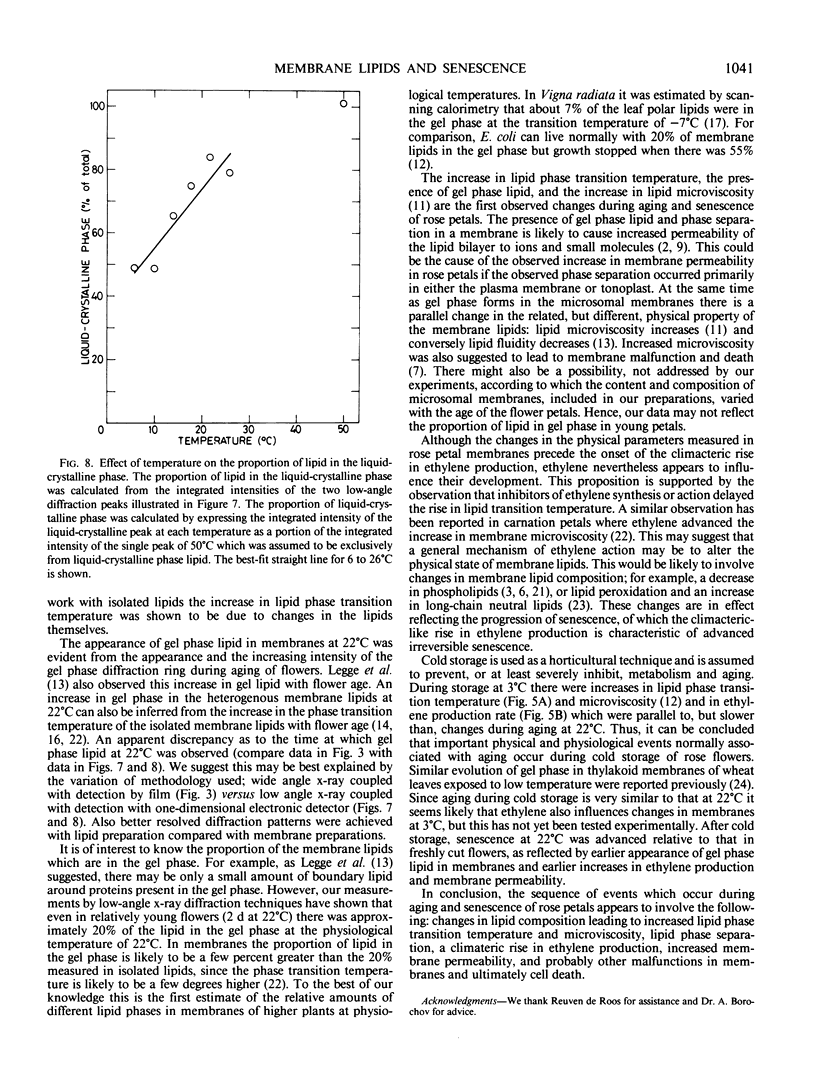
Abstract
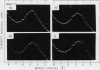
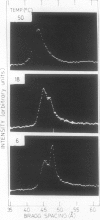
Changes in the physical state of microsomal membrane lipids during senescence of rose flower petals (Rosa hyb. L. cv Mercedes) were measured by x-ray diffraction analysis. During senescence of cut flowers held at 22°C, lipid in the ordered, gel phase appeared in the otherwise disordered, liquid-crystalline phase lipids of the membranes. This was due to an increase in the phase transition temperature of the lipids. The proportion of gel phase in the membrane lipids of 2-day-old flowers was estimated as about 20% at 22°C. Ethylene may be responsible, at least in part, for the increase in lipid transition temperature during senescence since aminooxyacetic acid and silver thiosulfate inhibited the rise in transition temperature. When flowers were stored at 3°C for 10 to 17 days and then transferrd to 22°C, gel phase lipid appeared in membranes earlier than in freshly cut flowers. This advanced senescence was the result of aging at 3°C, indicated by increases in membrane lipid transition temperature and ethylene production rate during the time at 3°C. It is concluded that changes in the physical state of membrane lipids are an integral part of senescence of rose petals, that they are caused, at least in part, by ethylene action and that they are responsible, at least in part, for the increase in membrane permeability which precedes flower death.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutelmann P., Kende H. Membrane Lipids in Senescing Flower Tissue of Ipomoea tricolor. Plant Physiol. 1977 May;59(5):888–893. doi: 10.1104/pp.59.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaurock A. E. Evidence of bilayer structure and of membrane interactions from X-ray diffraction analysis. Biochim Biophys Acta. 1982 May 12;650(4):167–207. doi: 10.1016/0304-4157(82)90016-8. [DOI] [PubMed] [Google Scholar]
- Borochov A., Halevy A. H., Shinitzky M. Senescence and the Fluidity of Rose Petal Membranes : RELATIONSHIP TO PHOSPHOLIPID METABOLISM. Plant Physiol. 1982 Feb;69(2):296–299. doi: 10.1104/pp.69.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M. Lipid bilayer structure in the membrane of Mycoplasma laidlawii. J Mol Biol. 1971 May 28;58(1):153–165. doi: 10.1016/0022-2836(71)90238-5. [DOI] [PubMed] [Google Scholar]
- Jackson M. B., Cronan J. E., Jr An estimate of the minimum amount of fluid lipid required for the growth of Escherichia coli. Biochim Biophys Acta. 1978 Oct 4;512(3):472–479. doi: 10.1016/0005-2736(78)90157-8. [DOI] [PubMed] [Google Scholar]
- McKersie B. D., Stinson R. H. Effect of Dehydration on Leakage and Membrane Structure in Lotus corniculatus L. Seeds. Plant Physiol. 1980 Aug;66(2):316–320. doi: 10.1104/pp.66.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn P. J. The fluidity of cell membranes and its regulation. Prog Biophys Mol Biol. 1981;38(1):1–104. doi: 10.1016/0079-6107(81)90011-0. [DOI] [PubMed] [Google Scholar]
- RENKONEN O., KOSUNEN T. U., RENKONEN O. V. EXTRACTION OF SERUM INOSITIDES AND OTHER PHOSPHATIDES. Ann Med Exp Biol Fenn. 1963;41:375–381. [PubMed] [Google Scholar]
- Thompson J. E., Mayak S., Shinitzky M., Halevy A. H. Acceleration of membrane senescence in cut carnation flowers by treatment with ethylene. Plant Physiol. 1982 Apr;69(4):859–863. doi: 10.1104/pp.69.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]