Abstract
Optical coherence tomography angiography (OCT-A) is an ocular imaging technology that has emerged as a non-invasive tool to evaluate retinal microvascular changes in neurodegenerative diseases including Parkinson’s disease (PD) and Alzheimer’s disease. While several studies have reported on the presence of pathologic retinal microvascular alterations in PD, the utility of OCT-A as a biomarker for PD evaluation is still unclear. A systematic review and meta-analysis were performed to explore the current evidence for the role of OCT-A in PD published up until June 2022. PubMed, Scopus, and Web of Science databases were used to systematically identify relevant papers and a meta-analysis was conducted using Stata16 software according to the level of heterogeneity applying a random- or fixed-effect model. Thirteen studies of 925 eyes in the PD group and 1501 eyes in the control group assessing OCT-A findings in PD patients were included. The meta-analyses revealed that the foveal region of PD patients had a significantly lower vessel density in the superficial capillary plexus (SCP) compared to healthy controls but that there were no significant differences in the foveal avascular zone, the SCP in whole, parafoveal, and perifoveal regions, and deep capillary plexus. OCT-A metrics may act as a potential biomarker for a more accurate and early PD diagnosis. Still, the OCT-A algorithms and interchangeability between OCT-A devices require further standardization to draw clinical conclusions regarding their utility.
Subject terms: Prognostic markers, Retinal diseases
摘要 摘要
相干光断层扫描血管成像 (Optical coherence tomography angiography, OCT-A) 是一种非侵入性眼部成像技术, 用于评估帕金森病 (Parkinson’s disease, PD) 和阿尔茨海默病等神经退行性疾病的视网膜微血管变化。虽然有几项研究报道了PD中存在视网膜病理性微血管改变, 但OCT-A用于评估PD的作用尚不清楚。我们进行了系统综述和荟萃分析, 检索了截至2022年6月发表的OCT-A在帕金森病中作用的现有证据。使用PubMed、Scopus和Web of Science数据库系统地检索相关文献, 并使用Stata16软件根据随机或固定效应模型的异质性水平进行meta分析。纳入13项研究, 包括PD组925只眼和对照组1501只眼, 评估PD患者OCT-A发现。荟萃分析显示, 与健康对照组相比, PD患者的中心凹区浅层毛细血管丛 (superficial capillary plexus, SCP) 的血管密度显著降低, 但在中心凹无血管区、整个、中心凹旁和中心凹周围区域的SCP和深层毛细血管丛中无统计学差异。OCT-A在PD早期精确诊断中可能作为一个潜在的生物标志物。但是OCT-A算法和OCT-A设备之间的互换性需要进一步标准化, 以得出关于其临床实用性的结论。
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder with increasing prevalence worldwide that poses an extensive economic and psychological burden to the healthcare system [1, 2]. Abnormal accumulation of cytoplasmic α-synuclein leads to loss of dopamine-producing cells within the substantia nigra, which causes the cardinal signs of the disease, such as resting tremor, bradykinesia, and rigidity. However, PD has a widespread pathophysiology involving several cell types in the neural network, leading to a variety of non-motor symptoms such as cognitive impairment, autonomic dysfunction, sleep disturbances, and visual symptoms [3, 4].
Dopamine plays a pivotal role in regulating visual signal transmission in retinal amacrine cells; thus, the dysregulation of dopamine in PD can lead to a variety ocular structural and functional alterations manifesting as visual acuity impairment, diminished contrast sensitivity, and colour vision disturbances. [5–8]. Postmortem and in vivo studies have confirmed retinal accumulation of α-synuclein in PD patients, possibly causing dopaminergic neuron loss [5–7].
Currently, there is no consensus over a biomarker for early detection of PD, and definitive diagnosis is only possible through histopathological examination. Recent studies have indicated that relying on clinical diagnosis for PD may often be unreliable due to other parkinsonian syndromes sharing similar motor symptoms such as multiple system atrophy (MSA), corticobasal degeneration (CBD), and progressive supranuclear palsy (PSP) (11, [8]). In addition, several studies have suggested that the neurodegeneration in PD is gradual and can begin years prior to onset of motor symptoms [9]. Therefore, a non-invasive and effective diagnostic test for early detection of PD is crucial to prevent late disease complications.
The contribution of vascular components to the pathophysiology of PD similar to other neurodegenerative conditions has been shown [10, 11]. In a postmortem histopathological evaluation of brain micro-vasculature, the capillaries in PD patients were shortened and widened with less branching, indicating damage to the capillary network, especially in the substantia nigra (SN) [12]. Furthermore, a close link between cerebral small vessel diseases and motor symptoms of PD has been found [13].
The brain and the retina share a common embryologic origin; thus, the retina exhibits striking vascular and anatomical similarities to the brain [14]. While neuroimaging is often expensive and requires specialized techniques, optical coherence tomography angiography (OCT-A) has emerged as a fast and non-invasive imaging modality, providing high-resolution and depth-selective images of the ocular vasculature [15, 16]. Due to the similarities between retinal and cerebral vasculature, several studies have proposed OCT-A as a potential method for early and accurate detection of various neurodegenerative diseases, e.g. Alzheimer’s disease and Parkinson’s disease [17–20].
Several optical coherence tomography (OCT) studies showed that the thickness of retinal nerve fibre layer (RNFL) and ganglion cell layer (GCL), and inner plexiform layer (IPL) along with choroidal parameters significantly decline in PD patients[21–23]. The retinal and choroidal degeneration have been linked to α-synuclein deposition [6, 7]. Emerging evidence suggest that retinal vasculopathy may contribute to PD pathology alongside retinal neurodegeneration [19].
Comparing OCT-A metrics between PD patients and healthy controls in different studies showed mixed results, possibly due to the low statistical power, variations in selection criteria, and imaging quality. Hence, it is necessary to integrate and arrange these findings for interpretation. Here, we conducted a systematic review and meta-analysis to explore the application of OCT-A imaging as a potential biomarker for PD.
Methods
This systematic review and meta-analysis follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [24]. The study protocol was developed and registered with the International Prospective Register of Systematic Reviews (PROSPERO) (Registration Number: CRD42021293758).
Search strategy
We performed a systematic search of PubMed, Scopus, and Web of Science databases from inception to June 3, 2022, and identified relevant studies using the following combination of key terms: (“Parkinson” OR “Parkinson’s Disease” OR “Idiopathic Parkinson’s Disease” OR “Lewy Body Parkinson’s Disease”) AND (“optical coherence tomography angiography” OR “OCT angiography” OR “OCTA” OR “optical coherence tomographic angiography”). No restrictions were applied regarding location of presented studies. We also conducted a manual citation search by screening the reference lists of the included studies.
Eligibility criteria
We included all published studies that used OCT-A to examine the retinal microvasculature in Parkinson’s disease patients and met the following inclusion criteria: (a) peer-reviewed original research; (b) written in English; (c) PD diagnosis made based on established criteria such as the Movement Disorder Society clinical criteria [25]; (d) inclusion of a control group. The exclusion criteria were: (a) non-human research; (b) non-original research; (c) written in a non-English language; (d) lack of control group. Three authors (AJ, SZ, and SJ) independently screened the identified studies by title and abstract. After removing duplicates and irrelevant studies, the remaining articles underwent a detailed full-text review according to the eligibility criteria. Disagreements were resolved by consulting with a third author (MAS).
Data extraction
Three authors (AJ, SZ, and SJ) independently extracted the following data: 1. first author and year of publication; 2. study design; 3. diagnostic criteria for PD; 4. Hoehn and Yahr clinical stage (H-Y scale); 5. Unified Parkinson’s Disease Rating Scale (UPDRS) score; 6. number, mean age, sex ratio, trait; 7. PD duration; 8. participant selection criteria; 9. OCT-A model; 10. OCT-A parameters such as vessel density, perfusion density, foveal avascular zone (FAZ), Any discrepancies were resolved through discussion with a third author (SM).
Statistical analysis
OCT-A parameters (mean ± standard deviation (SD)), were combined for data analysis if reported in at least two unique studies using a similar OCT-A model and software algorithm. Meta-analyses were conducted for all the continuous outcomes using the Stata version 16 (StataCorp, College Station, TX). We reported the effective sizes of difference in OCT-A parameters as mentioned above between PD and control groups as mean difference (MD) with a 95% confidence interval (CI), with P-values less than 0.05 considered as significant. For assessing heterogeneity across the studies, we used Higgin’s I² test. If the heterogeneity was less than 40%, then the difference was considered insignificant, and a fixed-effects model was used. However, if the heterogeneity level was more than 40%, a random-effect model was used. If a variable was reported in at least four studies, the effects of suspected confounding factors were assessed by conducting subgroup analysis and meta-regression.
Search Results
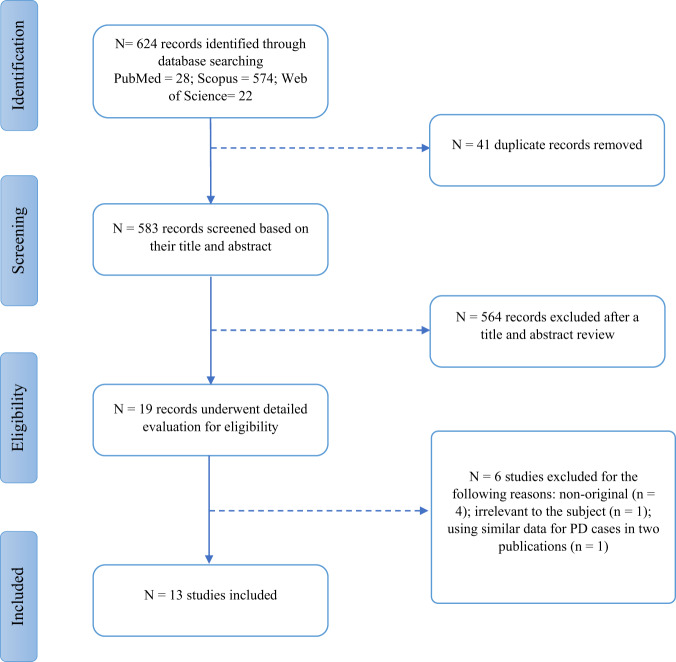
The systematic literature search yielded 624 results, of which 41 records were duplicates and removed. After a title and abstract screening, 564 articles that did not meet the selection criteria were removed. The remaining 19 studies underwent a detailed full-text evaluation [7, 15, 23, 26–41]. Four studies were not original and therefore were excluded [15, 27, 28, 31]. Another study was excluded due to using similar data for PD cases in two different publications [33]. A study that only assessed motion artifacts in OCT-A imaging in PD was excluded [36]. Figure 1 summarizes the study selection process. In total, thirteen observational studies with a total of 925 eyes in PD cases and 1501 eyes in controls were included in this meta-analysis.
Fig. 1. Study selection diagram.
This flow diagram shows the study selection process of studies on optical coherence tomography angiography on patients with Parkinson’s disease. N number, PD Parkinson’s disease.
Results
Study Characteristics
Table 1 presents a summary of the key characteristics and results of the included studies. All included studies were cross-sectional [7, 23, 26, 29, 30, 32, 34, 35, 37–41]. Three studies also described a prospective design [26, 36, 38], but no longitudinal studies had been published at the time of our review. PD was diagnosed according to either the Movement Disorder Society Criteria or the PD United Kingdom Brain Bank Criteria [25]. The average age of PD patients was 64.96 years (ranging from 55.9 to 71.7) and the average disease duration was 5.10 years, ranging from 2.0 to 9.7 years. Eight studies only recruited early-stage PD patients [26, 29, 30, 34, 38–41]. For assessing motor impairment severity, some studies used the Unified Parkinson’s Disease Rating Scale (UPDRS) [7, 32, 34, 35, 39–41], and some used the Hoehn and Yahr (HY) scale [7, 23, 29, 30, 34, 38–41], but two studies did not report either [26, 37]. Studies used varying exclusion criteria regarding the ophthalmologic or general health conditions of participants. These criteria are all summarized in Table 2 along with the eye selection method and applied statistical adjustments. Regarding OCT-A measurements, five studies used the Zeiss Cirrus 5000 (Carl Zeiss Meditec, Inc., Dublin, CA) along with AngioPlex software [30, 34, 35, 37, 38]. Five studies used the Optovue RTVue XR Avanti (Optovue Inc., Fremont, CA) along with AngioVue software [7, 26, 29, 40, 41]. One study used the Heidelberg Spectralis OCT-A (Heidelberg Engineering, Heidelberg, Germany) along with Heidelberg software [32]. Two studies used swept-source OCTA, comprising of two different devices, the VG200 (SVision Imaging, Ltd., Luoyang, China) along with VG200 software [39] and the Triton plus (Topcon Corporation, Tokyo, Japan) [23].
Table 1.
Study characteristics using optical coherence tomography angiography (OCT-A) to assess retinal microvasculature in Parkinson’s disease (PD) and healthy controls (HC).
| Author /Year | PD Criteria | Additional Scoring | trait | N of trait | Male (%) | Age (years) (mean ± SD) | Disease Duration (years) | BCVA of enrolled eyes (logMAR) (mean ± SD) | Treatment (%) | IOP of enrolled eyes (mean ± SD) | H&Y score | UPDRS score (mean ± SD) | MoCA or MMSE (mean ± SD) | Type of OCTA | Software OCTA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Robbins 2021(A) [37] | MDS-PD | MoCA, MMSE |
PD HC |
69(124 eyes) 137(248 eyes) |
56.5 56.2 |
71.7 ± 7 70.9 ± 6.7 |
- | - | - | - | - | - |
MMSE 28.4 ± 2.4 29.0 ± 2.8 |
Zeiss Cirrus 5000 | AngioPlex |
| Robbins 2021 (B) [38] | MDS-PD | MoCA, H&Y |
PD HC |
81(151 eyes) 266(514 eyes) |
62 28 |
70.08 ± 8.44 70.16 ± 7.23 |
- | - | 95 Levodopa |
15.42 ± 2.90 16.57 ± 3.04 |
2.12 ± 0.62 | - | - | Zeiss Cirrus 5000 | AngioPlex |
| Murueta-Goyena 2021 [32] | PD UK Brain Bank criteria | UPDRS, MoCA |
PD PD-MCI PD-NC HC |
49(87 eyes) 18 31 40(73 eyes) |
65.3 61.1 67.8 32.5 |
64.6 ± 7.9 67.1 ± 8.9 63.1 ± 6.9 62.1 ± 8 |
7.1 ± 4.1 (0.4-19.4) 6.3 ± 4.4 7.5 ± 4.0 |
- | 100 Levodopa | - | - | 27.7 ± 7.7 |
MoCA 24.4 ± 4.1 25.7 ± 2.5 |
Heidelberg Spectralis OCTA | Spectralis |
| Zhang 2021 [39] | PD UK Brain Bank criteria | UPDRS-III, H&Y |
PD** HC |
42(75 eyes) 75(150 eyes) |
44.6 42.6 (eyes) |
55.92 ± 7.53 54.68 ± 6.66 |
2.04 ± 1.23 |
1 1 |
- |
13.19 ± 1.76 13.21 ± 2.08 |
1.42 ± 0.55 | 16.92 ± 7.6 | - | SVision | VG200 |
| Zhou 2021 [34] | PD UK Brain Bank criteria | MMSE, UPDRS-III, H&Y, NEI VFQ-25 |
PD* HC |
24(24 eyes) 23(23 eyes) |
75 47.8 |
65.88 ± 6.5 63.43 ± 7.11 |
5.3 ± 4.2 |
0.088 ± 0.122 0.097 ± 0.117 |
- |
14.7 ± 2.4 15.6 ± 2.8 |
2.0 ± 0.3 | 26.5 ± 12.3 | MMSE 28.5 ± 1.6 | Zeiss Cirrus 5000 | AngioPlex |
| Zou 2020 [30] | PD UK Brain Bank criteria | H&Y |
PD*** HC |
35(35 eyes) 35(35 eyes) |
45.7 57.1 |
61.86 ± 5.46 60.2 ± 6.75 |
3.2 ± 2.0 |
1.2 (0.8-1.5) 1.2 (1.0-1.2) |
- |
16.85 ± 2.03 16.74 ± 2.82 |
2 (1–2) | - | - | Zeiss Cirrus | AngioPlexTM |
| Rascunà 2020 [40] | MDS-PD | UPDRS-III, H&Y, MoCA |
PD HC |
21(41 eyes) 17(33 eyes) |
57.1 52.9 |
61.5 ± 6.5 65.1 ± 10.7 |
2.3 ± 1.2 | - | - | - | 1.9 ± 0.4 |
25.0 ± 6.9 3.2 ± 2.7 |
MoCA 21.8 ± 5.1 23.4 ± 3.6 | RTVue XR Avanti | AngioAnalytics |
| Shi 2019 [29] | PD UK Brain Bank criteria | H&Y |
PD* HC |
25(50 eyes) 25(50 eyes) |
52 52 |
61.9 ± 7.6 59 ± 5.8 |
3.7 ± 2.4 |
0.01 ± 0.09 0.02 ± 0.04 |
- |
14.5 ± 2.6 13.3 ± 1.5 |
2.2 ± 1.0 | - | - | RTVue XR Avanti | AngioVue |
| Kwapong 2017 [26] | PD UK Brain Bank criteria | H&Y |
PD* HC |
38(49 eyes) 28(34 eyes) |
- |
62.95 ± 7.97 61.18 ± 5.74 |
3.84 ± 2.80 |
0.96 ± 0.23 0.97 ± 0.18 |
- |
14.73 ± 2.75 9.32 ± 6.68 |
- | - | - | RTVue XR Avanti | AngioVue |
| Xu 2022 [35] | MDS-PD | H&Y, UPDRS, UPDRS III, MMSE, NMSS |
PD H-Y I H-Y II H-Y III HC |
115(115 eyes) 50(50 eyes) 46(46 eyes) 19(19 eyes) 67(67 eyes) |
44.35 38 52.2 42.1 43.28 |
63.61 ± 6.92 62.08 ± 6.73 65.35 ± 7.07 63.47 ± 6.41 62.01 ± 7.74 |
6.00 (4.00–8.00) 4.00 (3.00–6.00) 7.00 (5.00–9.00) 9.00 (7.00–12.00) |
0.35 ± 0.13 0.33 ± 0.13 0.35 ± 0.13 0.38 ± 0.130.35 ± 0.13 |
- |
15.53 ± 2.02 15.42 ± 2.19 15.74 ± 1.97 15.32 ± 1.67 15.36 ± 2.07 |
- |
33 (22–54) 20.5 (12.75–26.25) 44.5 (31.75–56) 69 (55–76) |
28 (26–29) 28 (27–30) 27 (25.75–29) 26.5 (23.5–29) MMSE |
Zeiss Cirrus 5000 | AngioPlex |
| Lin 2022 [7] | PD UK Brain Bank criteria | MDS-UPDRS III, H&Y, MMSE |
PD H-Y I H-Y ll+III HC |
49 20 22 45 |
44.9 40.0 54.5 24.4 |
66.41 ± 7.37 64.20 ± 6.05 66.41 ± 7.00 64.33 ± 11.78 |
5.32 ± 3.12 | - | - | - | 2.3 ± 1.1 | 29.1 ± 17.3 |
MMSE 25.7 ± 4.8 - - 28.8 ± 2.1 |
RTVue XR Avanti | AngioVue |
| Li 2022 [41] | MDS-PD | UPDRS-III, H&Y, MMSE, MoCA |
PD HC |
45(83 eyes) 43(83 eyes) |
51.1 48.8 |
61.36 ± 8.68 60.24 ± 6.53 |
3.60 ± 3.11 |
0.89 ± 0.19 0.93 ± 0.13 |
- |
13.08 ± 2.97 13.65 ± 3.03 |
1.64 ± 0.68 | 30.58 ± 15.54 | MMSE (PD: 26.42 ± 2.67/HC: 28.65 ± 1.22) MoCA (PD: 22.21 ± 4.41/HC: 27.11 ± 1.70) | RTVue XR Avanti | AngioVue |
| Satue 2022 [23] | MDS-PD | H&Y |
PD HC |
42(42 eyes) 146(146 eyes) |
- |
67.33 ± 9.65 64.9 ± 7.45 |
8.95 ± 6.58 | - | 100**** |
16.1 ± 2.54 15.3 ± 2.32 |
2.67 ± 0.68 | - | - | Triton SS-OCT-Angio | Topcon IMAGEnet |
PD Parkinson’s disease, N Number, BCVA Best corrected visual acuity, logMAR Logarithm of the Minimum Angle of Resolution, IOP Intraocular pressure, H&Y Hoehn and Yahr scale, UPDRS Unified Parkinson’s Disease Rating Scale, MoCA Montreal Cognitive Assessment, MMSE Mini-Mental State Examination, OCTA Optical Coherence Tomography Angiography, MDS Movement Disorder Society, NEI VFQ-25 The National Eye Institute 25-Item Visual Function Questionnaire, MCI Mild cognitive impairment, NC Normal cognition, HC Healthy controls, NMSS Non-motor Symptoms Scale, SS-OCT Swept source optical coherence tomography.
-Data not reported. *Idiopathic PD/**early-stage PD/*** sporadic PD/****%95.24 treated with Levodopa, and %4.76 treated with Other without levodopa.
Table 2.
Ophthalmological and health condition exclusion criteria of included studies.
| Study | Health conditions exclusion criteria | Eyes | Ophthalmological exclusion criteria | Statistical adjustments |
|---|---|---|---|---|
| Robbins 2021 (A) [37] | History of DM, other dementias | Mixed | Retinal pathology, glaucoma, corrected ETDRS, visual acuity less than 20/40 (measured by staff at the time of image acquiring) | Age/Sex |
| Robbins 2021 (B) [38] | History of DM, dementia or cognitive disorder, neurologic disease (other than Parkinson’s) | Mixed | Glaucoma or suspected glaucoma, optic neuropathy, retinal pathology, known refractive error ≥ +6.0 diopters or -6.0 diopters, and corrected ETDRS visual acuity less than 20/40 (measured by staff at the time of image capturing) | Age/Sex |
| Murueta-Goyena 2021 [32] | Heavy smoker (more than 20 cigarettes per day), severe alcohol use (more than 4 drinks per day for men or 3 drinks per day for women), uncontrolled or resistant high blood pressure, diagnosis of any grade or type of DM, history of consumption of medications or drugs known to cause cognitive impairment retinal toxicity, chronic inflammatory systemic diseases (e.g. SLE, sarcoidosis, Bechet disease), and history of brain trauma or central nervous system diseases, except PD | Mixed | Spherical equivalent refractive error > 4.00 diopters or > 3.00 diopters of astigmatism or any systemic or ocular pathological condition influencing retinal OCT measures, except PD | Age/Sex/HTN |
| Zhang 2021 [39] | History of Alzheimer’s disease, stroke, muscular dystrophy, multiple system atrophy, HTN, or DM | Both (but some were excluded due to incompliance to the examination and suboptimal image quality) | Glaucoma; severe media opacities; uveitis; moderate to significant refractive errors (including spherical equivalent refraction or astigmatism>3 diopters); macular disease; optic nerve neuropathy | Age/Gender/IOP/Inter-eye correlation |
| Zhou 2021 [34] | Psychiatric or neurological diseases different from PD, such as dementia or MS; DM, uncontrolled HTN, or other systemic diseases which could affect the visual system | One | History of ocular trauma or ocular surgery; glaucoma history in family; elevated refractive error (± 6.00D spherical equivalent); media opacifications; IOP > 21 mmHg; concomitant ocular diseases such as glaucoma, corneal disease, or retinal disease | - |
| Zou 2020 [30] | History of hypertension or DM | One | BCVA < 0.5 evaluated with a Snellen chart; ophthalmic diseases, such as glaucoma, corneal diseases, and fundus diseases; presence of optic nerve or retinal diseases; diopter spherical power more than 6.00– 6.00 D and/or astigmatism more than 3.00–3.00 D; IOP > mmHg; history of intraocular surgery or ocular trauma; family history of glaucoma | Age |
| Rascunà 2020 [40] | Systemic conditions that can cause visual system impairment, such as DM, cardiovascular diseases, uncontrolled HTN or hypotension, and any other neurological disease | Both (but some were excluded due to low image quality) | History of ocular trauma, history of ocular surgery that could impair the macular morphology or visual pathway, concurrent ocular diseases (including optic nerve, retina, cornea, and macular diseases), media opacifications, IOP > 21 mmHg | Age/Sex/HTN |
| Shi 2019 [29] | History of systemic disease, e.g. DM, uncontrolled HTN/hypotension, history of cardiac diseases, and other neurologic diseases such as MS and peripheral nerve diseases, except PD | Both | A BCVA less than 20/30; The refractive error above +2.00 D or under -6.00 D; ocular disorders, such as a history of glaucoma, AMD, or other macular diseases affecting the retinal microvessels | Age/Sex |
| Kwapong 2017 [26] | Systemic conditions that could affect the visual system, such as uncontrolled HTN/ hypotension, DM, history of cardiac diseases, neurological diseases such as MS, and peripheral nerve diseases, except PD | Mixed | Patients with significant refractive errors (>5 diopters of spherical equivalent refraction or 3 diopters of astigmatism); apparent ocular changes during the ophthalmological evaluation, for example, retinal changes associated with high myopia; media opacifications; IOP ≥ 21 mm Hg; concomitant ocular diseases (such as a history of retinal pathology or glaucoma); suspected glaucomatous damage; history of previous ocular surgery that could affect the visual outcomes or the macular morphology | Age/IOP |
| Xu 2022 [35] | DM and uncontrolled HTN, concomitant neurological disorders, severe cardiac, pulmonary, renal, hepatic diseases, or any types of tumors, and insufficient clinical data | One (random) | Glaucoma, age-related macular degeneration, macular hole, epiretinal membrane, refractive error [> ±3 D sphere and ±2D cylinder], macular drusen, and pathological myopia | Age/Sex |
| Lin 2022 [7] | History of DM | - | Glaucoma, retinopathy, optic neuropathy, dense cataract, or ocular surgery | Age/Sex |
| Li 2022 [41] | Systemic diseases such as history of cardiac diseases, DM, and uncontrolled HTN/hypotension; other neurologic diseases such as MS and peripheral nerve diseases by processes other than PD; and patients meeting the diagnostic criteria of PD with dementia | Mixed | Ocular disorders, e.g., a history of glaucoma, high IOP (≥21 mmHg), age-related macular degeneration, and other macular diseases that could affect the retinal microvessels (n = 5) | Age/education duration/IOP |
| Satue 2022 [23] | systemic conditions potentially affecting the visual system—including DM and neurological diseases, such as, multiple sclerosis, dementia, and peripheral nerve disease attributable to processes other than PD— | One (random) | Significant refractive errors (>5 diopters of spherical equivalent refraction or 3 diopters of astigmatism); observable ocular changes during ophthalmological evaluation (e.g., retinal changes associated with a shallow chamber or high myopia); concomitant ocular disease, such as history of retinal pathology or glaucoma; intraocular pressure ≥21 mmHg; media opacification; eyes with suspected glaucomatous damage; amblyopic eyes | Age/IOP |
ETDRS Early Treatment Diabetic Retinopathy Study, DM Diabetes Mellitus, PD Parkinson’s Disease, OCT Optical coherence tomography, HTN Hypertension, SLE Systemic lupus erythematosus, IOP Intraocular pressure, MS Multiple sclerosis, BCVA Best corrected visual acuity, D Diopter, AMD Age-related macular degeneration.
-Data not reported.
Metrics and terminology
Different OCT-A devices applied various segmentation boundaries for the retinal layers. It was only possible to compare the results of studies that used the same OCT-A device and software; however, the foveal avascular zone (FAZ) area is comparable across various OCT-A platforms [42, 43]. Data from three studies that were not reported in mean ± SD format were not entered in the meta-analysis [30, 35, 41]. The terminology for the retinal layers was not unified across studies. For simplicity in this review, we refer to the superficial vascular complex or superficial (retinal) capillary plexus as the “superficial capillary plexus” (SCP) and the deep vascular complex or deep (retinal) capillary plexus as the “deep capillary plexus” (DCP); however, the layer segmentation algorithm may vary across studies. Table S1 presents the metrics, regions, and terminology applied by the included studies.
There were two approaches for measuring vessel density: (1) area-based (Optovue) and (2) length-based (Zeiss). The length-based measurement is more sensitive since all vessels of different lengths influence the result equally, whereas the area-based measurement is more influenced by larger vessels that perfuse a larger area. Studies that used the Zeiss device reported the following metrics: FAZ area, circulatory index, and perimeter; a length-based density measurement (vessel density), and an area-based density measurement (perfusion density). Studies that used the Optovue device reported the following metrics: FAZ area; an area-based density measurement that we will refer to as “vessel density”; retinal capillary skeleton density, and complexity. The study that applied the Heidelberg device reported: FAZ area; an area-based density measurement that we will call “vessel density”; skeleton density; vessel perimeter index and vessel diameter; fractal dimension; and lacunarity. The SS-OCTA study reported flow density and flow ratio.
Foveal Avascular Zone (FAZ) area
Six studies measured the FAZ area [7, 30, 32, 34, 35, 37]. Robbins et al. [37], Zhou et al. [34], and Zou et al. [30] used the Zeiss device and found no significant difference in the FAZ area between PD cases and controls (P-value = 0.29, 0.46, and 0.32, respectively). Zou et al. [30] measured two other FAZ metrics (perimeter and circulatory index) and revealed the FAZ circulatory index is significantly lower in PD patients (P-value = 0.037). Murueta-Goyena [32] applied a Heidelberg device and showed a significant reduction in the FAZ area both in the SCP and DCP (P-value = 0.004 and 0.014, respectively). However, they found that the FAZ circulatory index was not significantly different between PD patients and controls (P-value = 0.686); Meta-analysis of the five studies [7, 30, 32, 34, 37] showed no significant differences in the FAZ area between PD and controls (MD, −0.02; 95% CI [−0.07 to 0.03]; P-value = 0.49; I² = 88.35%) (Table 3). We assessed the effects of suspected confounding variables on the FAZ area measurements by conducting meta-regression and subgroup analysis. The univariate meta-regression revealed that none of the following variables including the number of participants, male percentage, age, and disease duration, show a significant correlation with the effect sizes (all P-values > 0.05). Also, we conducted a subgroup analysis according to the type of OCT-A device and software and found that the studies using the Heidelberg OCT-A (with the Spectralis software) or the RTVue XR Avanti OCT-A (with the AngioVue software) tend to show significantly lower measurements in the FAZ area (all P-values ≤ 0.05). Studies using the Zeiss Cirrus OCT-A device (with the AngioVue software) showed insignificant effects on the FAZ area measurements (P-value > 0.05).
Table 3.
Direction of effects in the included studies using optical coherence tomography angiography (OCT-A) to assess retinal microvasculature in Parkinson’s disease (PD) and its subgroups compared to healthy controls (HC).
| Study (Optovue) | FAZ area | Superficial parafoveal VD | Superficial inferior VD | Superficial superior VD | Superficial whole VD | Superficial foveal PFD | Superficial parafoveal PFD | Superficial inferior PFD | Superficial superior PFD | Deep parafoveal VD | Deep inferior VD | Deep superior VD | Deep Whole VD | Deep parafoveal PFD | Macular VD | Optic Disc | RPC peripapillary VD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rascuna et al. (2020) [40] | - | - | - | - | ∼ | - | - | - | - | - | - | - | ∼ | - | - | - | - |
| Shi et al. [29] | - | - | - | - | - | - | ↓ | ↓ | ↓ | - | - | - | - | ∼ | - | - | - |
| Kwapong et al. [26] | - | ↓ | ↓ | ↓ | - | - | - | - | - | ∼ | - | - | - | - | - | - | - |
| Lin et al. [7] | - | - | - | - | - | - | - | - | - | ↓H-Y II + III* | - | - | - | - | - | ↓H-Y II + III* | - |
| Li et al. [41] | - | - | - | - | ↑ | - | - | - | - | - | - | - | ↑ | - | - | - | ∼ |
| Study (Zeiss) | FAZ area | Superficial parafoveal VD | Superficial inferior VD | Superficial superior VD | Superficial whole VD | Superficial foveal PFD | Superficial parafoveal PFD | Superficial inferior PFD | Superficial superior PFD | Deep parafoveal VD | Deep inferior VD | Deep superior VD | Deep Whole VD | Deep parafoveal PFD | Macular VD | Optic Disc | RPC peripapillary VD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Robbins et al. (A) [37] | ∼ | ↓ | - | - | ↓ | - | ↓ | - | - | - | - | - | - | - | - | - | - |
| Robbins et al. (B) [38] | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ↑PFD average, sup, inf, temp, nas | - |
| Zhou et al. [34] | ∼ | - | - | - | - | - | - | - | - | - | - | - | - | - |
↓inner sup sector** ↓all sectors*** |
- |
↓outer sup sector ∼other sectors |
| Zou et al. [30] | ∼ | ↓ | - | - | ↓ | ↓ | ↓ | - | - | - | - | - | - | - | - | - | - |
| Xu et al. [35] |
↓ ↓H-Y III* ↓H-Y I |
↓ | ↓ | ↓ | - | - | - | - | - | - | - | - | - | - |
↓ all sectors ↓H-Y III: fovea, SI, II, NI, TI, TO* ∼ SO, IO, NO* ↓ H-Y III: all sectors |
- |
∼ ∼ between PD subgroups |
| Study (Heidelberg) | FAZ area | Superficial parafoveal VD | Superficial inferior VD | Superficial superior VD | Superficial whole VD | Superficial foveal PFD | Superficial parafoveal PFD | Superficial inferior PFD | Superficial superior PFD | Deep parafoveal VD | Deep inferior VD | Deep superior VD | Deep Whole VD | Deep parafoveal PFD | Macular VD | Optic Disc | RPC peripapillary VD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Murueta-Goyena et al. [32] |
↓ ↓PD-MCI**** |
- | - | - | - | ↑ | ∼ | - | - | - | - | - | - | ∼ | - | - | - |
| Study (SS-OCT) | FAZ area | Superficial parafoveal VD | Superficial inferior VD | Superficial superior VD | Superficial whole VD | Superficial foveal PFD | Superficial parafoveal PFD | Superficial inferior PFD | Superficial superior PFD | Deep parafoveal VD | Deep inferior VD | Deep superior VD | Deep Whole VD | Deep parafoveal PFD | Macular VD | Optic Disc | RPC peripapillary VD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhang et al. [39] | - | - | - | - | - | ↓ | ↓ | ↓ | ↓ | - | - | - | - | ↓ | - | - | - |
| Satue et al. [23] | - | - | ∼ | ∼ | - | - | - | - | - | - | - | - | - | - | ∼ | - | - |
(The information presented in the table is about the comparison of Parkinson’s disease and healthy controls except for the items specified in the table).
VD Vessel density, PFD Perfusion density, FAZ Foveal avascular zone, RPC Radial peripapillary capillary, sup Superior, inf Inferior, temp Temporal, nas Nasal, PD Parkinson’s disease, H-Y Hoehn-Yahr stage scale, SI Superior inner, II Inferior inner, NI Nasal inner, TI Temporal inner, SO Superior outer, IO Inferior outer, NO Nasal outer, TO Temporal outer, MCI Mild cognitive impairment, SS-OCT Swept source optical coherence tomography.
-Data not reported, ∼no significant difference, ↓significant decrease, ↑:significant increase.
*Compared to PD subgroups (H-Y I & II & III), **in 6x6mm2 field of view, ***in 3 x 3 mm2 field of view, ****compared to PD-NC (Normal cognition).
Density measurements in superficial capillary plexus
Five studies reported density measurements of the SCP in the whole macular region [30, 34, 37, 39, 40]. Robbins et al. [37] (vessel density, P-value = 0.03; perfusion density, P-value = 0.04), Zhou et al. [34] (vessel density, P-value = 0.01), and Zou et al. [30] (vessel density, P-value = 0.018; perfusion density, P-value = 0.007) all used a Zeiss device and found a significant decrease in PD cases independently. However, meta-analysis of two of these studies [34, 37] did not show any significant difference between PD case and control in the whole SCP (MD, −1.01; 95% CI [−2.59 to 0.57]; P-value = 0.21; I² = 78.58%). One Optovue study measured whole vessel density and did not find any significant changes in PD compared to control (P-value = 0.5) [40]. Zhang et al. revealed that PD cases had a significantly reduced flow density and flow ratio in the whole SCP (P-value < 0.0001) [39].
Density measurements in the parafoveal region were the most measured SCP metrics [7, 26, 29, 30, 32, 34, 35, 37, 39, 40]. This included four Zeiss studies [32, 36, 37, 39]; Robbins et al. [37] (vessel density, P-value = 0.003; perfusion density, P-value = 0.004), Zou et al. [30] (vessel density, P < 0.001; perfusion density, P-value < 0.001), and Xu et al. [37] (vessel density, P-value = 0.005) found a significantly decreased vessel density in PD patients. Zhou et al. [34] observed a significantly lower VD only in the superior sector of the parafoveal region when using a 6 × 6 mm scan; however, in the 3 × 3 mm scan protocol, the vessel density appeared to be significantly lower in all sectors (P-value = 0.03 and 0.01, respectively). The meta-analysis of two of these studies [34, 37] did not show any significant differences comparing PD patients and controls (MD, −1.14; 95% CI [−2.58 to 0.29]; P-value = 0.11; I² = 71.25%). Seven studies reported foveal SCP measurements [7, 30, 32, 34, 35, 39, 40]. Of these, three Zeiss studies found a significantly reduced SCP vessel density and perfusion density in the foveal region in PD (vessel density, P-value = 0.03 (Zou), 0.002 (Zhou), and <0.001(Xu); perfusion density, P-value = 0.002) [30, 34, 35]. Meta-analysis of two Zeiss studies [30, 34] revealed that the SCP vessel density was significantly reduced in PD patients (MD, −1.97; 95% CI [−2.97 to −0.97]; P-value < 0.001; I² = 0.00%). In contrast, Rascuna et al. [40] and Lin et al. [7] found no significant changes in foveal vessel density in PD (both P-value = 0.5); Meta-analysis on these two Optovue studies also showed no significant changes (MD, 0.56; 95% CI [−1.26 to 2.39]; P-value = 0.54; I² = 0.00%). Murueta-Goyena et al. [32] found a significantly greater perfusion density and skeleton density in the foveal region in PD (both P-values < 0.05). Zhang et al. [39] used swept-source OCTA and showed a significantly reduced flow density in the foveal region in PD (P-value = 0.001) (Table 3 and Table 4). Five Optovue studies reported parafoveal SCP density measurements [7, 26, 29, 40, 41]; Rascuna et al. [40] and Lin et al. [7] found no significant changes in the parafoveal vessel density in PD (P-value = 0.4 and 0.5, respectively); however, Shi et al. [29] and Kwapong et al. [26] and Li et al.[41] reported a significantly lower perfusion and vessel density in the parafoveal zone in PD individuals (all P-value < 0.05). Meta-analysis of three of these studies [7, 26, 40] showed no significant difference in the parafoveal vessel density comparing PD and controls (MD, −0.71; 95% CI [−2.81 to 1.37]; P-value = 0.5; I² = 77.56%). Shi et al. [29] also measured other retinal capillary parameters, such as skeleton density and complexity, which were both significantly lower in PD (P-value < 0.001 and 0.009, respectively). Murueta-Goyena et al. [32] used a Heidelberg device and measured lacunarity, fractal dimension, perfusion density, and skeleton density; which did not show any significant changes in the parafoveal region (all P-values > 0.05), except for parafoveal lacunarity that was significantly decreased in PD patients (P-value < 0.001). A recent study by Satue et al. [23] used swept-source OCTA and found PD patients had no significant changes in the SCP compared to controls (all P-values > 0.05).
Table 4.
Results of meta-analysis PD vs. control.
| Variables | Overall Effect | Heterogeneity | |||
|---|---|---|---|---|---|
| Mean Difference (95% CI) | P -value | I2 Test, % | Q Test (P) | ||
| Optovue | SCP VD | ||||
| parafoveal | -0.71 (-2.81 to 1.37) | 0.50 | 77.56 | 0.008 | |
| Superior | -0.78 (-3.77 to 2.20) | 0.60 | 79.170 | 0.02 | |
| Inferior | -0.72 (-4.50 to 3.06) | 0.70 | 87.37 | 0.004 | |
| Foveal | 0.56 (-1.26 to 2.39) | 0.54 | 0.00 | 0.73 | |
| DCP VD | |||||
| Parafoveal | -0.61 (-1.78 to 0.56) | 0.30 | 0.00 | 0.75 | |
| Superior | -0.39 (-1.96 to 1.16) | 0.61 | 0.00 | 0.64 | |
| Inferior | -0.24 (-1.75 to 1.26) | 0.74 | 0.00 | 0.53 | |
| Foveal | 0.64 (-1.27 to 2.55) | 0.51 | 0.00 | 0.85 | |
| RPC VD | 0.61 (-0.04 to 1.27) | 0.06 | 0.00 | 0.74- | |
| Zeiss | SCP VD | ||||
| Parafoveal | -1.14 (-2.58 to 0.29) | 0.11 | 71.25 | 0.06 | |
| Perifoveal | -0.29 (-0.59 to 0.01) | 0.06 | 0.00 | 0.66 | |
| Foveal | -1.97 (-2.97 to -0.97) | 0.0001 | 0.00 | 0.46 | |
| Whole | -1.01 (-2.59 to 0.57) | 0.21 | 78.58 | 0.03 | |
| FAZ area | -0.02 (-0.07 to 0.03) | 0.49 | 88.35 | 0.002 | |
SCP superficial capillary plexus, DCP deep capillary plexus, RPC radial peripapillary capillaries, FAZ foveal avascular zone, VD vessel density, PFD perfusion density, CI confidence interval.
Boldface values indicate the significance of the 95% confidence limit.
Four Zeiss studies reported perifoveal SCP density measurements [30, 34, 35, 37]. Two studies found that PD individuals had a significantly lower perfusion density (both P-values = 0.04) but did not find any significant changes in vessel density comparing PD and controls (P-values = 0.08 and 0.13) [30, 37]. In contrast, Xu et al. [35] found a significantly lower perifoveal vessel density in PD (P-value = 0.02). Meta-analysis of these two studies [34, 37] showed no significant changes in the perifoveal SCP in PD compared to controls (MD, −0.29; 95% CI [−0.59 to 0.01]; P-value = 0.60; I² = 0.00%).
Density measurements in deep capillary plexus
The most common DCP measurement was in the parafoveal region [7, 26, 29, 32, 39–41]. Lin et al. [7] and Li et al.[41] found a significantly lower parafoveal DCP vessel density in PD patients (P-value = 0.03). In contrast, Rascuna et al. [40] and Kwapong et al. [26] found no significant changes in the parafoveal DCP (P-value = 0.9 and 0.53, respectively). Meta-analysis of these two studies (26,40) also did not reveal any significant changes (MD, −0.61; 95% CI [−1.78 to 0.56]; P-value = 0.30; I² = 0.00). Two studies [7, 40] measured foveal DCP vessel density and found no significant differences between PD patients and controls (both P-values > 0.05). Meta-analysis of these studies also did not show any significant changes (MD, 0.064; 95% CI [−1.27 to 2.55]; P-value = 0.51; I² = 0.00). Shi et al. [29] and Murueta-Goyena et al. [32] compared the DCP perfusion density of the parafoveal region in PD and controls which did not reveal any significant differences (all P-values > 0.05). Using an Optovue device, Shi et al. [29] also measured the parafoveal retinal capillary complexity and skeleton density in DCP and found a significantly lower skeleton density and complexity in most sectors of the parafoveal DCP in PD (all P-values < 0.05). Murueta-Goyena et al. [32] revealed that the parafoveal DCP lacunarity in PD patients was significantly greater than that of controls (P-value < 0.001), but there were no significant changes in the skeleton density and perfusion density (both P-values > 0.05) (Tables 3 and 4)
Optic Disc Area
Four Optovue studies measured vessel density in the peripapillary region [7, 35, 40, 41]. Xu et al. [35] and Rascuna et al. [40], and Li et al. [41] found no significant changes in peripapillary vessel density (all P-value > 0.05). Lin et al. [7] discovered vessel density in the radial peripapillary capillaries is significantly lower in moderate-stage PD patients compared to early-stage patients. Meta-analysis of these studies [7, 40, 41] did not show any significant changes in PD individuals compared to controls (MD, 0.61; 95% CI [−0.04 to 1.27]; P-value = 0.06; I² = 0.00). The Zeiss study by Robbins et al. [38] discovered a significantly greater perfusion density in the average, temporal, and nasal sectors of the peripapillary region in PD cases (all P-values < 0.001); The capillary flux index of PD cases also appeared significantly greater in all peripapillary sectors (all P-values < 0.05) except for the inferior sector (P-value = 0.1) (Table 3).
Discussion
While deposition of α-synuclein and dopaminergic neuron loss in the SN is the characteristic pathological event in PD, recently there has been a growing appreciation that vascular alternations also contribute to the pathological process of PD and impact the disease onset and progression [44]. Reduced vessel length and branching along with a damaged capillary network and significantly decreased vessel density in the substantial nigra have been observed by direct examination of PD brains [12]. As retinal and cerebral vasculature share a common origin and features, evidence suggests that the α-synuclein accumulation is also present in the vessel walls of the retinal microvasculature in PD eyes, leading to vascular alternations and neuron loss in the retina causing visual disturbances [45].
In this study, we reviewed thirteen observational studies that used OCT-A to measure retinal microvascular parameters in PD patients, and we conducted a meta-analysis of their pooled results where possible. SCP was the most measured vascular layer using OCT-A. The results of the meta-analysis on two studies [30, 34] that used the same OCT-A device and software revealed that PD patients had a significantly lower vessel density in the foveal region. However, according to the pooled data, there were no significant differences in the density measurements of the parafoveal and perifoveal regions between PD and controls. Similarly, no significant changes in the FAZ area were observed in PD individuals. Our results suggest that while retinal microvascular alternations may exist in PD patients, these changes are more distinct in the foveal region.
In this study, we observed that the fovea is more sensitive to vessel density changes in PD, specifically in the SCP. Our results are in line with other studies that discovered significantly lower measurements of SCP vessel density in the fovea of PD patients [30, 34, 35]. Similarly, Murueta-Goyena et al. showed that the alternations in the retinal microvasculature appear more specifically in the foveal region. Though, they found that these microvascular parameters such as perfusion and skeleton density, lacunarity of capillaries, and fractal dimension are greater in PD [32]. In contrast, Rascuna et al. did not report any significant changes in the foveal SCP. However, they found an association between intraretinal layers thickness and SCP vessel density which was present only in the foveal region in PD patients that were at an early stage [40]. Recent evidence has suggested that the vascular changes in PD are dynamic and depend on the stage of the disease; in the early stages of the disease, angiogenesis plays a more important role whereas in later stages vascular degeneration is more evident [44, 46]. A study by Xu et al. showed that the reduction in the SCP density was correlated with higher disease duration, H-Y score, and UPDRS score which highlights the stage-dependent nature of the microvascular changes in PD retina [35, 41]. In addition, they found no significant changes in the SCP vessel density in the early-stage PD group that had a lower H-Y score and disease duration [35]. Another study by Lin et al. found lower microvascular densities in moderate-stage PD patients compared to early-stage cases and suggested a link between disease severity and vessel density changes. However this correlation was only observed in the DCP and radial peripapillary capillaries [7].
The fovea contains the most cortical neurons per unit area compared to other retinal regions, and the fovea accommodates the highest concentration of retinal photoreceptors, which allows to mediate the highest contrast sensitivity [47]. Dysfunction of foveal vision and remodelling has been previously described in PD [47, 48]. Several studies have reported an association between intra-retinal neuron loss and vessel density changes, particularly in the fovea of individuals with PD, which is present even in the early stages[26, 32, 40]. OCT studies suggest that the whole thickness of central retina as well as the thickness of GCL and RNFL are significantly lower in the foveal region of PD patients[49]. How the dopamine depletion processes in each PD stage manifest in the retinal structure and vessels remains unclear. Some studies reported alternations in both the retinal thickness and vasculature of PD patients [23]. They suggest that reduction in the thicknesses of retinal layers happens primarily and the retinal microvasculature would regress in response to the retinal thinning [39, 50]. This is in line with the findings of Chen et al, which demonstrated that the RNFL loss preceded the decrease in vessel density in optic neuropathies rather than being a consequent of this phenomenon [50]. Chen and colleagues hypothesized that OCT-A in the current form only detects areas of no RBC flow in the capillaries and is not able to detect any degree of decrease in the flow until there is absolutely no flow in the capillaries. According to this binary point of view, the authors emphasized that the decrease in capillary density occurs secondarily to the atrophy of the nerve tissue, and does not precede it [50]. In contrast, some studies failed to find any detectable changes in the thickness of retinal layers in PD, despite the vascular alternations [37, 38]. Robbins et al. found that in early-stage PD patients, RNFL changes may not reach a detectable threshold on OCT imaging, while OCT-A markers may be more useful even in PD patients with mild symptomology [38]. The accumulation of α-synuclein in the retina and around retinal vessels and subsequent neuroinflammation could provoke a retinal inflammatory response similar to the CNS, which could lead to apoptosis at the early stages of the disease; the vessel density changes may reflect the retinal neurodegeneration earlier and encompass better diagnostic ability compared to the retinal thinning [37, 39, 45].
Our analysis did not show any significant changes in the vessel density of the parafoveal region, which were in line with the results of the studies by Rascuna et al. [40], Murueta-Goyena et al. [32], and Satue et al. [23]. In contrast to our results, Kwapong et al. [26] found that PD causes a reduction in parafoveal vessel density in the early stages. This contradicting result could be due to a lack of control for inter-eye correlations in their statistical analysis that could increase the false-positive results in the parafovea. In addition, due to the differences in the segmentation boundaries between different OCT-A algorithms, we were unable to pool the results of all the studies that measured parafoveal vessel density, which limited the chances of comparing their results.
In addition to OCT-A metrics, studies have shown that alternations in OCT parameters could serve as PD biomarkers [21, 23, 30, 51]. Several studies suggest that the thicknesses of the macula, RNFL, and GCL-IPL decline in PD patients [21–23, 30]. The choriocapillaris flow density, choroidal vascular index, and choroidal vascular volume significantly decrease in early-stage PD [22]. Powel et al. [52] found that the RNFL thickness negatively correlates with PD severity and duration, suggesting OCT is a valuable tool for monitoring the gradual morphological changes in PD retinas. Similarly, Garcia et al. [53] discovered a negative correlation between GCL thickness and the severity of PD. The macula consumes the greatest amount of oxygen than any other tissue in the body, while only a single-layered capillary arcade in the parafovea supplies it; therefore, the macula is highly susceptible to hypoxia [54]. Accumulation of α-synuclein around retinal arteries in PD subjects implies that microvascular degeneration (appeared as reduced VD and FAZ metrics) may reflect the retinal degeneration processes [45, 55]. The SCP parameters might even be a more sensitive and earlier PD indicator compared to RNFL [22]. OCT and OCT-A findings are both closely associated with PD progression, a combination of their findings improves the diagnostic accuracy more than OCT or OCT-A alone [30].
As a novel diagnostic tool, OCT-A technology is now of great use in clinical practice as well as research to visualize ocular microvasculature [56]. Several studies have reported on the interchangeability of OCT-A parameters across devices, yielding inconsistent results [56–58]. The results of most of these quantitative studies agree vessel density measurements in both SCP and DCP aren’t comparable across devices [56, 58]. Each OCT-A device utilizes a different algorithm and a unique set point for retinal layers segmentation which could lead to variability across devices [56]. Similarly, scanning speed and the number of B-scans in each field of view could alter the image resolution and act as a source of variation in vessel detection [56]. Several studies suggest FAZ area measurements are consistent across OCT-A devices [42, 56, 59]; however, Corvi et al. suggested none of the FAZ metrics or vessel density measurements are interchangeable between different devices [58]. In this study, the subgroup analysis showed, that the Heidelberg Spectralis and RTVue XR Avanti (with AngioVue software) OCT-A devices, tend to show significantly lower measures for the FAZ area. As OCT-A incorporation in daily practice is growing, these controversial results highlight the necessity of standardizing OCT-A metrics across devices for better clinical interpretation and research purposes.
Studies reported inconsistent results regarding FAZ measurements. Most of them observed no significant changes in the FAZ area in PD, and our analysis confirms their data. In contrast, Xu et al. [35] suggested the FAZ area as a sensitive biomarker for PD as they found a significant decline even in early-stage patients. They suggested that foveal dopaminergic neuron loss promotes vasculogenesis and leads to a smaller FAZ area accompanied by decreased foveal vessel density.
The limitation of our study was diversity in the OCT-A device model and software applied by the included studies prevented a complete comparison between their results. We were only able to analyse the studies that used the same OCT-A software and model because segmentation algorithms for retinal microvascular layers vary immensely between devices. Therefore, it is necessary to standardize the defined retinal boundaries among different devices. Furthermore, OCT-A imaging is prone to motion artifacts in PD cases with longer disease duration and severe motor disabilities [36, 60]. In severe PD cases these artifacts appear to be higher, which could lower the image quality and make it challenging to obtain artifact-free images from patients. This could explain why most of the included studies only recruited early-stage PD patients [36]. Another source of heterogeneity was the different fields of view that studies employed (6 × 6 mm or 3 × 3 mm). These scan protocols both provide good reproducibility for FAZ area and density measurements; however, a recent study showed that a direct comparison is not possible between them, especially the FAZ metrics, since different scan densities may produce quantitative differences in the microvascular measurements [61]. The 3 × 3 mm scan protocol reflects a more detailed evaluation of the microvasculature and is more useful for the early assessment of retinal microvascular biomarkers in PD [34]. The fields of view utilized in our included studies can be found in Table S2. Our literature search did not yield any longitudinal studies and since it is necessary to examine the dynamics of retinal vascular changes over time of PD progression, we recommend that future studies apply a longitudinal design.
In this systematic review and meta-analysis, we observed that clinically diagnosed PD patients had a significantly lower vessel density in the SCP, which is more distinct in the foveal region. OCT-A provides a non-invasive evaluation of PD effects on the retinal microvasculature and could potentially offer a reliable biomarker for more accurate and early PD detection and monitoring. Our study highlights the need for standardization of the OCT-A algorithms between devices to increase the comparability of their data. Longitudinal studies and recruiting higher populations of PD individuals from all disease stages are necessary to distinguish between disease effects on retinal microvasculature during each stage of the disease course.
Supplementary information
Author contributions
Author contributions included conception and study design (SM and MAS), article screening (AJ and MAS and SM), data collection or acquisition (SSZ, MAS, and SM), statistical analysis (MAS and SM), interpretation of results (MAS and SM and FR), drafting the manuscript work or revising it critically for important intellectual content (MAS, SM, FR, MG, GY, IS, SJ, and RS) and approval of the final version to be published and agreement to be accountable for the integrity and accuracy of all aspects of the work (MAS, SM, FR, MG, GY, IS, SJ, and RS).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
Authors declare no conflicts of interest except Dr. Rishi P. Singh who is a consultant for Genentech, Regeneron, Alcon, Bausch and Lomb, Asclepix, Gyroscope, Novartis.
Footnotes
The original online version of this article was revised:. In the sub-section ‘Density measurements in deep capillary plexus’, the following sentence “Meta-analysis of these three studies also did not reveal any significant changes (MD, −0.61; 95% CI [−1.78 to 0.56]; P-value = 0.30; I² = 0.00)” was corrected to read “Meta-analysis of these two studies (26,40) also did not reveal any significant changes (MD, −0.61; 95% CI [−1.78 to 0.56]; P-value = 0.30; I² = 0.00)”. Additionally, the reference numbers in the tables 1, 2, 3, as well as the supplementary tables s1 and s2 have been corrected.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Mohammad Amin Salehi, Fateme Rezagholi, Soheil Mohammadi.
Change history
9/15/2023
A Correction to this paper has been published: 10.1038/s41433-023-02691-w
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-023-02483-2.
References
- 1.Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 2.Chen RC, Chang SF, Su CL, H. Chen TH, Yen MF, Wu HM, et al. Prevalence, incidence, and mortality of PD. Neurology. 2001;57:1679. doi: 10.1212/WNL.57.9.1679. [DOI] [PubMed] [Google Scholar]
- 3.Lotharius J, Brundin P. Pathogenesis of Parkinson’s disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci. 2002;3:932–42. doi: 10.1038/nrn983. [DOI] [PubMed] [Google Scholar]
- 4.Pfeiffer RF. Non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord. 2016;22:S119–22. doi: 10.1016/j.parkreldis.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Guo L, Normando EM, Shah PA, De Groef L, Cordeiro MF. Oculo-visual abnormalities in Parkinson’s disease: Possible value as biomarkers. Mov Disord. 2018;33:1390–406. doi: 10.1002/mds.27454. [DOI] [PubMed] [Google Scholar]
- 6.Mammadova N, Summers CM, Kokemuller RD, He Q, Ding S, Baron T, et al. Accelerated accumulation of retinal α-synuclein (pSer129) and tau, neuroinflammation, and autophagic dysregulation in a seeded mouse model of Parkinson’s disease. Neurobiol Dis. 2019;121:1–16. doi: 10.1016/j.nbd.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Lin CW, Lai TT, Chen SJ, Lin CH. Elevated α-synuclein and NfL levels in tear fluids and decreased retinal microvascular densities in patients with Parkinson’s disease. Geroscience. 2022;44:1551–62. doi: 10.1007/s11357-022-00576-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams DR, Litvan I. Parkinsonian syndromes. Continuum (Minneap Minn) 2013;19:1189–212. doi: 10.1212/01.CON.0000436152.24038.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Postuma RB, Aarsland D, Barone P, Burn DJ, Hawkes CH, Oertel W, et al. Identifying prodromal Parkinson’s disease: pre-motor disorders in Parkinson’s disease. Mov Disord. 2012;27:617–26. doi: 10.1002/mds.24996. [DOI] [PubMed] [Google Scholar]
- 10.Zhang C, Wu B, Wang X, Chen C, Zhao R, Lu H, et al. Vascular, flow and perfusion abnormalities in Parkinson’s disease. Parkinsonism Relat Disord. 2020;73:8–13. doi: 10.1016/j.parkreldis.2020.02.019. [DOI] [PubMed] [Google Scholar]
- 11.Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37:56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan J, Pavlovic D, Dalkie N, Waldvogel HJ, O’Carroll SJ, Green CR, et al. Vascular degeneration in Parkinson’s disease. Brain Pathol. 2013;23:154–64. doi: 10.1111/j.1750-3639.2012.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan Y, Hu W, Gan J, Song L, Wu N, Chen Y, et al. Exploring the association between Cerebral small-vessel diseases and motor symptoms in Parkinson’s disease. Brain Behav. 2019;9:e01219. doi: 10.1002/brb3.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol. 2013;9:44–53. doi: 10.1038/nrneurol.2012.227. [DOI] [PubMed] [Google Scholar]
- 15.Asanad S, Mohammed I, Sadun AA, Saeedi OJ. OCTA in neurodegenerative optic neuropathies: emerging biomarkers at the eye-brain interface. Ther Adv Ophthalmol. 2020;12:2515841420950508. doi: 10.1177/2515841420950508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pellegrini M, Vagge A, Ferro Desideri LF, Bernabei F, Triolo G, Mastropasqua R, et al. Optical coherence tomography angiography in neurodegenerative disorders. J Clin Med. 2020;9:1706. doi: 10.3390/jcm9061706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsokolas G, Tsaousis KT, Diakonis VF, Matsou A, Tyradellis S. Optical coherence tomography angiography in neurodegenerative diseases: a review. Eye Brain. 2020;12:73–87. doi: 10.2147/EB.S193026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rifai OM, McGrory S, Robbins CB, Grewal DS, Liu A, Fekrat S, et al. The application of optical coherence tomography angiography in Alzheimer’s disease: A systematic review. Alzheimers Dement. 2021;13:e12149. doi: 10.1002/dad2.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christou EE, Asproudis I, Asproudis C, Giannakis A, Stefaniotou M, Konitsiotis S. Macular microcirculation characteristics in Parkinson’s disease evaluated by OCT-Angiography: a literature review. Semin Ophthalmol. 2022;37:399–407. doi: 10.1080/08820538.2021.1987482. [DOI] [PubMed] [Google Scholar]
- 20.Mardin CY, Hosari S. Optical coherence tomography angiography in neuronal diseases: Preliminary findings. Ophthalmologe. 2019;116:714–21. doi: 10.1007/s00347-019-0883-5. [DOI] [PubMed] [Google Scholar]
- 21.Zhou WC, Tao JX, Li J. Optical coherence tomography measurements as potential imaging biomarkers for Parkinson’s disease: A systematic review and meta-analysis. Eur J Neurol. 2021;28:763–74. doi: 10.1111/ene.14613. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Yang L, Gao Y, Zhang D, Tao Y, Xu H, et al. Choroid and choriocapillaris changes in early-stage Parkinson’s disease: a swept-source optical coherence tomography angiography-based cross-sectional study. Alzheimers Res Ther. 2022;14:116. doi: 10.1186/s13195-022-01054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Satue M, Castro L, Vilades E, Cordon B, Errea JM, Pueyo A, et al. Ability of Swept-source OCT and OCT-angiography to detect neuroretinal and vasculature changes in patients with Parkinson’s disease and essential tremor. Eye. 2022. 10.1038/s41433-022-02112-4. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 24.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30:1591–601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 26.Kwapong WR, Ye H, Peng C, Zhuang X, Wang J, Shen M, et al. Retinal microvascular impairment in the early stages of Parkinson’s disease. Invest Ophthalmol Vis Sci. 2018;59:4115–22. doi: 10.1167/iovs.17-23230. [DOI] [PubMed] [Google Scholar]
- 27.Grewal DS, Fekrat S, Fine HF. Is OCT angiography useful in neurodegenerative diseases? Ophthalmic Surg Lasers Imaging Retin. 2019;50:269–73. doi: 10.3928/23258160-20190503-02. [DOI] [PubMed] [Google Scholar]
- 28.Zafar S, McCormick J, Giancardo L, Saidha S, Abraham A, Channa R. Retinal imaging for neurological diseases: a window into the brain. Int Ophthalmol Clin. 2019;59:137–54. doi: 10.1097/IIO.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 29.Shi C, Chen Y, Kwapong WR, Tong Q, Wu S, Zhou Y, et al. Characterization by fractal dimension analysis of the retinal capillary network in parkinson disease. Retina. 2020;40:1483–91. doi: 10.1097/IAE.0000000000002641. [DOI] [PubMed] [Google Scholar]
- 30.Zou J, Liu K, Li F, Xu Y, Shen L, Xu H. Combination of optical coherence tomography (OCT) and OCT angiography increases diagnostic efficacy of Parkinson’s disease. Quant Imaging Med Surg. 2020;10:1930–9. doi: 10.21037/qims-20-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin JB, Apte RS. Seeing Parkinson’s disease in the retina. JAMA Ophthalmol. 2021;139:189–90. doi: 10.1001/jamaophthalmol.2020.5719. [DOI] [PubMed] [Google Scholar]
- 32.Murueta-Goyena A, Barrenechea M, Erramuzpe A, Teijeira-Portas S, Pengo M, Ayala U, et al. Foveal remodeling of retinal microvasculature in Parkinson’s disease. Front Neurosci. 2021;15:708700. doi: 10.3389/fnins.2021.708700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rascunà C, Cicero CE, Chisari CG, Russo A, Giuliano L, Castellino N, et al. Retinal thickness and microvascular pathway in Idiopathic Rapid eye movement sleep behaviour disorder and Parkinson’s disease. Parkinsonism Relat Disord. 2021;88:40–5. doi: 10.1016/j.parkreldis.2021.05.031. [DOI] [PubMed] [Google Scholar]
- 34.Zhou M, Wu L, Hu Q, Wang C, Ye J, Chen T, et al. Visual impairments are associated with retinal microvascular density in patients with Parkinson’s disease. Front Neurosci. 2021;15:718820. doi: 10.3389/fnins.2021.718820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu B, Wang X, Guo J, Xu H, Tang B, Jiao B, et al. Retinal microvascular density was associated with the clinical progression of Parkinson’s disease. Front Aging Neurosci. 2022;14:818597. doi: 10.3389/fnagi.2022.818597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lauermann JL, Sochurek JAM, Plöttner P, Alten F, Kasten M, Prasuhn J, et al. Applicability of optical coherence tomography angiography (OCTA) imaging in Parkinson’s disease. Sci Rep. 2021;11:5520. doi: 10.1038/s41598-021-84862-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robbins CB, Thompson AC, Bhullar PK, Koo HY, Agrawal R, Soundararajan S, et al. Characterization of retinal microvascular and choroidal structural changes in Parkinson disease. JAMA Ophthalmol. 2021;139:182–8. doi: 10.1001/jamaophthalmol.2020.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robbins CB, Grewal DS, Thompson AC, Soundararajan S, Yoon SP, Polascik BW, et al. Identifying peripapillary radial capillary plexus alterations in Parkinson’s disease using OCT Angiography. Ophthalmol Retin. 2022;6:29–36. doi: 10.1016/j.oret.2021.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Zhang D, Gao Y, Yang L, Tao Y, Xu H, et al. Retinal flow density changes in early-stage Parkinson’s disease investigated by swept-source optical coherence tomography angiography. Curr Eye Res. 2021;46:1886–91. doi: 10.1080/02713683.2021.1933054. [DOI] [PubMed] [Google Scholar]
- 40.Rascunà C, Russo A, Terravecchia C, Castellino N, Avitabile T, Bonfiglio V, et al. Retinal thickness and microvascular pattern in early Parkinson’s disease. Front Neurology. 2020;11:533375. doi: 10.3389/fneur.2020.533375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Wang X, Zhang Y, Zhang P, He C, Li R, et al. Retinal microvascular impairment in Parkinson’s disease with cognitive dysfunction. Parkinsonism Relat Disord. 2022;98:27–31. doi: 10.1016/j.parkreldis.2022.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Lu Y, Wang JC, Zeng R, Katz R, Vavvas DG, Miller JW, et al. Quantitative comparison of microvascular metrics on three optical coherence tomography angiography devices in chorioretinal disease. Clin Ophthalmol. 2019;13:2063–9. doi: 10.2147/OPTH.S215322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munk MR, Giannakaki-Zimmermann H, Berger L, Huf W, Ebneter A, Wolf S, et al. OCT-angiography: A qualitative and quantitative comparison of 4 OCT-A devices. PLoS One. 2017;12:e0177059. doi: 10.1371/journal.pone.0177059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paul G, Elabi OF. Microvascular changes in Parkinson’s disease- focus on the neurovascular unit. Front Aging Neurosci. 2022;14:853372. doi: 10.3389/fnagi.2022.853372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ortuño-Lizarán I, Beach TG, Serrano GE, Walker DG, Adler CH, Cuenca N. Phosphorylated α-synuclein in the retina is a biomarker of Parkinson’s disease pathology severity. Mov Disord. 2018;33:1315–24. doi: 10.1002/mds.27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elabi O, Gaceb A, Carlsson R, Padel T, Soylu-Kucharz R, Cortijo I, et al. Human α-synuclein overexpression in a mouse model of Parkinson’s disease leads to vascular pathology, blood-brain barrier leakage and pericyte activation. Sci Rep. 2021;11:1120. doi: 10.1038/s41598-020-80889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bodis-Wollner I. Foveal vision is impaired in Parkinson’s disease. Parkinsonism Relat Disord. 2013;19:1–14. doi: 10.1016/j.parkreldis.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 48.Spund B, Ding Y, Liu T, Selesnick I, Glazman S, Shrier EM, et al. Remodeling of the fovea in Parkinson disease. J Neural Transm. 2013;120:745–53. doi: 10.1007/s00702-012-0909-5. [DOI] [PubMed] [Google Scholar]
- 49.Huang L, Zhang D, Ji J, Wang Y, Zhang R. Central retina changes in Parkinson’s disease: a systematic review and meta-analysis. J Neurol. 2021;268:4646–54.. doi: 10.1007/s00415-020-10304-9. [DOI] [PubMed] [Google Scholar]
- 50.Chen JJ, AbouChehade JE, Iezzi R, Jr, Leavitt JA, Kardon RH. Optical coherence angiographic demonstration of retinal changes from chronic optic neuropathies. Neuroophthalmology. 2017;41:76–83. doi: 10.1080/01658107.2016.1275703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deng Y, Jie C, Wang J, Liu Z, Li Y, Hou X. Evaluation of retina and microvascular changes in the patient with Parkinson’s disease: A systematic review and meta-analysis. Front Med. 2022;9:957700. doi: 10.3389/fmed.2022.957700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Powell A, Muller AJ, O’Callaghan C, Sourty M, Shine JM, Lewis SJG. Dopamine and functional connectivity in patients with Parkinson’s disease and visual hallucinations. Mov Disord. 2020;35:704–5. doi: 10.1002/mds.27995. [DOI] [PubMed] [Google Scholar]
- 53.Garcia-Martin E, Larrosa JM, Polo V, Satue M, Marques ML, Alarcia R, et al. Distribution of retinal layer atrophy in patients with Parkinson’s disease and association with disease severity and duration. Am J Ophthalmol. 2014;157:470–8.e2. doi: 10.1016/j.ajo.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 54.Yu DY, Cringle SJ, Yu PK, Balaratnasingam C, Mehnert A, Sarunic MV, et al. Retinal capillary perfusion: Spatial and temporal heterogeneity. Prog Retin Eye Res. 2019;70:23–54. doi: 10.1016/j.preteyeres.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 55.Price DL, Rockenstein E, Mante M, Adame A, Overk C, Spencer B, et al. Longitudinal live imaging of retinal α-synuclein::GFP deposits in a transgenic mouse model of Parkinson’s Disease/Dementia with Lewy Bodies. Sci Rep. 2016;6:29523. doi: 10.1038/srep29523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu Y, Wang JC, Cui Y, Zhu Y, Zeng R, Lu ES, et al. A quantitative comparison of four optical coherence tomography angiography devices in healthy eyes. Graefes Arch Clin Exp Ophthalmol. 2021;259:1493–501. doi: 10.1007/s00417-020-04945-9. [DOI] [PubMed] [Google Scholar]
- 57.Corvi F, Cozzi M, Barbolini E, Nizza D, Belotti M, Staurenghi G, et al. Comparison between several optical coherence tomography angiography devices and indocyanine green angiography of choroidal neovascularization. Retina. 2020;40:873–80. doi: 10.1097/IAE.0000000000002471. [DOI] [PubMed] [Google Scholar]
- 58.Corvi F, Pellegrini M, Erba S, Cozzi M, Staurenghi G, Giani A. Reproducibility of vessel density, fractal dimension, and Foveal Avascular zone using 7 different optical coherence tomography angiography devices. Am J Ophthalmol. 2018;186:25–31. doi: 10.1016/j.ajo.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 59.Mihailovic N, Brand C, Lahme L, Schubert F, Bormann E, Eter N, et al. Repeatability, reproducibility and agreement of foveal avascular zone measurements using three different optical coherence tomography angiography devices. PLoS One. 2018;13:e0206045. doi: 10.1371/journal.pone.0206045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ranjbar M, Plöttner P, Sochurek JAM, Lauermann JL, Alten F, Prasuhn J, et al. The impact of motion artifacts on quantitative optical coherence tomography angiography analysis in Parkinson’s disease. Parkinsonism Relat Disord. 2022;95:57–8. doi: 10.1016/j.parkreldis.2022.01.006. [DOI] [PubMed] [Google Scholar]
- 61.Xiao H, Liu X, Liao L, Tan K, Ling Y, Zhong Y. Reproducibility of Foveal Avascular zone and superficial macular retinal vasculature measurements in healthy eyes determined by two different scanning protocols of optical coherence tomography angiography. Ophthalmic Res. 2020;63:244–51. doi: 10.1159/000503071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.