availability
The datasets analysed in this work are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-023-02504-0.
References
- 1.Centers for Disease Control and Prevention. Table 24. All Stages of Syphilis: Sexually Transmitted Disease Surveillance 2019. Atlanta: Centers for Disease Control and Prevention; 2021.
- 2.Chen X-S. Special theme—Strengthening linkages between sexual and reproductive health and HIV. Bull World Health Organ. 2009;87:814–5. [Google Scholar]
- 3.Rowley J, Hoorn S, Korenromp E, Low N, Unemo M, Abu-Raddad L, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97:548–562P. doi: 10.2471/BLT.18.228486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiteri G, Unemo M, Mårdh O, Amato-Gauci A. The resurgence of syphilis in high-income countries in the 2000s: a focus on Europe. Epidemiol Infect. 2019;147:e143. doi: 10.1017/S0950268819000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sex workers with active syphilis (%). The Global Health Observatory: World Health Organization. 2020. https://www.who.int/data/gho/data/indicators/indicator-details/GHO/sex-workers-with-active-syphilis-(-) (accessed Dec 2021).
- 6.Tsuboi M, Evans J, Davies EP, Rowley J, Korenromp EL, Clayton T, et al. Prevalence of syphilis among men who have sex with men: a global systematic review and meta-analysis from 2000-20. Lancet Glob Health. 2021;9:1110–28. doi: 10.1016/S2214-109X(21)00221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tucker JD, Li JZ, Robbins GK, Davis BT, Lobo AM, Kunkel J, et al. Ocular syphilis among HIV-infected patients: a systematic analysis of the literature. Sex Transm Infect. 2010;87:4–8. doi: 10.1136/sti.2010.043042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stokes J, Beerman H, Ingraham N. Modern Clinical Syphilology. 3rd ed. W.B. Saunders Company: Philadelphia, PA; 1945.
- 9.Dombrowski JC, Pedersen R, Marra CM, Kerani RP, Golden MR. Prevalence estimates of complicated syphilis. Sex Transm Dis. 2015;42:702–4. doi: 10.1097/OLQ.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 10.Neurosyphilis, Ocular Syphilis, and Otosyphilis. Centers for Disease Control and Prevention. 2021. https://www.cdc.gov/std/syphilis/neuro-ocular-oto.htm (accessed Dec 2021).
- 11.Kiss S, Damico FM, Young LH. Ocular manifestations and treatment of syphilis. Semin Ophthalmol. 2005;20:161–7. doi: 10.1080/08820530500232092. [DOI] [PubMed] [Google Scholar]
- 12.Salado-Rasmussen K, Hoffmann S, Cowan S, Jensen J, Benfield T, Gerstoft J, et al. Serological response to treatment of syphilis with doxycycline compared with penicillin in HIV-infected individuals. Acta Derm Venereol. 2016;96:807–11. doi: 10.2340/00015555-2289. [DOI] [PubMed] [Google Scholar]
- 13.St. Louis M, Levine W, Wasserheit J, DeCock K, West G, Holtgrave D, et al. HIV Prevention Through Early Detection and Treatment of Other Sexually Transmitted Diseases - United States: Recommendations of the Advisory Committee for HIV and STD Prevention. MMWR Recomm. 1998;47:1–24. [PubMed] [Google Scholar]
- 14.Rasoldier V, Gueudry J, Chapuzet C, Bodaghi B, Muraine M, Tubiana R, et al. Early symptomatic neurosyphilis and ocular syphilis: a comparative study between HIV-positive and HIV-negative patients. Infect Dis Now. 2021;51:351–6. doi: 10.1016/j.medmal.2020.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Bollemeijer JG, Wieringa WG, Missotten TO, Meenken I, ten Dam-van Loon NH, Rothova A, et al. Clinical manifestations and outcome of syphilitic uveitis. Investig Ophthalmol Vis Sci. 2016;57:404–11. doi: 10.1167/iovs.15-17906. [DOI] [PubMed] [Google Scholar]
- 16.Pinto TVL, Gomes Neto AP, Cunha MN, Bernardino LM, Christo PP. Spectrum of ocular manifestations and visual outcomes of neurosyphilis among 53 patients. Arq Neuropsiquiatr. 2021;79:584–9. doi: 10.1590/0004-282x-anp-2020-0332. [DOI] [PubMed] [Google Scholar]
- 17.Vadboncoeur J, Labbé AC, Fortin C, Serhir B, Rabia Y, Najem K, et al. Ocular syphilis: case series (2000–2015) from 2 tertiary care centres in Montreal, Canada. Can J Ophthalmol. 2020;55:30–37. doi: 10.1016/j.jcjo.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Haddaway N, Grainger M, Gray C. citationchaser: an R package for forward and backward citations chasing in academic searching. Zenodo 2021. 10.5281/ZENODO.4533747.
- 19.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of Observational Studies in Epidemiology: A Proposal for Reporting. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 20.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/S13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, editors. 2020 10.46658/JBIMES-20-08.
- 22.Munn Z, Barker TH, Moola S, Tufanaru C, Stern C, McArthur A, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18:2127–33. doi: 10.11124/JBISRIR-D-19-00099. [DOI] [PubMed] [Google Scholar]
- 23.Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: Systematic reviews of etiology and risk. JBI Manual for Evidence Synthesis. 2020. https://synthesismanual.jbi.global (accessed 17 Jan 2022).
- 24.Jabs DA, Nussenblatt RB, Rosenbaum JT, Atmaca LS, Becker MD, Brezin AP, et al. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005;140:509–16. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Day AC, Donachie PHJ, Sparrow JM, Johnston RL. The Royal College of Ophthalmologists’ National Ophthalmology Database study of cataract surgery: report 1, visual outcomes and complications. Eye. 2015;29:552–60. doi: 10.1038/eye.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holladay JT. Proper method for calculating average visual acuity. J Refract Surg. 1997;13:388–91. doi: 10.3928/1081-597X-19970701-16. [DOI] [PubMed] [Google Scholar]
- 27.Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual Acuities “Hand Motion” and “Counting Fingers” Can Be Quantified with the Freiburg Visual Acuity Test. Investig Ophthalmol Vis Sci. 2006;47:1236–40. doi: 10.1167/iovs.05-0981. [DOI] [PubMed] [Google Scholar]
- 28.Moussa G, Bassilious K, Mathews N. A novel excel sheet conversion tool from Snellen fraction to LogMAR including ‘counting fingers’, ‘hand movement’, ‘light perception’ and ‘no light perception’ and focused review of literature of low visual acuity reference values. Acta Ophthalmol. 2021;99:e963–e965.. doi: 10.1111/aos.14659. [DOI] [PubMed] [Google Scholar]
- 29.Afonso VCC, Nascimento H, Belfort RM, Sato EI, Muccioli C, Belfort R. Visual loss resulting from immunosuppressive therapy in patients with syphilitic uveitis. Arq Bras Oftalmol. 2015;78:185–6. doi: 10.5935/0004-2749.20150047. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal M, Ranjan R, Paul L, Sharma D. Syphilitic uveitis misdiagnosed as viral retinitis—a misleading history. J Ophthalmic Inflamm Infect. 2018;8:22. doi: 10.1186/S12348-018-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agostini FA, Queiroz RP, Azevedo DO, Henriques JF, Campos WR, Vasconcelos-Santos DV. Intravenous Ceftriaxone for Syphilitic Uveitis. Ocul Immunol Inflamm. 2018;26:1059–65. doi: 10.1080/09273948.2017.1311926. [DOI] [PubMed] [Google Scholar]
- 32.Aguilar-González M, Fernández-Santodomingo AS, Marín-Payá E, Rahhal-Ortuño M, Udaondo P. Bilateral exudative retinal detachment in undiagnosed ocular syphilis after treatment with corticosteroids ocular syphilis after treatment with corticosteroids. Eur J Ophthalmol. 2021;31:NP86–NP90. doi: 10.1177/1120672119889007. [DOI] [PubMed] [Google Scholar]
- 33.Albini T, Davis JL, Tuda CD. Challenging cases discussed by experts: retinal vasculitis following coinfection with HIV and syphilis. J Ophthalmic Inflamm Infect. 2011;1:89–93. doi: 10.1007/s12348-011-0022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almekhlafi M, Williams G, Costello F. Clinical reasoning: optic disc swelling in a patient with AIDS. Neurology. 2011;77:e28–e32. doi: 10.1212/WNL.0b013e318227b1d4. [DOI] [PubMed] [Google Scholar]
- 35.Apinyawasisuk S, Poonyathalang A, Preechawat P, Vanikieti K. Syphilitic optic neuropathy: re-emerging cases over a 2-year period. Neuroophthalmology. 2016;40:69–73. doi: 10.3109/01658107.2015.1134586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aranda S, Amer R. Sequential spontaneous resolution of acute syphilitic posterior placoid chorioretinitis. Eur J Ophthalmol. 2014;25:263–5. doi: 10.5301/ejo.5000530. [DOI] [PubMed] [Google Scholar]
- 37.Artaechevarria Artieda J, Estébanez-Corrales N, Muñoz N, Rodríguez-Olleros Rodríguez C, Cabello Úbeda A, Carreño E. Spectrum of syphilitic chorioretinitis and its evolution based on multimodal imaging. Ocul Immunol Inflamm 2021;30:1639–50. [DOI] [PubMed]
- 38.Azar G, Wolff B, Azam S, Mauget-Faÿsse M. Acute syphilitic posterior placoid chorioretinopathy presenting as atypical multiple evanescent white dot syndrome. Eur J Ophthalmol. 2021;31:NP141–NP144. doi: 10.1177/1120672120957589. [DOI] [PubMed] [Google Scholar]
- 39.Baek J, Kim KS, Lee WK. Natural course of untreated acute syphilitic posterior placoid chorioretinitis. Clin Exp Ophthalmol. 2016;44:431–3. doi: 10.1111/ceo.12679. [DOI] [PubMed] [Google Scholar]
- 40.Bakhsh SR, Ali MH, Bhat P. Chorioretinal lesions and symptoms of elevated intracranial pressure. JAMA Ophthalmol. 2017;135:889–90. doi: 10.1001/jamaophthalmol.2016.5829. [DOI] [PubMed] [Google Scholar]
- 41.Balci SY, Vural ET, Ozcaliskan S. Intermediate uveitis as the initial and only presentation of syphilis. Turk J Ophthalmol. 2019;49:297–9. doi: 10.4274/tjo.galenos.2019.72558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burkholder BM, Leung TG, Ostheimer TA, Butler NJ, Thorne JE, Dunn JP. Spectral domain optical coherence tomography findings in acute syphilitic posterior placoid chorioretinitis. J Ophthalmic Inflamm Infect. 2014;4:1–5. doi: 10.1186/1869-5760-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen JJ, Bhatti MT, Bradley E, Garrity J, Thurtell MJ. Incipient Syphilitic Papillitis. Neuroophthalmology. 2019;44:11–15. doi: 10.1080/01658107.2019.1615959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng Y, Wang C, Su G. Necrotizing retinitis in a patient with syphilis: a case report. Medicine. 2021;100:e24452–e24452. doi: 10.1097/MD.0000000000024452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christakopoulos C, Munch IC. The ‘pitchfork sign’ on optical coherence tomography in a case of acute syphilitic posterior placoid chorioretinitis. Acta Ophthalmol. 2019;97:e942–e943.. doi: 10.1111/aos.14094. [DOI] [PubMed] [Google Scholar]
- 46.Cillino S, di Pace F, Trizzino M, Vecchi VL, di Carlo P. Chancre of the eyelid as manifestation of primary syphilis, and precocious chorioretinitis and uveitis in an HIV-infected patient: a case report. BMC Infect Dis. 2012;12:226–226. doi: 10.1186/1471-2334-12-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Curi AL, Sarraf D, Cunningham ET. Multimodal imaging of syphilitic multifocal retinitis. Retin Cases Brief Rep. 2015;9:277–80. doi: 10.1097/ICB.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 48.de Aragao R, Barreira I, Muccioli C, Carneiro G, de Araujo C, de Almeida E, et al. Good visual outcome in a bilateral multifocal syphilitic chorioretinitis, despite late diagnosis. Retin Cases Brief Rep. 2019;13:84–87. doi: 10.1097/ICB.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 49.de Simone L, Pellegrini F, Cirone D, Cimino L, Falardeau J. The unfaithful neuritis. Surv Ophthalmol. 2018;63:875–9. doi: 10.1016/j.survophthal.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 50.Deibert B, Wark K, Diaz R, Blodi C. Spontaneous hyphema associated with ocular syphilis. J Ophthalmic Inflamm Infect. 2020;10:17. doi: 10.1186/s12348-020-00209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeVience EX, Schechet SA, Carney M, Kaleem M, DeVience S, Chang L, et al. Syphilitic retinitis presentations: punctate inner retinitis and posterior placoid chorioretinitis. Int Ophthalmol. 2021;41:211–9. doi: 10.1007/s10792-020-01569-0. [DOI] [PubMed] [Google Scholar]
- 52.Dua J, Nori A, Elliot E, Goh B, Orkin C. Blinded by love: ocular syphilis as the initial manifestation of HIV. J Infect. 2011;63:e119. doi: 10.1016/j.jinf.2011.04.200. [DOI] [Google Scholar]
- 53.Eliott D, Papaliodis GN, Durand ML, Turbett SE. Case Records of the Massachusetts General Hospital. Case 20-2016. A 50-Year-Old Man with Cloudy Vision, Hearing Loss, and Unsteadiness. N Engl J Med. 2016;374:2586–93. doi: 10.1056/NEJMcpc1600611. [DOI] [PubMed] [Google Scholar]
- 54.Etheridge T, Bowen RC, Raven M, Snow KB, Urban AW, Chang JS. Ocular syphilis: clinical manifestations and treatment course. WMJ. 2019;118:191–5. [PubMed] [Google Scholar]
- 55.Fénolland J-R, Bonnel S, Rambaud C, Froussart-Maille F, Rigal-Sastourné J-C. Syphilitic scleritis. Ocul Immunol Inflamm. 2016;24:93–95. doi: 10.3109/09273948.2014.916307. [DOI] [PubMed] [Google Scholar]
- 56.Ghanimi Zamli A, Irma Ngah N, Chew-Ean T, Muhammed J, Hitam W, Hussein A, et al. Clinical profile and visual outcomes of ocular syphilis: a five-year review in Hospital Universiti Sains, Malaysia. Cureus. 2019;11:e4015–e4015. doi: 10.7759/cureus.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonzalez Collazo MP, Rebollo Rodriguez NP, Santiago-Vazquez M, Crespo-Ramos SM, Marcos-Martinez MJ, Villegas VM, et al. Bilateral hypopyon in syphilitic uveitis. Am J Ophthalmol Case Rep. 2021;21:101007. doi: 10.1016/j.ajoc.2020.101007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamill MM, Seppings L, Kit V, Antao S. Concurrent primary chancre and ocular syphilis in an human immunodeficiency virus-negative man. Sex Transm Infect. 2018;45:e109–e112. doi: 10.1097/OLQ.0000000000000900. [DOI] [PubMed] [Google Scholar]
- 59.Haug SJ, Takakura A, Jumper JM, Heiden D, McDonald HR, Johnson RN, et al. Rhegmatogenous retinal detachment in patients with acute syphilitic panuveitis. Ocul Immunol Inflamm. 2016;24:69–76. doi: 10.3109/09273948.2014.925122. [DOI] [PubMed] [Google Scholar]
- 60.Hay MW, Emami-Naeini P. A middle-aged man with bilateral eye redness and pain. JAMA Ophthalmol. 2021;139:1319–20. doi: 10.1001/jamaophthalmol.2021.1451. [DOI] [PubMed] [Google Scholar]
- 61.Herbort C, Papasavvas I, Mantovani A. Choriocapillaris involvement in acute syphilis posterior placoid chorioretinitis is responsible for functional impairment and points towards an immunologic mechanism: A comprehensive clinicopathological approach. J Curr Ophthalmol. 2020;32:381–9. doi: 10.4103/JOCO.JOCO_184_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Horng C-T, Tsai P-F, Tsai M-L. Multiple preretinal yellowish dots in a patient with syphilis. Tzu Chi Med J. 2018;30:255–6. doi: 10.4103/tcmj.tcmj_79_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jahnke S, Sunderkötter C, Lange D, Wienrich R, Kreft B. Ocular syphilis—a case series of four patients. J Dtsch Dermatol Ges. 2021;19:987–91. doi: 10.1111/ddg.14464. [DOI] [PubMed] [Google Scholar]
- 64.Ji Y-S, Yang JM, Park S-W. Early resolved acute syphilitic posterior placoid chorioretinitis. Optom Vis Sci. 2015;92:S55–S58.. doi: 10.1097/OPX.0000000000000531. [DOI] [PubMed] [Google Scholar]
- 65.Kansal V, Dollin M. Acute and painless monocular vision loss in a human immunodeficiency virus-positive man. JAMA Ophthalmol. 2017;135:811–2. doi: 10.1001/jamaophthalmol.2016.5679. [DOI] [PubMed] [Google Scholar]
- 66.Karti O, Top Karti D, Ozkan Ozdemir H, Eskut N, Zengin MO, Kusbeci T, et al. Coexistence of papillitis and posterior placoid chorioretinopathy as the presenting symptoms of syphilis-human immunodeficiency virus coinfection. Neuroophthalmology. 2019;43:196–200. doi: 10.1080/01658107.2018.1493515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karunaratne I, Sharma S, Dick A, Carrington D, Horner P. Shared care approach to managing ophthalmological disease in patients with positive treponemal serology: a case series. Int J STD AIDS. 2012;23:291–6. doi: 10.1258/ijsa.2011.011210. [DOI] [PubMed] [Google Scholar]
- 68.Khan MS, Kuruppu DK, Popli TA, Moorthy RS, Mackay DD. Unilateral optic neuritis and central retinal vasculitis due to ocular syphilis. Retin Cases Brief Rep. 2020;14:35–38. doi: 10.1097/ICB.0000000000000614. [DOI] [PubMed] [Google Scholar]
- 69.Kim J, Ussher JG. Ocular syphilis: connecting the dots. J Prim Health Care. 2019;11:12–13. doi: 10.1071/HC18065. [DOI] [PubMed] [Google Scholar]
- 70.Klein A, Fischer N, Goldstein M, Shulman S, Habot‐Wilner Z. The great imitator on the rise: ocular and optic nerve manifestations in patients with newly diagnosed syphilis. Acta Ophthalmol. 2019;97:e641–e647. doi: 10.1111/aos.13963. [DOI] [PubMed] [Google Scholar]
- 71.Kumar A, Kumar P, Mishra SK, Goyal S. Unilateral hypopyon associated acute syphilitic posterior placoid chorioretinitis: Unusual presentation leading to HIV diagnosis. Trop Doct. 2021;51:444–6. doi: 10.1177/0049475521998178. [DOI] [PubMed] [Google Scholar]
- 72.Kurtz SD, Rollin F. Ocular syphilis in a patient with HIV. JAAPA. 2014;27:32–35. doi: 10.1097/01.JAA.0000444734.39540.fc. [DOI] [PubMed] [Google Scholar]
- 73.Latif N, Janani M, Sudharshan, Selvamuthu P, Dutta Majumder P. Triple trouble: a case of retinochoroiditis in a patient with syphilis, tuberculosis, and human immunodeficiency virus infection. Indian J Ophthalmol. 2020;68:1995–7. doi: 10.4103/ijo.IJO_2170_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee SB, Kim KS, Lee WK, Kim YJ, Kang MW. Ocular syphilis characterised by severe scleritis in a patient infected with HIV. Lancet Infect Dis. 2013;13:994. doi: 10.1016/S1473-3099(13)70198-1. [DOI] [PubMed] [Google Scholar]
- 75.Lee SY, Cheng V, Rodger D, Rao N. Clinical and laboratory characteristics of ocular syphilis: a new face in the era of HIV co-infection. J Ophthalmic Inflamm Infect. 2015;5:26. doi: 10.1186/s12348-015-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li SY, Birnbaum AD, Tessler HH, Goldstein DA. Posterior syphilitic uveitis: clinical characteristics, co-infection with HIV, response to treatment. Jpn J Ophthalmol. 2011;55:486–94. doi: 10.1007/s10384-011-0053-z. [DOI] [PubMed] [Google Scholar]
- 77.Lim YW, Lott PW, Mohamad NF, Begam Iqbal T. A case series with literature review on adjunctive usage of intravitreal ceftazidime in ocular syphilis with human immunodeficiency virus infection. Int J STD AIDS. 2021;32:968–73. doi: 10.1177/09564624211011917. [DOI] [PubMed] [Google Scholar]
- 78.Lima BR, Mandelcorn ED, Bakshi N, Nussenblatt RB, Nida Sen H. Syphilitic outer retinopathy. Ocul Immunol Inflamm. 2014;22:4–8. doi: 10.3109/09273948.2013.841960. [DOI] [PubMed] [Google Scholar]
- 79.Loureiro MM, Sepúlveda PA. Bilateral chorioretinitis as syphilis presentation: multimodal characterization and therapy response. J Clin Diagn Res. 2016;10:ND01–ND02. doi: 10.7860/JCDR/2016/19359.8406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mathews B, George R, Ulrich JN. Ocular syphilis causing panophthalmitis. Ophthalmic Plast Reconstr Surg. 2021;37:e69–e71. doi: 10.1097/IOP.0000000000001780. [DOI] [PubMed] [Google Scholar]
- 81.Milger K, Fleig V, Kohlenberg A, Discher T, Lohmeyer J. Neurosyphilis manifesting with unilateral visual loss and hyponatremia: a case report. BMC Infect Dis. 2011;11:17. doi: 10.1186/1471-2334-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mitchell JP, Huang LL, Rosberger DF. Ocular syphilis in patients with Human Immunodeficiency Virus infection. J Natl Med Assoc. 2015;107:130–2. doi: 10.1016/S0027-9684(15)30037-7. [DOI] [PubMed] [Google Scholar]
- 83.Monica F, Luisa C, Cristina P, Joana N, Raquel S. Secondary syphilis presenting as optic neuritis in an immunocompetent patient: case report. J Clin Res Ophthalmol. 2014;1:19–21.. doi: 10.17352/2455-1414.000005. [DOI] [Google Scholar]
- 84.Morris JE, Ivos M, Axe D. Recurrent ocular syphilis in a patient living with HIV. Int J STD AIDS. 2020;31:1114–6. doi: 10.1177/0956462420906923. [DOI] [PubMed] [Google Scholar]
- 85.Motlagh MN, Javid CG. Presentation of ocular syphilis in a HIV-positive patient with false-negative serologic screening. Case Rep Infect Dis. 2019;2019:1–5. doi: 10.1155/2019/8191724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mustapha M, Abdollah Z, Ahem A, Mohd Isa H, Bastion M-LC, Din N. Ocular syphilis: resurgence of an old disease in modern Malaysian society. Int J Ophthalmol. 2018;11:1573–6. doi: 10.18240/ijo.2018.09.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nguyen A, Berngard SC, Lopez JP, Jenkins TC. A case of ocular syphilis in a 36-year-old HIV-positive male. Case Rep Infect Dis. 2014;2014:1–4. doi: 10.1155/2014/352047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nolan NS, Gibbons LE, Hepburn MA, Elkeeb A, Regunath H. Optic neuritis caused by the re-emerging great masquerader. BMJ Case Rep. 2018;11:e225635. doi: 10.1136/bcr-2018-225635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Northey LC, Skalicky SE, Gurbaxani A, McCluskey PJ. Syphilitic uveitis and optic neuritis in Sydney, Australia. Br J Ophthalmol. 2015;99:1215–9. doi: 10.1136/bjophthalmol-2014-306168. [DOI] [PubMed] [Google Scholar]
- 90.Nurfahzura M-J, Hanizasurana H, Zunaina E, Adil H. Successful treatment of syphilitic uveitis in HIV-positive patients. Clin Ophthalmol. 2013;7:1651–4. doi: 10.2147/OPTH.S46876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Odendaal LN, Smit DP. A triple case series of uveitis caused by HIV, syphilis and tuberculosis coinfection. AIDS. 2022;36:155–6. doi: 10.1097/QAD.0000000000003073. [DOI] [PubMed] [Google Scholar]
- 92.Ormaechea MS, Hassan M, Nguyen QD, Schlaen A. Acute syphilitic posterior placoid chorioretinopathy: An infectious or autoimmune disease? Am J Ophthalmol Case Rep. 2019;14:70–73. doi: 10.1016/j.ajoc.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Parija S, Lalitha C. Ocular syphilis presenting as acute necrotizing retinitis in a human immunodeficiency virus-positive patient. J Glob Infect Dis. 2020;12:149–51. doi: 10.4103/jgid.jgid_105_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Patel A, Papakostas T, Vavvas D. Under the Guise of Retinitis. JAMA Ophthalmol. 2015;133:1205–6. doi: 10.1001/jamaophthalmol.2015.1926. [DOI] [PubMed] [Google Scholar]
- 95.Pichi F, Ciardella AP, Cunningham ET, Morara M, Veronese C, Jumper JM, et al. Spectral domain optical coherence tomography findings in patients with acute syphilitic posterior placoid chorioretinopathy. Retina. 2014;34:373–84. doi: 10.1097/IAE.0b013e3182993f11. [DOI] [PubMed] [Google Scholar]
- 96.Pirani V, Pelliccioni P, De Turris S, Rosati A, Franceschi A, Cesari C, et al. The Eye as a Window to Systemic Infectious Diseases: Old Enemies, New Imaging. J Clin Med. 2019;8:1392. doi: 10.3390/jcm8091392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ploysangam P, Mattern RM. Perforating peripheral ulcerative keratitis in syphilis. Case Rep Ophthalmol. 2019;10:267–73. doi: 10.1159/000501996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rishi E, Govindarajan M, Biswas J, Agarwal M, Sudharshan S, Rishi P. Syphilitic uveitis as the presenting feature of HIV. Indian J Ophthalmol. 2016;64:149–50. doi: 10.4103/0301-4738.179714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rodrigues RAM, do Nascimento HM, Muccioli C. Yellowish dots in the retina: a finding of ocular syphilis? Arq Bras Oftalmol. 2014;77:324–6. doi: 10.5935/0004-2749.20140081. [DOI] [PubMed] [Google Scholar]
- 100.Rodrigues RP, Correia N, Lopes AV. Neurosyphilis with optical involvement in an immunocompetent patient: a case report. Int Med Case Rep J. 2012;5:5–11. doi: 10.2147/IMCRJ.S26281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rodríguez-Uña I, Serrador-García M, Santos-Bueso E, Díaz-Valle D, García-Feijóo J. Simultaneous optic and vestibulocochlear syphilitic neuropathy in a patient with HIV infection. J Ophthalmic Inflamm Infect. 2013;3:1–4. doi: 10.1186/1869-5760-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Romao EA, Bolella VR, Nardin MEP, Habib-Simao ML, Furtado JM, Moyses-Neto M. Ocular syphilis in a kidney transplant recipient. Rev Inst Med Trop Sao Paulo. 2016;58:46–46. doi: 10.1590/S1678-9946201658046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sahin O, Ziaei A. Clinical and laboratory characteristics of ocular syphilis, co-infection, and therapy response. Clin Ophthalmol. 2015;10:13–28. doi: 10.2147/OPTH.S94376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schlaen A, Aquino MP, Ormaechea MS, Couto C, Saravia M. Spectral optical coherence tomography findings in an adult patient with syphilitic bilateral posterior uveitis and unilateral punctate inner retinitis. Am J Ophthalmol Case Rep. 2019;15:100489. doi: 10.1016/j.ajoc.2019.100489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schulz DC, Orr SMA, Johnstone R, Devlin MK, Sheidow TG, Bursztyn LLCD. The many faces of ocular syphilis: case-based update on recognition, diagnosis, and treatment. Can J Ophthalmol. 2021;56:283–93. doi: 10.1016/j.jcjo.2021.01.006. [DOI] [PubMed] [Google Scholar]
- 106.Shen J, Feng L, Li Y. Ocular syphilis: an alarming infectious eye disease. Int J Clin Exp Med. 2015;8:7770–7. [PMC free article] [PubMed] [Google Scholar]
- 107.Shinha T, Weaver BA. Necrotizing retinitis due to syphilis in a patient with AIDS. IDCases. 2016;6:17–19. doi: 10.1016/j.idcr.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sidiqi A, Navajas E. Spontaneous improvement of syphilis chorioretinitis: case report and review of the literature. Retin Cases Brief Rep. 2020;14:170–3. doi: 10.1097/ICB.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 109.Silpa-Archa S, Preble JM, Foster CS. Vitreous treponemal antibody as a supplementary test to serology for the confirmation of syphilitic chorioretinitis. Retin Cases Brief Rep. 2020;14:166–9. doi: 10.1097/ICB.0000000000000676. [DOI] [PubMed] [Google Scholar]
- 110.Sood AB, Pearce WA, Workowski KA, Lockwood J, Yeh S. Combined intravitreal and systemic antibiotic therapy in a patient with syphilitic uveitis. Ocul Immunol Inflamm. 2019;27:131–3. doi: 10.1080/09273948.2017.1385817. [DOI] [PubMed] [Google Scholar]
- 111.Świerczyńska MP, Sedlak LS, Nowak MA, Wyględowska-Promieńska D. Choroidal neovascularization secondary to ocular syphilis. Rom J Ophthalmol. 2021;65:406–10. doi: 10.22336/rjo.2021.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Teh BL, Khan MK, Butt A, Kuffova L. Bilateral ocular involvement with syphilis in a known HIV patient. Am J Ophthalmol Case Rep. 2020;18:100638. doi: 10.1016/j.ajoc.2020.100638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tsai P-F, Horng C-T, Tsai M-L. Acute syphilitic posterior placoid chorioretinopathy with typical placoid edge in a Taiwanese male. Tzu Chi Med J. 2021;33:200–1. doi: 10.4103/tcmj.tcmj_135_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tsen C-L, Chen S-C, Chen Y-S, Sheu S-J. Uveitis as an initial manifestation of acquired immunodeficiency syndrome. Int J STD AIDS. 2017;28:1224–8. doi: 10.1177/0956462417694569. [DOI] [PubMed] [Google Scholar]
- 115.Tsuboi M, Nishijima T, Yashiro S, Teruya K, Kikuchi Y, Katai N, et al. Prognosis of ocular syphilis in patients infected with HIV in the antiretroviral therapy era. Sex Transm Infect. 2016;92:605–10. doi: 10.1136/sextrans-2016-052568. [DOI] [PubMed] [Google Scholar]
- 116.Turchetti P, Pacella F, Pacella E, Mirisola C, Uccella I. An immunocompetent migrant presenting with neurosyphilis with an unusual unilateral papillitis: a case report. Eur J Med Res. 2012;17:1–8. doi: 10.1186/2047-783X-17-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vidal-Villegas B, Arcos-Villegas G, Fernández-Vigo JI, Díaz-Valle D. Atypical syphilitic outer retinitis and severe retinal vasculitis as onset manifestations in a patient with concurrent HIV and syphilis infection. Ocul Immunol Inflamm. 2020. 10.1080/09273948.2020.1787464. [DOI] [PubMed]
- 118.Vignesh A, Srinivasan R, Vijitha S. Ocular syphilis masquerading as bilateral peripheral ulcerative keratitis. Taiwan J Ophthalmol. 2016;6:204–5. doi: 10.1016/j.tjo.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang P, Zhang N, Li F, Chen Y, Kijlstra A. Ocular manifestations of syphilitic uveitis In Chinese patients. Retina. 2012;32:1906–14. doi: 10.1097/IAE.0b013e3182509796. [DOI] [PubMed] [Google Scholar]
- 120.Yap SC, Tan YL, Chio MT, Teoh SC. Syphilitic uveitis in a Singaporean population. Ocul Immunol Inflamm. 2014;22:9–14. doi: 10.3109/09273948.2013.829106. [DOI] [PubMed] [Google Scholar]
- 121.Yosar J. Neurosyphilis presenting as visually asymptomatic bilateral optic perineuritis. BMJ Case Rep. 2019;12:e232520. doi: 10.1136/bcr-2019-232520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang R, Qian J, Guo J, Yuan Y, Xue K, Yue H, et al. Clinical manifestations and treatment outcomes of syphilitic uveitis in a chinese population. J Ophthalmol. 2016;2016:2797028. doi: 10.1155/2016/2797028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhu J, Jiang Y, Shi Y, Zheng B, Xu Z, Jia W. Clinical manifestations and treatment outcomes of syphilitic uveitis in HIV-negative patients in China. Medicine. 2017;96:e8376. doi: 10.1097/MD.0000000000008376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Peterman T, Schillinger J, Blank S, Berman S, Ballard R, Cox D, et al. Syphilis testing algorithms using treponemal tests for initial screening—four laboratories, New York City, 2005–2006. MMWR Morb Mortal Wkly Rep. 2008;57:872–5. [PubMed] [Google Scholar]
- 125.Hernández-Aguado I, Bolumar F, Moreno R, Pardo FJ, Torres N, Belda J, et al. False-positive tests for syphilis associated with human immunodeficiency virus and hepatitis B virus infection among intravenous drug abusers. Valencian Study Group on HIV Epidemiology. Eur J Clin Microbiol Infect Dis. 1998;17:784–7. doi: 10.1007/s100960050186. [DOI] [PubMed] [Google Scholar]
- 126.Tuddenham S, Ghanem KG. Ocular syphilis: opportunities to address important unanswered questions. Sex Transm Infect. 2016;92:563–5. doi: 10.1136/sextrans-2016-052570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schurmann D, Bergmann F, Bertelmann E, Padberg J, Liekfeld A, Pleyer U. Early diagnosis of acquired ocular syphilis requires a high index of suspicion and may prevent visual loss. AIDS. 1999;13:623–4. doi: 10.1097/00002030-199904010-00014. [DOI] [PubMed] [Google Scholar]
- 128.Mathew RG, Goh BT, Westcott MC. British Ocular Syphilis Study (BOSS): 2-year national surveillance study of intraocular inflammation secondary to ocular syphilis. Investig Ophthalmol Vis Sci. 2014;55:5394–5400. doi: 10.1167/iovs.14-14559. [DOI] [PubMed] [Google Scholar]
- 129.Oliver S, Aubin M, Atwell L, Matthias J, Cope A, Mobley V, et al. Ocular Syphilis - Eight Jurisdictions, United States, 2014-5. MMWR Morb Mortal Wkly Rep. 2016;65:1185–8. doi: 10.15585/mmwr.mm6543a2. [DOI] [PubMed] [Google Scholar]
- 130.Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:35. [PMC free article] [PubMed] [Google Scholar]
- 131.Public Health Agency of Canada. Syphilis guide: Screening and diagnostic testing. https://www.canada.ca/en/public-health/services/infectious-diseases/sexual-health-sexually-transmitted-infections/canadian-guidelines.html. 2017.
- 132.Solebo AL, Westcott M. Corticosteroids in ocular syphilis. Ophthalmology. 2007;114:1593. doi: 10.1016/j.ophtha.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 133.Kingston M, French P, Higgins S, McQuillan O, Sukthankar A, Stott C, et al. UK national guidelines on the management of syphilis 2015. Int J STD AIDS. 2016;27:421–46. doi: 10.1177/0956462415624059. [DOI] [PubMed] [Google Scholar]
- 134.Klein A, Fischer N, Goldstein M, Shulman S, Habot‐Wilner Z. The great imitator on the rise: ocular and optic nerve manifestations in patients with newly diagnosed syphilis. Acta Ophthalmol. 2019;97:e641–e647.. doi: 10.1111/aos.13963. [DOI] [PubMed] [Google Scholar]
- 135.Curi AL, Sarraf D, Cunningham ET. Multimodal imaging of syphilitic multifocal retinitis. Retin Cases Brief Rep. 2015;9:277–80. doi: 10.1097/ICB.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 136.Gu X, Gao Y, Yan Y, Marks M, Zhu L, Lu H, et al. The importance of proper and prompt treatment of ocular syphilis: a lesson from permanent vision loss in 52 eyes. J Eur Acad Dermatol Venereol. 2020;34:1569–78. doi: 10.1111/jdv.16347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Oliver GF, Stathis RM, Furtado JM, Arantes TE, McCluskey PJ, Matthews JM, et al. Current ophthalmology practice patterns for syphilitic uveitis. Br J Ophthalmol. 2019;103:1645–9. doi: 10.1136/bjophthalmol-2018-313207. [DOI] [PubMed] [Google Scholar]
- 138.Groft JN, Robinson Vollman A. Seeking serenity: living with HIV/AIDS in rural Western Canada. Rural Remote Health. 2007;7:677. [PubMed] [Google Scholar]
- 139.Berry DE. Rural acquired immunodeficiency syndrome in low and high prevalence areas. South Med J. 2000;93:36–43. doi: 10.1097/00007611-200093010-00007. [DOI] [PubMed] [Google Scholar]
- 140.Moramarco A, Mallone F, Pirraglia MP, Bruscolini A, Giustolisi R, la Cava M, et al. Clinical features of ocular syphilis: a retrospective clinical study in an Italian Referral Centre. Semin Ophthalmol. 2020;35:50–55. doi: 10.1080/08820538.2020.1723651. [DOI] [PubMed] [Google Scholar]
- 141.Ly V, Elhusseiny AM, Sallam AB. Syphilis-related ocular inflammation in HIV-positive and HIV-negative patients. Ocul Immunol Inflamm. 2022. 10.1080/09273948.2022.2082987. [DOI] [PubMed]
- 142.Cope AB, Mobley VL, Oliver SE, Larson M, Dzialowy N, Maxwell J, et al. Ocular syphilis and human immunodeficiency virus coinfection among syphilis patients in North Carolina, 2014–6. Sex Transm Dis. 2019;46:80–85. doi: 10.1097/OLQ.0000000000000910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sudharshan S, Menia N, Selvamuthu P, Tyagi M, Kumarasamy N, Biswas J. Ocular syphilis in patients with human immunodeficiency virus/acquired immunodeficiency syndrome in the era of highly active antiretroviral therapy. Indian J Ophthalmol. 2020;68:1887–93. doi: 10.4103/ijo.IJO_1070_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1 - Risk of bias assessment summary
Supplemental Table 2 - Full electronic search strategy of databases
Data Availability Statement
The datasets analysed in this work are available from the corresponding author upon reasonable request.