Abstract
Adults with Down syndrome (DS) experience high risk for Alzheimer’s disease (AD), but there is variability in the timing of transition from a cognitively stable state to prodromal AD and dementia. The present study examined the association between a modifiable lifestyle factor, employment complexity, and cognitive decline across two time points in adults with DS. Employment complexity, defined as the degree of problem-solving or critical thinking required for employment activities, was operationalized using the Dictionary of Occupational Titles, a system which classifies occupations based on three categories: Data, People, and Things. Eighty-seven adults with DS (M = 36.28 years, SD = 6.90 years) were included in analyses. Partial correlations revealed that lower employment complexity involving People and Things were associated with increased dementia symptoms. Lower employment complexity involving Things was also associated with memory decline. These findings have implications for vocational programs focused on job training and placement for adults with DS.
Keywords: Alzheimer’s disease, cognition, dementia, down syndrome, employment
Identifying ways to promote healthy cognitive aging, defined as improving or maintaining cognitive functioning during adulthood, is of critical importance to people with Down syndrome (DS) and their families (e.g., Fick, 2021). DS is a neurodevelopmental condition that is caused by an extra copy of chromosome 21 (full, partial, or translocation) and occurs in 1 in 700 live births in the U.S. (Centers for Disease Control and Prevention, 2022). People with DS have a unique phenotype that includes an early onset and high risk for Alzheimer’s disease (AD) (Mann and Esiri, 1989; Wiseman et al., 2015). Beginning in their 30s, people with DS evidence brain β-amyloid plaques (e.g., Lao et al., 2016; Keator et al., 2020), an early hallmark feature of AD, and this is followed by pathological changes such as neurofibrillary tangles of tau, reduced hippocampal volume, and altered brain metabolism that are thought to cause AD (Fortea et al., 2021; Lott and Head, 2019). Despite a shared genetic risk for AD due to trisomy 21, there is variability in the timing of the transition from a cognitive stable state to prodromal AD and dementia among people with DS. Some people with DS evidence prodromal AD in their mid to late 40s, whereas others live into their 70s without evidencing dementia (Krinsky-McHale et al., 2008; Holland et al., 2000; Iulita et al., 2022). In part, lifestyle factors may explain this variability (e.g., Mihaila et al., 2019; Yu et al., 2020). The present study sought to determine if employment complexity, a modifiable aspect of lifestyle was associated with AD-related cognitive decline across two time points (16–20 months apart) in adults with DS.
Employment complexity is the extent to which employment activities are cognitively challenging and require problem-solving, critical thinking, perspective taking, and sustained focus (e.g., Andel et al., 2005). The protective effect of employment complexity on aging- and AD-related cognitive decline is thought to relate to the theory of cognitive reserve (Stern, 2006), which posits that cognitive stimulation helps people tolerate aging- and AD-related brain pathology (e.g., decreases in hippocampal volume and altered brain metabolism) because they can draw from a broader range of preexisting cognitive strategies or use compensatory approaches to mitigate the adverse effects of this pathology for a longer period of time. Research on the theory of cognitive reserve has most commonly indexed how cognitively stimulating lifestyles are through employment complexity and/or education level (Boots et al., 2015; Schultz et al., 2015; Stern, 2012).
Across studies, engagement in highly complex employment is related to less decline in memory and processing speed (Smart et al., 2014) and reduced risk for AD (Andel et al., 2005; Krӧger et al., 2008) in middle-aged and older adults without DS. Moreover, among older adults without DS, many of whom were genetically at-risk for AD due to their APOE allele status, those with higher employment complexity maintained better cognitive functioning in the face of AD pathology (more cerebral hypometabolism and lower hippocampal volume and more whole-brain atrophy) than those with lower employment complexity (Boots et al., 2015; Garibotto et al., 2008; 2013). Among older adults newly diagnosed with AD and with comparable clinical presentation, those with higher education had higher brain Aβ in the lateral frontal cortex and lower glucose metabolic rate in the temporoparietal cortical regions compared to those with low education. This finding suggests that higher-educated older adults, which can be a proxy for employment complexity, remained in a preclinical stage (i.e., AD pathology but no cognitive decline) longer prior to diagnosis than lower-educated older adults (Kemppainen et al., 2008). Higher (versus lower) education has also been associated with a slower rate of cognitive decline in the years prior to dementia onset in adults with autosomal dominant AD due to a single PSEN1 E280A mutation (e.g., Aguirre-Acevadu et al., 2016). Together these studies suggest that lifestyles involving more cognitive stimulation may preserve cognitive functioning with age and could mitigate the adverse effects of early AD pathology on cognition including in populations at genetic-risk for AD. Virtually nothing is known about whether employment complexity similarly confers beneficial effects on cognitive aging in DS.
Much of the research examining the potential benefit of employment complexity on cognitive aging or risk of AD in general population samples of middle-aged or older adults has used the Dictionary of Occupational Titles (DOT; United States Employment Service, 1991), which is a catalog of occupation ratings based on observations performed by job analysts. The DOT rates employment complexity in three domains: complexity with Data (i.e., synthesizing, coordinating, analyzing); complexity with People (i.e., mentoring, negotiating, instructing); complexity with Things (i.e., setting up, precision working, operating-controlling). Higher scores in complexity with Data and People are most strongly linked with high cognitive stimulation (and the idea of cognitive reserve) and have the strongest associations with reduced risk of aging-related cognitive decline and AD (Andel et al., 2005; Smart et al., 2014).
Historically, adults with DS had limited employment opportunities; sheltered workshops involving assembly or sorting work were often the only options (Browder and Cooper, 1994; Pruchno and McMullen, 2004). More recently, employment for adults with disabilities has shifted away from sheltered workshops to community jobs, providing a pathway for more cognitively-complex work (Migliore et al., 2008). In a sample of 511 adults with DS, 57% reported having a paid job, and 26% worked as a volunteer, with the most common paid or volunteer positions involving food services, janitorial work, and office work (Kumin and Schoenbrodt, 2016). College programs have also been created for adults with intellectual and developmental disabilities including DS (Lee, Day, Carter, and Taylor, 2021), which involve cognitive training to build capacity for complex employment positions. Opportunities within the community such as employment have been documented to have numerous benefits for people with intellectual disabilities including increased feelings of self-determination (Lindsay et al., 2018; Shogren et al., 2015; Vicente et al., 2020), better psychological well-being, and a higher self-reported quality of life (Lindsay et al., 2018). However, virtually nothing is known about whether employment complexity, including engagement in post-secondary education programs, protect against aging and AD-related cognitive decline in people with DS.
The goal of the present study was to determine if employment complexity is related to cognitive decline in people with DS. The study aims were to 1) describe the employment complexity of adults with DS and 2) determine whether employment complexity at cycle 1 predicted change in memory and dementia symptoms across two time points spaced approximately 2 years apart (cycle 1 and 2). Analyses included 87 adults with DS aged 25–57 years who did not evidence AD dementia. The DOT rating system was used to code employment complexity in terms of Data, People, and Things but was modified to fit employment relevant to adults with DS and to include engagement in postsecondary education programs. Cognitive functioning was assessed using directly-administered measures of memory and dementia symptoms and a caregiver-reported measure of dementia symptoms. Higher employment complexity at cycle 1 was hypothesized to be associated with less decline in memory and less increase in dementia symptoms across cycle 1 to cycle 2 in models that controlled for age and intellectual disability level.
Method
Participants
Analyses drew on a sample of 104 adults with DS recruited from two sites involved in the Alzheimer’s Biomarker Consortium in DS (ABC-DS). As part of ABC-DS study visits (Handen et al., 2020 describes full protocol), adults with DS were administered a neuropsychological battery and caregivers reported on the adult with DS’s dementia symptoms. Study inclusion criteria were: 1) age ≥ 25 years; 2) mental age ≥ 30 months; 3) no conditions that preclude brain imaging (e.g., pregnant or breastfeeding or metal in the body); 4) genetic confirmation of trisomy 21; and 5) no untreated medical or psychiatric conditions that alter cognitive functioning. Seven adults with DS had a clinical status of mild cognitive impairment or AD dementia and were not included in analyses, resulting in a sample of 97 adults with DS. Ten participants were not given an employment complexity code due to a lack of sufficient information on job responsibilities; therefore, employment complexity data was available for 87 participants. Clinical status was determined by a case consensus process involving at least 3 research staff (including a licensed psychologist and a physician) who reviewed information from directly-administered cognitive measures, caregiver-reported measures of functioning, behavior, and cognition, a physical exam, and medical and psychiatric history. All participants provided written informed consent.
Procedure
Study activities occurred in 2017 to 2019 and were part of a larger research protocol that has been previously described (Handen et al., 2020). As part of the larger research study, caregivers completed socio-demographic and employment information about the adult with DS at the first cycle of data collection. Updates on employment were provided at cycle 2. Adults with DS were administered a battery of cognitive measures and caregivers completed informant measures on dementia symptoms at both cycle 1 and 2. Cycle 2 of data collection occurred 16–20 months after cycle 1.
Measures
Socio-demographics.
Caregivers reported on the age, biological sex, and type of residence. Intellectual disability level was based on the Stanford-Binet, fifth edition abbreviated battery IQ score (Roid and Pomplun, 2012) and coded as 1 = mild (mental age ≥8 years), 2 = moderate (mental age of 5–7 years), 3 = severe (mental age ≤4 years). Blood karyotype testing was performed to assess type of DS (full trisomy, mosaic, or translocation).
Cognitive functioning.
There were three measures of cognitive functioning. The Modified Cued Recall Test (CRT) assesses episodic memory and is reliable and valid in adults with DS (Zimmerli and Devenny, 1995). In the initial learning phase, participants learn 12 items (e.g., grapes) displayed in pictures and their category (e.g., fruit). Participants are asked to freely recall the objects. There are also cued trials, that prompt participants with category cues (e.g., what was the fruit) for objects not freely recalled. The CRT Total score is the sum of pictures recalled across three free and cued trials. The CRT Intrusion score is the number of inaccurately recalled pictures in the cued trials. Higher CRT Total scores and lower CRT Intrusion scores are representative of better memory performance.
The Down Syndrome Mental Status Examination (DSMSE; Haxby, 1989) is a directly-administered measure of dementia symptoms that assesses: personal information, object memory, location memory, apraxia, language, visuospatial, and knowledge of the examiner. The Total score is calculated by adding the scores across the domains. This measure is deemed reliable and valid for measuring neuropsychological functioning in adults with DS (Haxby, 1989) and higher scores indicate better cognitive functioning.
The Dementia Questionnaire for People with Learning Disabilities (DLD; Evenhuis, 2018) was completed by the caregiver and assesses short- and long-term memory, temporal and spatial orientation, speech, practical skills, mood, interests, and behavioral disturbances. The sum of cognitive (memory and orientation) and the sum of social (speech, practical skills, mood, interest in activity, behavioral disturbance) scores were used in analyses. The DLD is a valid and reliable measure for use with the DS population (Elliott-King et al., 2016; Koehl et al., 2020). Lower scores on the DLD are indicative of fewer dementia symptoms.
Employment complexity.
Caregivers reported on the employment activities of the adult with DS. Some participants were employed in multiple jobs or participated in both employment and day programs and/or volunteer activities. Total number of hours spent in employment activities was used in these cases and the complexity rating was based on the activity with the highest level of complexity. A modified version of the rating system from the DOT was used to code participants’ employment complexity. The DOT uses a 9-digit coding system to classify jobs (e.g., 920–687-014; grocery bagger). The first three digits represent an occupational group (e.g., professional, technical, clerical) and the last three digits differentiate particular occupations from all others. The fourth, fifth, and sixth digits represent the complexity scores. Complexity scores are divided into three categories: 1) complexity of working with Data (4th digit); 2) complexity of working with People (5th digit); 3) complexity of working with Things (6th digit). In the original DOT, Data complexity is composed of a 7-point rating scale (0–6), People complexity is composed of a 9-point rating scale (0–8), and Things complexity is composed of an 8-point rating scale (0–7). Ratings for the DOT coding system are coded so that lower scores reflect higher occupational complexity, with (0,0,0) representing the highest possible complexity and (6,8,7) representing the lowest possible complexity of the scale. Modifications were made to the coding system to account for common activities for adults with DS that are similar to employment but are not captured in the DOT coding system. We modified the coding system in the following ways: 1) created an additional complexity code for sheltered workshop activities (7,9,8) that represented lower complexity than the least complex jobs (6,8,7); 2) in order to account for participants that stayed at home, created the code (8,10,9), representing lower complexity than both the least complex jobs as well as sheltered workshop activities; 3) college students were given the code (3,2,1) to represent a highly complex activity, similar to the highest level of complexity for employment positions in our sample.
Data analysis plan
Descriptive analyses were used to determine the mean, standard deviation, and range in demographic, cognitive, and employment variables. Histograms and scatterplots were used to examine variable spread. Change scores were calculated for the cognitive measures (CRT, DSMSE, and DLD) by subtracting the study cycle 2 score from the study cycle 1 score. For the CRT and DSMSE, a high positive change score indicates cognitive decline and a high negative number means scores improved from study cycle 1 to study cycle 2. The opposite is true for DLD scores, with high negative scores indicating cognitive decline. Scores near zero represent no change in scores at the two time points. Bivariate Pearson correlations were conducted to examine associations among socio-demographic variables, employment complexity, and cognitive change. Partial correlations were then completed to adjust for the effect of relevant socio-demographic variables (i.e., those significantly associated with employment complexity or cognitive change) when examining the association between employment complexity at study cycle 1 and the cognitive change scores.
Results
The 87 participants had a mean age of 36.28 years (SD = 6.90) at cycle 1. Approximately half were male (n = 44), and half were female (n = 43). Forty-six (53%) participants had a mild level of intellectual disability, 29 (33%) had a moderate level of intellectual disability, and 12 (14%) had a severe level of intellectual disability. Additional demographic information is in Table 1.
Table 1.
Sample socio-demographics (n = 87).
| Cycle 1 age in years, M(SD) | 36.28 (6.90) |
| Cycle 2 age in years, M(SD) | 38.09 (7.29) |
| Female, n (%) | 43 (49%) |
| Residence, n (%) | |
| Family or caregiver | 52 (60%) |
| Independent | 16 (18%) |
| Group home | 5 (6%) |
| N/A | 14 (16%) |
| Intellectual disability level, n (%) | |
| Mild | 46 (53%) |
| Moderate | 29 (33%) |
| Severe | 12 (14%) |
| Karyotype, n (%) | |
| Full trisomy | 76 (87%) |
| Mosaicism | 1 (1%) |
| Translocation | 9 (10%) |
| N/A | 1 (1%) |
| Employment Activity, n (%) | |
| Employed/Student | 59 (68%) |
| Student | 2 (2%) |
| Sheltered workshop | 23 (26%) |
| Day program | 1 (1%) |
| Stay at home | 2 (2%) |
| Employment Complexity, M(SD) | |
| Data | 5.70 (1.25) |
| People | 7.25 (1.67) |
| Things | 6.31 (1.98) |
As shown in Table 1, Data complexity had an average score of 5.70 (SD = 1.25, range = 3–8), People complexity had a mean score of 7.25 (SD = 1.67, range = 2–10), and Things complexity had an average score of 6.31 (SD = 1.98, range = 1–9). A summary of employment complexity scores is also provided in Figure 1. Overall, 70% (n = 61) of participants were primarily employed (n = 59) or attending college (n = 2), 26% (n = 23) attended a sheltered workshop, 1% (n = 1) attended a day program, and 2% (n = 2) stayed at home. Forty-four participants (51%) spent greater than 20 hours/week participating in employment activities, whereas 29 (33%) spent 10–12 hours per week, and 11 (13%) spent less than 10 hours per week. Participants were employed in a variety of positions including janitor, cafeteria attendant, and grocery store attendant. Some participants (n =16) were involved in multiple jobs or activities (e.g., job and day program) (see Table 2). Nearly all (n = 84, 97%) participants participated in the same employment activities at cycle 2 as they had at cycle 1. Two (2%) participants changed employment activities but received the same People, Data, and Things complexity scores at cycle 2 as at cycle 1. One (1%) participant changed employment activities and received the same Data and Things complexity scores but their People complexity score went from a 9 to an 8.
Figure 1.
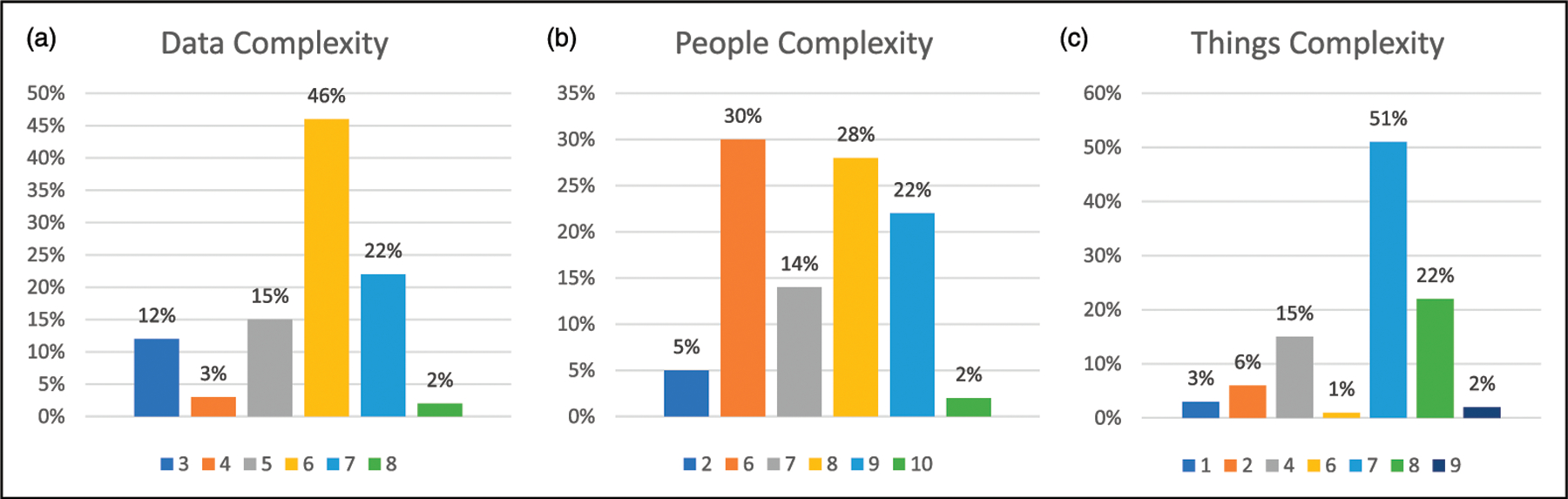
Distribution of employment complexity scores in Data (1a), People (1b), and Things (1c). Lower score indicates higher complexity.
Table 2.
Examples of job titles, responsibilities, and complexity codes.
| Complexity |
||||
|---|---|---|---|---|
| Job Title | Responsibilities | Data | People | Things |
| Assembler (n = 2) | Assembly line job | 6 | 8 | 7 |
| Bakery Helper (n = 1) | Helps bake; lift and carry things | 6 | 8 | 6 |
| Bracelet Maker (n =1) | Makes bracelets | 6 | 8 | 1 |
| Cafeteria Attendant or coffee shop assistant [n = 8]) | Cleans tables; organizes, washes dishes | 6 | 7 | 7 |
| Clerical Assistant (n = 4) | Filing; office Work | 5 | 6 | 2 |
| College Student (n = 2) | Attends college | 3 | 2 | 1 |
| Day Program (n = 6) | Assembly; sorting; shredding | 7 | 9 | 8 |
| Dishwasher (n = 5) | Washes Dishes | 6 | 8 | 7 |
| Dry Cleaning Attendant (n = 1) | Gets stains out of clothes | 6 | 7 | 7 |
| Fast Food Worker (n = 4) | Cleans, stocks, clears dishes | 4 | 7 | 2 |
| Garment Sorter (n = 1) | Sorts merchandise; hangs clothes | 6 | 8 | 7 |
| Hand Packager (n = 5) | Folds and packages items | 5 | 8 | 7 |
| Information Clerk (n = 2) | Office clerical work; self-advocate; | 3 | 6 | 7 |
| Janitor (n =15) | Cleaning; minor maintenance | 6 | 6 | 4 |
| Kitchen Helper (Hotel/Restaurant) (n = 3) | prep; lifting and carrying; cleaning and rearranging; wash dishes | 6 | 8 | 7 |
| Library Aid (n = 2) | Puts book away; inventory; organize books; help children | 3 | 6 | 7 |
| Mailroom Clerk (n = 1) | Sorts and delivers mail; makes up gift packages | 6 | 8 | 7 |
| Nursery School Attendant (n = 2) | Monitors and plays with children | 6 | 7 | 7 |
| Office Helper (n = 5) | Shreds; sorts; creates labels; filing; order supplies | 5 | 6 | 7 |
| Scanner/Bagger, grocery store (n = 5) | Scans items; puts items into bags | 4 | 6 | 2 |
| Sheltered Workshop (n = 27) | Sorting; assembly; shredding; cleaning; packaging | 7 | 9 | 8 |
| Stays at Home (n = 2) |
|
8 | 10 | 9 |
| Stock Clerk (n = 1) | Stock and Shelve Items | 3 | 6 | 7 |
| Teacher’s Assistant (n = 3) | Grades; reads to children; organizes; recess monitor; copying; shredding | 3 | 2 | 7 |
| Walmart Associate (n = 1) | Greets; assists customers | 3 | 6 | 7 |
| Warehouse Worker (n = 1) | Sorting; organizing | 6 | 8 | 7 |
Note: The total n for this table is 110 as some participants (n = 16) listed more than one job/activity.
The means, standard deviations, and range for cognitive variables at study cycles 1 and 2 are reported in Table 3. Next, the relation among socio-demographic variables, employment complexity, and cognitive change were investigated using bivariate Pearson correlations. Premorbid intellectual disability level was positively associated with Data employment complexity (r =.243, p = .023) and People employment complexity (r = .229, p = .033), indicating that individuals with more severe intellectual disability were more likely to have less complex employment activity. Premorbid intellectual disability was also positively correlated with the CRT Total change score (r =.264, p = .016) and negatively correlated with the CRT Intrusions change score (r = −.218, p = .048). This means that participants with lower intellectual ability evidenced greater decline in CRT scores over time than those with higher intellectual ability. Chronological age was positively correlated with the CRT Total change score (r =.319, p = .003) and negatively correlated with CRT Intrusions change score (r = −.274, p = .012); thus, older participants evidenced greater memory decline than younger participants. Hours spent in employment activities per week were positively correlated with the CRT Total change score (r =.241, p = .033) and negatively correlated with the CRT Intrusions change score (r = −.236, p = .036), indicating that participants who engaged in more employment activities had greater memory decline than those who spent less time. Biological sex was not significantly correlated with the employment complexity scores or cognitive change scores. Given these associations, partial correlations controlled for premorbid intellectual impairment, chronological age, and hours spent in activities each week.
Table 3.
Means, standard deviations, and range for cycles 1 & 2 of cognitive functioning measures.
| M(SD) Cycle 1 | Range Cycle 1 | M(SD) Cycle 2 | Range Cycle 2 | t | df | p | |
|---|---|---|---|---|---|---|---|
| CRT Total | 33.61 (3.37) | 19–36 | 29.77 (6.62) | 7–36 | 5.806 | 73 | <.001 |
| CRT Intrusions | 1.94 (2.40) | 0–12 | 5.05 (5.60) | 0–27 | −5.514 | 73 | <.001 |
| DSMSE | 66.11 (11.16) | 33.5–83 | 65.30 (11.63) | 35.5–82 | .201 | 73 | .842 |
| DLD Sum of Social | 3.22 (3.44) | 0–15 | 3.78 (4.02) | 0–20 | −.413 | 72 | .681 |
| DLD Sum of Cognitive | 2.94 (4.94) | 0–26 | 3.11 (5.27) | 0–24 | .576 | 72 | .566 |
Note. CRT = Modified Cued Recall Test; DSMSE = Down Syndrome Mental Status. Examination Total score; DLD = Dementia Questionnaire for People with Learning. Disabilities; t = t-value for paired sample t-test; df = degrees of freedom.
Table 4 provides the results for the partial correlations between employment complexity and change in cognitive functioning controlling for age, intellectual disability level, and hours spent per week in employment activities. Employment complexity involving People was negatively associated with the DLD Sum of Social change score (r = −.262, p = .032), indicating that those with lower employment complexity involving People were more likely to have greater declines in social skills over time. Employment complexity involving Things was significantly positively associated with the DSMSE change score (r = .263, p = .032) and the CRT Total change score (r = .280, p = .022), and negatively correlated with the DLD Sum of Social change score (r = −.242, p = .049). This means that individuals with greater employment complexity involving things were less likely to experience decline in DSMSE, CRT scores, and social skills over time. Employment complexity involving Data was not significantly correlated with any of the cognitive change scores.
Table 4.
Partial correlations between employment complexity and change in cognitive functioning.
| Change in Memory |
Change in Dementia Symptoms |
||||
|---|---|---|---|---|---|
| CRT Total | CRT Intrusions | DSMSE | DLD Sum of Social | DLD Sum of Cognitive | |
| Data Complexity |
r=−.007 p=.953 |
r=−.026 p=.836 |
r=.080 p=.518 |
r=−.100 p=.419 |
r=−.117 p=.345 |
| People Complexity |
r=.023 p=.853 |
r=−.075 p=.545 |
r=.237 p=.053 |
r=−.262 p=.032 |
r=−.228 p=.064 |
| Things Complexity |
r=.280 p=.022 |
r=−.214 p=.082 |
r=.263 p=.032 |
r=−.242 p=.049 |
r=−.137 p=.271 |
Note. Partial correlations control for age, level of intellectual disability, and hours spent in employment per week. DSMSE = Down Syndrome Mental Status Examination Total score; DLD = Dementia Questionnaire for People with Learning Disabilities; CRT = Modified Cued Recall Test.
Discussion
The present study investigated the employment complexity of adults with DS to determine whether lifestyles with high employment complexity were associated with better cognitive aging outcomes (i.e., less decline in memory and fewer new dementia symptoms across two time points). In recent decades, shifts in educational and vocational rehabilitation programs and societal attitudes have provided adults with intellectual and developmental disabilities, including DS, opportunities to engage in a variety of employment positions as well as post-secondary education programs (Kumin and Schoenbrodt, 2016; Lee et al., 2021; Migliore et al., 2008). The lives of many adults with DS may include a high level of cognitive stimulation through these activities. Employment complexity is a modifiable aspect of lifestyle that has been shown to alter the risk of AD and influence the timing of aging-related cognitive decline in other populations (Andel et al., 2005; Krӧger et al., 2008; Smart et al., 2014). Employment complexity may provide a target for policy and intervention aimed at prolonging healthy cognitive functioning in DS.
In the present study, participation in employment activities as broadly construed was high for the adults with DS. The majority (70%) of adults with DS were employed or enrolled in college (2% college, 68% for competitive employment, 26% sheltered workshop) and about half (51%) spent greater than 20 hours a week in employment activities. These findings corroborate those of Migliore and colleagues (2008) and Kumin and Schoenbrodt (2016), highlighting that employment is now a central part of adult life for people with DS. However, the high employment rate of our sample is likely, in part, driven by sampling methods; adults with DS who are employed may be more likely to participant in research studies and the study inclusion criteria included a mental age of ≥ 30 months. Employment activities ranged from sorting to grading papers and assisting in classrooms. The most common employment-related activities were janitorial work and sheltered workshop employment activities such as assembly work.
The employment activities of the adults with DS in the present study had varying levels of complexity across the three employment complexity categories: Data, People, and Things. The most complex jobs reported in the current study were Teacher’s Assistant and College Student, whereas the least complex jobs were Assembler, Kitchen Assistant and those who participated in sheltered workshops/day programs. Certain jobs also had low complexity codes in one category and high complexity in another. For example, some of the positions within the category of clerical assistant had low complexity in terms of People but high complexity related to Things.
Employment complexity, in terms of Data and People, differed by level of intellectual disability such that adults with DS with higher intellectual functioning levels (e.g., mild or upper range of moderate intellectual disability) had greater employment complexity than adults with DS with lower intellectual functioning levels (e.g., lower range of moderate to severe intellectual disability). This could represent appropriate placement of adults with DS to work environments that match their overall cognitive abilities. It is also possible that adults with DS with lower intellectual levels have fewer opportunities to engage in complex employment activities, even if accommodations could be made to allow participation. Surprisingly, a higher total number of hours spent in employment activities per week was connected to greater decline in memory performance. However, this finding could be driven by the tendency for sheltered workshop positions to be intertwined with adult day programming in terms of hours; thus, adults with DS who spent the most time in work-related activities tended to be in employment positions that were less complex (e.g., sheltered workshops). Older adults with DS also experienced greater cognitive decline than younger participants, which is expected due to age-related risks for memory decline in adults with DS (Cole et al., 2017; Hartley et al., 2020; Hithersay et al., 2017). Biological sex was not related to employment complexity or cognitive decline in the study sample, indicating that opportunities related to the complexity of employment were comparable for males and females in the current study.
After controlling for chronological age, intellectual disability level, and hours spent in employment activities, adults with DS who were involved in more complex employment in terms of People and Things evidenced less cognitive decline across 16–20 months than did adults with DS involved in no employment activities or less complex activities. This was true on both caregiver-reported measures and directly-administered measures of cognition. Specifically, adults with DS involved in more complex employment related to People had fewer increases in dementia-related social behaviors (DLD Sum of Social change score) such as social withdrawal. This finding builds on prior evidence that social inclusion and navigating frequent and diverse interpersonal interactions may have important benefits for adults with disabilities (e.g., Clifford et al., 2015), and potentially include promoting healthy aging in DS. Adults with DS involved in more complex employment related to Things had fewer increases in dementia-related cognitive problems (DSMSE change score) such as difficulties tracking verbal instructions, and social behaviors (DLD Sum of Social change score), and less decline in memory ability (CRT Total change score) over time. Thus, these findings suggest that employment activities that involve assembling, moving, cleaning, and organizing objects may help maintain memory abilities across time in adults with DS.
In contrast, employment complexity related to Data did not predict cognitive decline in adults with DS. This category of employment complexity had a smaller range (3–8) than did the People and Things categories, which may reflect that employment activities of adults with DS do not involve high amounts of synthesizing, coordinating, or analyzing of information. Although the associations in this study were modest, the significant correlations are in line with the theory of cognitive reserve (Stern, 2006), relating cognitive stimulation to reduced or delayed cognitive decline. The current study also corroborates previous findings linking greater employment complexity with healthy aging outside of DS (Krӧger et al., 2008; Smart et al., 2014). Employment complexity is likely one aspect of lifestyle that provides cognitive stimulation in adulthood for people with DS.
Strengths, Limitations and future directions
One strength of the current study was the inclusion of a longitudinal design, which allowed for cognitive decline to be measured across two time points. The implementation of the modified DOT coding scheme also had strengths. The DOT coding system has been utilized to categorize employment complexity since its original publication in 1977 (United States Employment Service, 1977), and has been used in previous research (e.g., Andel et al., 2005; Boots et al., 2015; Smart et al., 2014). The modifications to the DOT coding system implemented in the present study were aimed at better aligning the coding system with the employment activities of people with DS. This modified approach considered both paid and unpaid (e.g., volunteer) activities as well as post-secondary education. Incorporating unpaid positions allowed for more adults with DS to be assigned a complexity code for activities that are comparable to paid employment. One limitation of this coding system is that it does not account for support provided by a job coach or support staff, which could reduce the level of required complexity.
In the current sample, most adults with DS reported the same employment activities across the 16–20 months. However, the study did not collect information on when participants began the employment activities (i.e., length of employment). Benefits of highly complex employment for cognitive aging may require long-term engagement. The current study assessed cognitive decline over a short period of time (16–20 months). Longer-term longitudinal studies would be better suited to capture early cognitive declines and dementia symptoms associated with AD pathology, as such changes are not expected to be marked until the late 30s and beyond (e.g., Hartley et al., 2020; Videla et al., 2022).
Conclusions
People with DS are at high risk for AD dementia due to trisomy 21 (Fortea et al., 2021; Lott and Head, 2019; Wiseman et al., 2015), however, there is variability in the age of onset of cognitive decline and AD dementia (Krinsky-McHale et al., 2008; Holland et al., 2000; Iulita et al., 2022). Employment complexity is a modifiable lifestyle factor that could potentially be used to mitigate cognitive decline in adults with DS. The present study was, to our knowledge, the first examination of employment complexity in adults with DS and its link to AD-related cognitive change. Findings suggest that higher employment complexity may serve as a protective factor that could delay AD-related cognitive decline and dementia symptoms. These findings highlight that despite having a genetic risk for AD, there may be ways to modify lifestyles to promote healthy cognitive aging for longer in people with DS. These findings have implications for social policy and vocational rehabilitation programs aimed at job training and placement. Efforts to prepare, train, and place adults with DS into employment positions (paid or unpaid) that involve more complex work with others and objects in particular may be key to supporting successful cognitive aging in DS.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research is funded by the National Institute on Aging (U19 AG070043, U01AG051406, R01AG70028) and Eunice Kennedy Shriver National Institute of Child Health and Human Development (5T32 HD007489, U19 AG070043, U54 HD090256).
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Brianna Piro-Gambetti, Waisman Center, University of Wisconsin-Madison, Madison, WI, USA, Department of Human Development & Family Studies, University of Wisconsin-Madison, Madison, WI, USA.
Emily K. Schworer, Waisman Center, University of Wisconsin-Madison, Madison, WI, USA, Department of Human Development & Family Studies, University of Wisconsin-Madison, Madison, WI, USA
Benjamin Handen, Department of Psychiatry, University of Pittsburgh, Pittsburgh, PA, USA.
Masha Glukhovskaya, Department of Psychiatry, University of Pittsburgh, Pittsburgh, PA, USA.
Sigan L. Hartley, Waisman Center, University of Wisconsin-Madison, Madison, WI, USA, Department of Human Development & Family Studies, University of Wisconsin-Madison, Madison, WI, USA
References
- Aguirre-Acevedo DC, Lopera F, Henao E, Tirado V, Muñoz C, Giraldo M, Bangdiwala SI, Reiman EM, Tariot PN, Langbaum JB, Quiroz YT and Jaimes F. Cognitive Decline in a Colombian Kindred With Autosomal Dominant Alzheimer Disease: A Retrospective Cohort Study. JAMA Neurol 2016;73(4): 431–438. doi: 10.1001/jamaneurol.2015.4851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andel R, Crowe M, Pedersen NL, Mortimer J, Crimmins E, Johansson B and Gatz M (2005).Complexity of work and risk of Alzheimer’s disease: A population-based study of Swedish twins. Journal of Gerontology: Psychological Sciences, 60B(5), 251–258. doi: 10.1093/geronb/60.5.p251 [DOI] [PubMed] [Google Scholar]
- Boots EA, Schultz SA, Almeida RP, Oh JM, Koscik RL, Dowling MN, Gallagher CL, Carlsson CM, Rowley HA, Bendlin BB, Asthana S, Sager MA, Hermann BP, Johnson SC and Okonkwo OC (2015). Occupational complexity and cognitive reserve in a middle-aged cohort at risk for Alzheimer’s disease. Archives of Clinical Neuropsychology, 30, 634–642. doi: 10.1093/arclin/acv041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browder DM and Cooper KJ (1994). Inclusion of older adults with mental retardation in leisure opportunities. Mental Retardation, 32(2), 91–99. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2022). Down syndrome Available at: https://www.cdc.gov/ncbddd/birthdefects/downsyndrome.html
- Clifford S, Geraldine L, Kosciulek J and Leahy M (2015).Defining social inclusion of people with intellectual and developmental disabilities: An ecological model of social networks and community participation, Research in Developmental Disabilities, 38, 18–29. 10.1016/j.ridd.2014.10.008 [DOI] [PubMed] [Google Scholar]
- Cole JH, Annus T, Wilson LR, Remtulla R, Hong YT, Fryer TD, Acosta-Cabronero J, Cardenas-Blanco A, Smith R, Menon DK, Zaman SH, Nestor PJ and Holland AJ (2017). Brain-predicted age in Down syndrome is associated with beta amyloid deposition and cognitive decline. Neurobiology of Aging, 56, 41–49. doi: 10.1016/j.neurobiolaging.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick DM (2021). Aging with Down Syndrome & Tips for Promoting Cognitive Brain Health at Every Age [Down Syndrome Association of Wisconsin, Inc. Webinar]. https://www.dsaw.org/events/agingwithds21
- Elliott-King J, Shaw S, Bandelow S, Devshi R, Kassam S and Hogervorst E (2016). A critical literature review of the effectiveness of various instruments in the diagnosis of dementia in adults with intellectual disabilities. Alzheimer’s & Dementia, 4(1), 126–148. doi: 10.1016/j.dadm.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenhuis HM (2018). The Dementia Questionnaire for People with Learning Disabilities. In: Prasher V (eds) Neuropsychological Assessments of Dementia in Down Syndrome and Intellectual Disabilities Springer, Cham. doi: 10.1007/978-3-319-61720-6_3 [DOI] [Google Scholar]
- Fortea J, Zaman SH, Hartley S, Rafii MS, Head E and Carmona-Iragui M (2021). Alzheimer’s disease associated with Down syndrome: A genetic form of dementia. The Lancet Neurology, 20(11), 930–942. doi: 10.1016/S1474-4422(21)00245-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garibotto V, Borroni B, Kalbe E, Herholz K, Salmon E, Holtoff V, Sorbi S, Cappa SF, Padovani A, Fazio F and Perani D (2008). Education and occupation as proxies for reserve in aMCI converters and AD: FDG-PET evidence. Neurology, 71, 1342–1349. doi: 10.1212/01.wnl.0000327670.62378.c0 [DOI] [PubMed] [Google Scholar]
- Garibotto V, Borroni B, Sorbi S, Cappa SF, Padovani A and Perani D (2013). Education and occupation provide reserve in both ApoE e4 carrier and noncarrier patients with probable Alzheimer’s disease. Neurological Sciences, 33, 1037–1042. doi: 10.1007/s10072-011-0889-5 [DOI] [PubMed] [Google Scholar]
- Handen B, Lott IT, Christian BT, Schupf N, O’Bryant S, Mapstone M, Fagan AM, Lee JH, Tudorascu D, Wang M-C, Head E, Klunk W, Ances B, Lai F, Zaman S, Krinsky-McHale S, Brickman AM, Rosas HD, Cohen A, Andrews H, Hartley S and Silverman W. (2020). The Alzheimer’s Biomarker Consortium-Down Syndrome: Rationale and methodology. Alzheimer’s & Dementia, 12(1), e12065. doi: 10.1002/dad2.12065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley SL, Handen BL, Devenny D, Tudorascu D, Piro-Gambetti B, Zammit MD, Laymon CM, Klunk WE, Zaman S, Cohen A and Christian BT (2020). Cognitive indicators of transition to preclinical and prodromal stages of Alzheimer’s disease in Down syndrome. Alzheimer’s & Dementia, 12(1), e12096. doi: 10.1002/dad2.12096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV (1989). Neuropsychological evaluation of adults with Down’s syndrome: Patterns of selective impairment in non-demented old adults. Journal of Mental Deficiency Research, 33(Pt3), 193–210. doi: 10.1111/j.1365-2788.1989.tb01467.x [DOI] [PubMed] [Google Scholar]
- Hithersay R, Hamburg S, Knight B and Strydom A (2017). Cognitive decline and dementia in Down syndrome. Current Opinion Psychiatry, 30(2), 102–107. doi: 10.1097/YCO.0000000000000307 [DOI] [PubMed] [Google Scholar]
- Holland AJ, Hon J, Huppert FA and Stevens F (2000). Incidence and course of dementia in people with Down’s syndrome: Findings from a population-based study. Journal of Intellectual Disabilities Research, 44(Pt2), 138–146. doi: 10.1046/j.1365-2788.2000.00263.x [DOI] [PubMed] [Google Scholar]
- Iulita MF, Chavez DG, Christensen MK, Tamayo NV, Plana-Ripoll O, Rasmussen SA, Figuls MR, Alcolea D, Videla L, Barroeta I, Benejam B, Altuna M, Padilla C, Pegueroles J, Fernandez S, Belbin O, Carmona-Iragui M, Blesa R, Lleó A, Benjanin A and Fortea J (2022). Association of Alzheimer disease with life expectancy in people with Down syndrome. JAMA Neurology, 5(5), e2212910. doi: 10.1001/jamanetworkopen.2022.2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keator DB, Doran E, Taylor L, Phelan MJ, Hom C, Tseung K, van Erp TGM, Potkin SG, Brickman AM, Rosas DH, Yassa MA, Silverman W and Lott IT (2020). Brain amyloid and transition to dementia in Down syndrome. Alzheimer’s & Dementia, 12(1), e12126. doi: 10.1002/dad2.12126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen NM, Aalto S, Karrasch M, Någren K, Savisto N, Oikonen V, Viitanen M, Parkkola R and Rinne JO (2008). Cognitive research hypothesis: Pittsburgh Compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer’s disease. Annals of Neurology, 63, 112–118. doi: 10.1002/ana.21212 [DOI] [PubMed] [Google Scholar]
- Koehl L, Harp J, Van Pelt KL, Head E and Schmitt FA (2020). Longitudinal assessment of dementia measures in Down syndrome. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 12, e12075. doi: 10.1002/dad2.12075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinsky-McHale SJ, Devenny DA, Gu H, Jenkins EC, Kittler P, Murty VV, Schupf N, Scotto L, Tycko B, Urv TK, Ye L, Zigman WB and Silverman W (2008). Successful aging in a 70-year-old man with Down syndrome: A case study. Intellectual and Developmental Disabilities, 46(3) 215–228. doi: 10.1352/2008.46: 215–228 [DOI] [PubMed] [Google Scholar]
- Krӧger E, Andel R, Lindsay J, Benounissa Z, Verreault R and Laurin D (2008). Is complexity of work associated with risk of dementia? The Canadian study of health and aging. American Journal of Epidemiology, 167(7), 820–830. doi: 10.1093/aje/kwm382 [DOI] [PubMed] [Google Scholar]
- Kumin L and Schoenbrodt L (2016). Employment in adults with Down syndrome in the United States: Results from a national survey. Journal of Applied Research in Intellectual Disabilities, 29(4), 330–345. doi: 10.1111/jar.12182 [DOI] [PubMed] [Google Scholar]
- Lao PJ, Betthauser TJ, Hillmer AT, Price JC, Klunk WE, Mihaila I, Higgins AT, Bulova PD, Hartley SL, Hardison R, Tumuluru RV, Murali D, Mathis CA, Cohen AD, Barnhart TE, Devenny DA, Mailick MR, Johnson SC, Handen BL and Christian BT (2016). The effects of normal aging on amyloid-β deposition in nondemented adults with Down syndrome as imaged by carbon 11-labeled Pittsburgh compound B. Alzheimer’s & Dementia, 12(4), 380–390. doi: 10.1016/j.jalz.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CE, Day TL, Carter EW and Taylor JL (2021). Examining growth among college students with intellectual and developmental disability: A longitudinal study. Behavior Modification, 45(2). 324–348. doi: 10.1177/0145445520982968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay S, Cagliostro E, Albarico M, Mortaji N and Karon L (2018). A systematic review of the benefits of hiring people with disabilities. Journal of Occupational Rehabilitation, 28, 634–655. doi: 10.1007/s10926-018-9756-z [DOI] [PubMed] [Google Scholar]
- Lott IT and Head E (2019). Dementia in Down syndrome: Unique insights for Alzheimer disease research. National Reviews: Neurology, 15(3), 135–147. doi: 10.1038/s41582-018-0132-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DM and Esiri MM (1989). The pattern of acquisition of plaques and tangles in the brains of patients under 50 years of age with Down’s syndrome. Journal of Neurological Science, 89(2–3), 169–179. doi: 10.1016/0022-510x(8990019-1) [DOI] [PubMed] [Google Scholar]
- Migliore A, Grossi T, Mank D and Rogan P (2008). Why do adults with intellectual disabilities work in sheltered workshops? Journal of Vocational Rehabilitation, 28, 29–40. doi: 10.1052-2263/08 [DOI] [Google Scholar]
- Mihaila I, Handen B, Christian BT, Lao PJ, Cody K, Klunk WE, Tudorascu DL, Cohen AD, Okonkwo O and Hartley SL (2019). Leisure activity, brain β-amyloid, and Episodic memory in adults with Down syndrome. Developmental Neurobiology, 79(7), 738–749. doi: 10.1002/dneu.22677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruchno RA and McMullen WF (2004). Patterns of service utilization by adults with a developmental disability: Type of service makes a difference. American Journal of Mental Retardation, 109(5), 362–378. doi: [DOI] [PubMed] [Google Scholar]
- Roid GH and Pomplun M (2012). The Stanford Binet Intelligence Scales, Fifth Edition. In Flanagan DP and Harrison PL (Eds.), Contemporary intellectual assessment: Theories, tests, and issues (pp. 249–268). The Guilford Press. [Google Scholar]
- Schultz SA, Larson J, Oh J, Koscik R, Dowling MN, Gallagher CL, Carlsson CM, Rowley HA, Bendlin BB, Asthana S, Hermann BP, Johnson SC, Sager M, LaRue A and Okonkwo OC (2015). Participation in cognitively-stimulating activities is associated with brain structure and cognitive function in preclinical Alzheimer’s disease. Brain Imaging and Behavior, 9, 729–736. doi: 10.1007/s11682-014-9329-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shogren KA, Wehmeyer ML, Palmer SB, Rifenbark GG and Little TD (2015). Relationships between self-determination and postschool outcomes for youth with disabilities. The Journal of Special Education, 48(4), 256–267. doi: 10.1177/0022466913489733 [DOI] [Google Scholar]
- Smart EL, Gow AJ and Deary IJ (2014). Occupational complexity and lifetime cognitive abilities. Neurology, 83(24), 2285–2291. doi: 10.1212/WNL.0000000000001075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y (2006). Cognitive reserve and Alzheimer’s disease. Alzheimer Disease and Associated Disorders, 20, 112–117. doi: 10.1016/S1474-4422(1270191-6) [DOI] [PubMed] [Google Scholar]
- Stern Y (2012). Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol, 11(11), 1006–1012. doi: 10.1016/S1474-4422(12)70191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Employment Service. (1977). Dictionary of Occupational Titles Washington, DC: Superintendent of Documents, US Government Printing Office. [Google Scholar]
- United States Employment Service. (1991). Dictionary of Occupational Titles Washington, D.C.: The administration. [Google Scholar]
- Vicente E, Mubbardó-Adam C, Guillén VM, Coma-Roselló T, Bravo-Álvarez M-Á and Sάnchez S (2020). Self-determination in people with intellectual disability: The mediating role of opportunities. International Journal of Environmental Research and Public Health, 17, 1–14. doi: 10.3390/ijerph17176201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videla L, Benejam B, Pegueroles J, et al. Longitudinal Clinical and Cognitive Changes Along the Alzheimer Disease Continuum in Down Syndrome, JAMA Network Open 2022;5(8):e2225573. doi: 10.1001/jamanetworkopen.2022.25573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman FK, Al-Janabi T, Hardy J, Karmiloff-Smith A, Nizetic D, Ty-bulewicz VLJ, Fisher EMC and Strydom A (2015). A genetic cause of Alzheimer disease: mechanistic insights from down syndrome. National Reviews Neuroscience, 16(9), 564–574. doi: 10.1038/nrn3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Feng Q, Yu J, Zeng Y and Feng L (2020). Late-life cognitive trajectories and their associated lifestyle factors. Journal of Alzheimer’s Disease: JAD, 73(4), 1555–1563. doi: 10.3233/JAD-191058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli E and Devenny DA. 1995. Cued recall as a screen for dementia in the MR population. Paper presented at the Gatlinburg conference on research and theory in mental retardation and developmental disabilities, 1995 March 11–14, Gatlinburg, TN.


