Abstract
The effects of bovine lactoferrin (LF) or the LF-derived antimicrobial peptide lactoferricin B (LFcin B) on the growth of Candida albicans hyphae, including those of three azole-resistant strains, were investigated by a crystal violet staining method. The hyphae of two highly azole-resistant strains were more susceptible to inhibition by LF or LFcin B than the azole-susceptible strains tested. One moderately azole-resistant strain was defective in the formation of hyphae and showed a susceptibility to LF greater than that of the susceptible strains but a susceptibility to LFcin B similar to that of the susceptible strains. The highly azole-resistant strain TIMM3317 showed trailing growth in the presence of fluconazole or itraconazole, while the extent of growth was reduced by the addition of LF or LFcin B at a sub-MIC. Thus, the addition of LF or LFcin B at a sub-MIC resulted in a substantial decrease in the MICs of fluconazole and itraconazole for two highly azole-resistant strains; e.g., the MIC of fluconazole for TIMM3317 was shifted from >256 to 0.25 μg/ml by LF, but the MICs were not decreased for the susceptible strains. The combination effects observed with triazoles and LF-related compounds in the case of the two highly azole-resistant strains were confirmed to be synergistic by the fractional inhibitory concentration index. These results demonstrate that for some azole-resistant C. albicans strains, LF-related compounds combined with triazoles can inhibit the growth of hyphae, an important form of this organism in pathogenesis.
Candida albicans is an opportunistic pathogen that causes systemic and mucosal infectious diseases in humans. Among mucosal Candida infections, oropharyngeal candidiasis has become a serious clinical problem in immunocompromised hosts such as AIDS patients (18). The hyphal form of C. albicans is more capable of adhering to mucosal cells than the yeast form and, thus, is more likely to invade host tissues and initiate clinical disease (16). Therapeutic strategies should include agents that target hyphal development.
The triazole antifungal agents fluconazole and itraconazole are widely used to treat infections caused by Candida spp. because of their demonstrated efficacy and low levels of toxicity (8). Azole antifungal agents are known to affect the development of C. albicans hyphae at doses that result in only a relatively small degree of inhibition of the growth rate (17). However, in severely immunocompromised patients, such as those with late-stage AIDS or chronic mucocutaneous candidiasis, repeated treatments with fluconazole have led to the appearance of Candida isolates resistant to these agents (21). Although several antifungal agents have been reported to have efficacy against azole-resistant C. albicans, studies with those agents have dealt mainly with cells growing in the yeast form (12, 22). Studies focusing on cells growing in the hyphal form are essential considering the importance of this form in pathogenesis.
Lactoferrin (LF) is an antimicrobial protein found in various exocrine secretions of mammals and in the secondary granules of neutrophils. Many investigators have reported on the anti-Candida activities of LF (15, 19, 23). The antimicrobial peptide lactoferricin is derived from the N-terminal region of the LF molecule (2), and this lactoferricin region is responsible for the antimicrobial activity of LF (11). Lactoferricin B (LFcin B), derived from bovine LF, exhibits potent disruptive effects on the fungal cell membrane and has fungicidal activity against C. albicans (25).
From studies with yeast forms, we have reported that LF or LFcin B combined with azole antifungal agents has synergistic antifungal activity against C. albicans (24). To assess the value of combination therapy, we have studied the susceptibilities of hyphal forms of azole-resistant C. albicans to LF or LFcin B alone or in combination with triazole antifungal agents.
MATERIALS AND METHODS
Materials.
Bovine LF was produced by Morinaga Milk Industry Co. (Tokyo, Japan). The antimicrobial peptide LFcin B was produced by the method reported previously (2). Amphotericin B was purchased from Sigma Chemical Co. (St. Louis, Mo.). Fluconazole and itraconazole were extracted from Diflucan capsules (Pfizer Pharmaceuticals Inc., Tokyo, Japan) and Itrizole capsules (Janssen Kyowa Co., Tokyo, Japan), respectively.
C. albicans strains.
The following azole-susceptible C. albicans strains were used: ATCC 90028, recommended by National Committee for Clinical Laboratory Standards document M27-T (14), and TIMM1768, a clinically isolated serotype A strain (Teikyo University Institute of Medical Mycology, Tokyo, Japan). The azole-resistant strains were as follows: moderately azole-resistant strain TIMM3164 was isolated from an AIDS patient with oropharyngeal candidiasis, and highly azole-resistant strains TIMM3315 and TIMM3317 were sequentially isolated from a patient with a hematological disease. Stock cultures were transferred onto Sabouraud glucose agar and were incubated at 28°C before use. The susceptibilities of the strains to antifungal agents were confirmed by the standard method described in document M27-T (14).
Measurement of hyphal growth.
RPMI 1640 medium supplemented with 2.5% heat-inactivated fetal calf serum, 20 mM HEPES, 2 mM l-glutamine, and 16 mM sodium hydrogen carbonate (pH 7.0) was used as the hyphal growth-promoting medium (RP medium) for C. albicans. Yeast-form cells of C. albicans were collected from cultures on Sabouraud glucose agar, washed with saline, and suspended in RP medium at 105 cells/ml. Each well of a 96-well flat-bottom microplate received a mixture of 20 μl of Candida suspension, 10 μl of a stock solution of LF or LFcin B, 2 μl of a stock solution of antifungal agent, and 168 μl of RP medium, and all microplates were incubated at 37°C in a 5% CO2 atmosphere for 15 h. To determine the extent of growth of C. albicans hyphae, the crystal violet (CV) staining assay was performed as described previously (1, 19). Briefly, the medium in the wells was discarded and the adhesive Candida mycelia were sterilized by treatment with 70% ethanol. The mycelia were stained with 0.02% CV and washed with water. After the microplates were dried, 150 μl of isopropanol containing 0.04 N HCl and 50 μl of 0.25% sodium dodecyl sulfate were added to the wells and mixed. The absorbances at 550 to 630 nm of triplicate samples were measured spectrophotometrically. For fluconazole and LF-related compounds, the MIC was defined as the lowest drug concentration that reduced growth by 80% compared with the growth in the drug-free well. For itraconazole, the 90% inhibitory concentration was chosen because this drug caused partial inhibition of azole-resistant C. albicans.
The fractional inhibitory concentration (FIC) index was calculated as follows (6): [(A)/MICA]+[(B)/MICB] = FIC index, where MICA and MICB are the MICs of drugs A and B, respectively, determined separately, and (A) and (B) are the MICs of drugs A and B, respectively, when the MIC of the combination was determined. The effects of the drugs were interpreted to be indicative of synergy or indifference when the FIC index was <1 or 1 to 4, respectively.
RESULTS
Inhibition of hyphal growth by antifungal agents, LF, or LFcin B.
The effects of antifungal agents, LF, and LFcin B on the growth of the hyphae of the test strains in RP medium were quantified by the CV staining method except in the case of TIMM3164, for which the MIC was determined by monitoring the increase in the optical density at 630 nm of the culture because of the defective growth of the strain’s hyphae (Table 1). High levels of resistance of TIMM3315 and TIMM3317 to fluconazole and itraconazole were observed in this assay, and a moderate level of resistance of TIMM3164 to these agents was observed. The MIC of LF for the azole-resistant strains TIMM3164, TIMM3315, and TIMM3317 was lower than that for the azole-susceptible strains ATCC 90028 and TIMM1768. The MIC of LFcin B for TIMM3315 and TIMM3317 was also lower than that for the azole-susceptible strains.
TABLE 1.
MICs of antifungal agents, LF, and LFcin B determined by the CV staining assay
| C. albicans strain | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| Ampho- tericin B | Fluco- nazole | Itracon- azole | LF | LFcin B | |
| Azole susceptible | |||||
| ATCC 90028 | 0.13 | 0.25 | 0.0063 | >6,400 | 400 |
| TIMM1768 | 0.13 | 0.25 | 0.0063 | 6,400 | 400 |
| Azole resistant | |||||
| TIMM3164a | 0.13 | 1 | 0.05 | 1,600 | 400 |
| TIMM3315 | 0.25 | 128 | >51 | 200 | 50 |
| TIMM3317 | 0.13 | >256 | >51 | 1,600 | 200 |
The MIC for this strain was determined by measuring the increase in the optical density at 630 nm of the culture.
Effect of triazole antifungal agents combined with LF-related compounds against C. albicans TIMM3317.
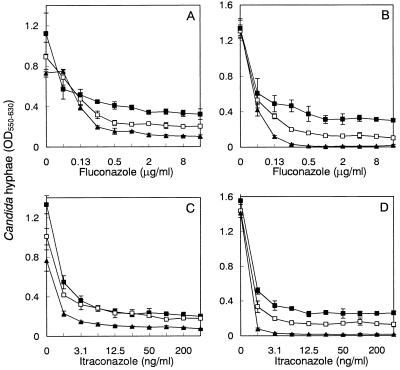
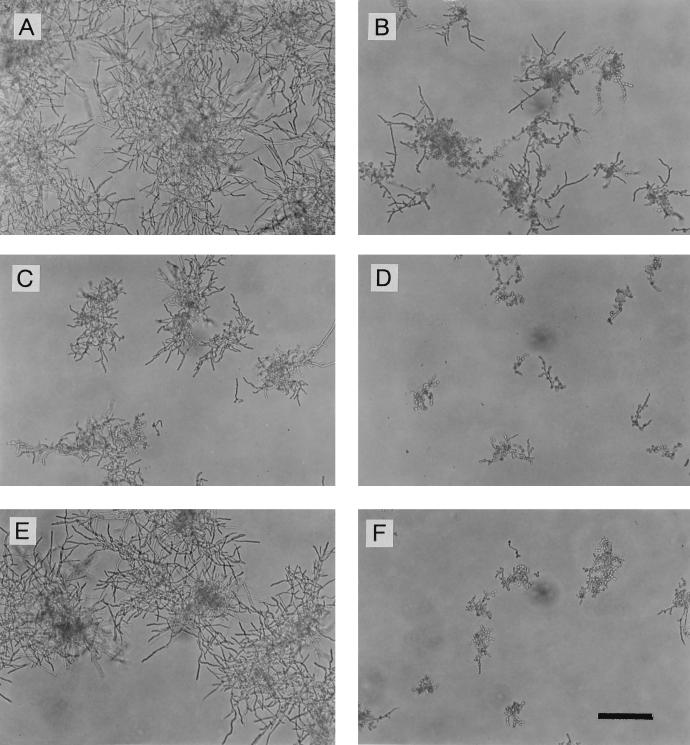
Inhibition of the growth of the hyphae of azole-resistant strain TIMM3317 by fluconazole or itraconazole was observed in the presence or absence of LF or LFcin B (Fig. 1). At higher concentrations of fluconazole and itraconazole, the isolate showed trailing growth, as indicated by disappearance of the endpoint. The addition of LF or LFcin B decreased the extent of trailing growth in the presence of fluconazole or itraconazole. In particular, LFcin B at 100 μg/ml with fluconazole or itraconazole completely inhibited hyphal growth, although the peptide alone had almost no effect. To examine the effect of the combination of LF or LFcin B with fluconazole, the growth of TIMM3317 treated with these agents was monitored microscopically (Fig. 2). After 15 h of incubation, the strain grown in drug-free medium showed substantial development of hyphae and yeast cells were not evident. In the presence of fluconazole at 1 μg/ml, some hyphae and yeast cells were observed. In the presence of LF at 200 μg/ml, fewer hyphae were seen. When exposed to the combination of fluconazole and LF, the strain developed significantly fewer hyphae than were observed in the presence of each drug, although some yeast cells were evident. When fluconazole and LFcin B together were added to the culture, a few yeast cells but almost no hyphae were observed, although LFcin B alone had little effect on the development of hyphae. These microscopic observations corresponded well to the results of the quantification of the hyphae obtained by the CV staining method (Fig. 1).
FIG. 1.
Inhibition of growth of C. albicans TIMM3317 hyphae by triazole antifungal agents in the presence of LF or LFcin B after 15 h of incubation. Fluconazole (A) and itraconazole (C) were tested in the absence (▪) or presence (□, 200 μg/ml; ▴, 800 μg/ml) of LF. Fluconazole (B) and itraconazole (D) were tested in the absence (▪) or presence (□, 25 μg/ml; ▴, 100 μg/ml) of LFcin B. The values are the means ± standard deviations for three determinations. OD550–630, optical density at 550 to 630 nm.
FIG. 2.
Phase-contrast micrographs of C. albicans TIMM3317 grown in the presence of fluconazole and/or LF-related compounds. (A) Control culture with no drug showing substantial development of hyphae and no yeast cells; (B) culture treated with fluconazole (1 μg/ml) showing some hyphae and yeast cells; (C) culture treated with LF (200 μg/ml) showing fewer hyphae; (D) culture treated with fluconazole (1 μg/ml) plus LF (200 μg/ml) showing significantly fewer hyphae and some yeast cells; (E) culture treated with LFcin B (25 μg/ml) showing unchanged development of hyphae; (F) culture treated with fluconazole (1 μg/ml) plus LFcin B (25 μg/ml) showing a few yeast cells and almost no hyphae. Bar, 100 μm.
Comparison of the effects of combinations against azole-susceptible and -resistant strains.
The effects of the combinations on the development of hyphae by azole-susceptible and -resistant strains were compared with respect to reduction of the MIC and the FIC index (Table 2). The MICs of fluconazole for the azole-susceptible strains ATCC 90028 and TIMM1768 did not decrease upon the addition of LF or LFcin B at concentrations of less than one-fourth the MIC, whereas for the azole-resistant strains TIMM3315 and TIMM3317, decreases in the MICs were evident (Tables 1 and 2). From examination of the FIC indices for both strains, it was interpreted that the combination of fluconazole and LF, as well as the combination of fluconazole and LFcin B, was synergistic (Table 2). With strain TIMM3164 growing in the yeast form, a reduction of the MIC of fluconazole was not evident after the addition of LF or LFcin B. In tests with itraconazole, a decrease in the MIC and a synergistic effect were observed when itraconazole was combined with LF or LFcin B in the case of strains TIMM3164, TIMM3315, and TIMM3317.
TABLE 2.
MIC and FIC index of fluconazole or itraconazole in combination with LF-related compounds as determined by the CV staining assay
| C. albicans strain | Fluconazole with the following:
|
Itraconazole with the following
|
||||||
|---|---|---|---|---|---|---|---|---|
| LF
|
LFcin B
|
LF
|
LFcin B
|
|||||
| MIC (μg/ml)a | FIC indexb | MIC (μg/ml) | FIC index | MIC (μg/ml) | FIC index | MIC (μg/ml) | FIC index | |
| Azole susceptible | ||||||||
| ATCC 90028 | 0.5 (800) | 2.06 (I) | 0.25 (25) | 1.06 (I) | 0.0063 (800) | 1.06 (I) | 0.0031 (25) | 0.56 (S) |
| TIMM1768 | 0.25 (800) | 1.13 (I) | 0.25 (25) | 1.06 (I) | 0.0063 (800) | 1.13 (I) | 0.0063 (25) | 1.06 (I) |
| Azole resistant | ||||||||
| TIMM3164c | 1 (200) | 1.13 (I) | 2 (25) | 2.06 (I) | 0.025 (200) | 0.63 (S) | 0.025 (25) | 0.56 (S) |
| TIMM3315 | 0.12 (25) | 0.13 (S) | 0.25 (6.3) | 0.13 (S) | ≤0.0016 (25) | 0.13 (S) | 0.025 (6.3) | 0.13 (S) |
| TIMM3317 | 0.25 (400) | 0.25 (S) | 0.25 (25) | 0.13 (S) | 0.0031 (400) | 0.25 (S) | 0.0063 (25) | 0.13 (S) |
LF or LFcin B was added at a concentration of less than its one-fourth its MIC, and the concentration (in micrograms per milliliter) is indicated in parentheses.
The FIC index was calculated as described in the text, and its interpretation is indicated in parentheses: S, synergy (<1); I, indifference (1 to 4).
The MIC for this strain was determined by measuring the increase in the optical density at 630 nm of the culture.
DISCUSSION
By examining hyphal growth, we have found that (i) azole-resistant strains of C. albicans are more susceptible to inhibition by LF and LFcin B than azole-susceptible strains and (ii) some azole-resistant strains are inhibited by fluconazole or itraconazole to a greater extent in the presence of relatively low concentrations of LF or LFcin B. These findings indicate that LF or LFcin B may play a valuable role in the inhibition of the mycelial form of azole-resistant C. albicans.
The finding that azole-resistant strains showed higher susceptibilities to LF or LFcin B than the azole-susceptible strains is not surprising. Fluconazole-resistant Candida glabrata strains lacking cytochrome P-450, an enzyme involved in ergosterol biosynthesis and the target of azole action, are highly susceptible to killing by H2O2 and human neutrophils (9). The absence of cytochrome P-450 activity and the resultant membrane sterol alterations may be associated with membrane perturbations in these azole-resistant strains. LF interacts with the cell surface causing extracellular leakage of proteins and the formation of surface blebs in Candida spp. (15), and LFcin B has been found to disrupt cell membrane functions in C. albicans (25). Thus, some azole-resistant Candida spp. which have alterations in their cell membranes appear to be more susceptible than other strains to nonspecific host defense factors such as active oxygen and LF-related compounds that target the cell membrane.
Azole resistance in C. albicans is correlated mainly with enhanced azole efflux mediated by multidrug efflux transporters such as Cdr1 energized by ATP and Benr energized by the proton motive force (4, 20). It has been demonstrated that LFcin B dissipates the proton gradient across the cell membrane of C. albicans (25) and inhibits uptake of glucose by Trichophyton rubrum (3), suggesting that it may reduce the levels of ATP production in fungi. Although these effects of LFcin B and those of LF may inhibit the activity of multidrug efflux transporters and thereby reduce the extent of azole resistance in the resistant strains, further studies will be necessary to clarify this possibility.
An effect of the combination of triazoles and LF-related compounds was not observed against the moderately azole-resistant strain TIMM3164, which is defective in the formation of hyphae. It is unknown whether the low level of susceptibility of TIMM3164 to these agents compared to those of the other strains examined is indicative of a difference in the mechanism of azole resistance or whether it results from a defect in the formation of hyphae. Upon testing of the azole-susceptible strains ATCC 90028 and TIMM1768, no effect of triazoles and LF-related compounds combined was evident, although an effect of such a combination was demonstrated for TIMM1768 in our previous study (24). This discrepancy may have been due to the different culture conditions and differences in the growth form of the organisms tested. In fact, the MIC of fluconazole for TIMM1768 was 0.25 μg/ml when the MIC was determined by quantification of hyphal growth in RP medium, whereas it was 4 to 16 μg/ml when the MIC was determined by measuring growth in Sabouraud glucose broth, in which yeast-form cells are dominant. LF-related compounds may enhance the susceptibility of Candida to azole agents under growth conditions that make the organisms less susceptible to these drugs.
Plasma LF is derived from neutrophils, and during microbial infections the levels of LF in plasma may increase 100-fold (up to 200 μg/ml) (7). LF is also present in mucus secretions at variable concentrations ranging from 5 μg/ml in saliva (10) to 1,000 μg/ml in cervical mucus (13). The presence of LF proteolysis products in bronchoalveolar lavage samples from patients with inflammatory lung diseases has been reported (5). LF derived from neutrophils or mucosal surfaces such as the cervix uteri and active fragments of LF may have the potential to augment the efficacy of azole antifungal chemotherapy and thereby inhibit colonization of the host by azole-resistant C. albicans. The LF concentration in oral mucus is low, and the oral cavity is the primary site of infection in the case of azole-resistant C. albicans (21). The therapeutic effects of LF-related compounds against experimental murine candidiasis due to azole-resistant C. albicans are now being assessed.
ACKNOWLEDGMENTS
We thank Katsuhisa Uchida for providing azole-resistant C. albicans and Kazunori Maebashi and Michinari Kudoh for useful discussions.
REFERENCES
- 1.Abe S, Satoh T, Tokuda Y, Tansho S, Yamaguchi H. A rapid colorimetric assay for determination of leukocyte-mediated inhibition of mycelial growth of Candida albicans. Microbiol Immunol. 1994;38:385–388. doi: 10.1111/j.1348-0421.1994.tb01795.x. [DOI] [PubMed] [Google Scholar]
- 2.Bellamy W, Takase M, Yamauchi K, Wakabayashi H, Kawase K, Tomita M. Identification of the bactericidal domain of lactoferrin. Biochim Biophys Acta. 1992;1121:130–136. doi: 10.1016/0167-4838(92)90346-f. [DOI] [PubMed] [Google Scholar]
- 3.Bellamy W, Yamauchi K, Wakabayashi H, Takase M, Takakura N, Shimamura S, Tomita M. Antifungal properties of lactoferricin B, a peptide derived from the N-terminal region of bovine lactoferrin. Lett Appl Microbiol. 1994;18:230–233. [Google Scholar]
- 4.Ben-Yaacov R, Knoller S, Caldwell G A, Becker J M, Koltin Y. Candida albicans gene encoding resistance to benomyl and methotrexate is a multidrug resistance gene. Antimicrob Agents Chemother. 1994;38:648–652. doi: 10.1128/aac.38.4.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britigan B E, Hayek M B, Doebbeling B N, Fick R B., Jr Transferrin and lactoferrin undergo proteolytic cleavage in the Pseudomonas aeruginosa-infected lungs of patients with cystic fibrosis. Infect Immun. 1993;61:5049–5055. doi: 10.1128/iai.61.12.5049-5055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eliopoulos G M, Moellering R C. Antimicrobial combinations. In: Lorian V, editor. Antibiotics in laboratory medicine. 3rd ed. Baltimore, Md: The Williams & Wilkins Co.; 1991. pp. 432–492. [Google Scholar]
- 7.Gutteberg T J, Haneberg B, Jorgensen T. The latency of serum acute proteins in meningococcal septicaemia, with special emphasis on lactoferrin. Clin Chim Acta. 1984;136:173–178. doi: 10.1016/0009-8981(84)90289-4. [DOI] [PubMed] [Google Scholar]
- 8.Hay, R. J. 1990. Overview of studies of fluconazole in oropharyngeal candidiasis. Rev. Infect. Dis. 12(Suppl. 3):334–337. [DOI] [PubMed]
- 9.Kan V L, Geber A, Bennett J E. Enhanced oxidative killing of azole-resistant Candida glabrata strains with ERG11 deletion. Antimicrob Agents Chemother. 1996;40:1717–1719. doi: 10.1128/aac.40.7.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenander-Lumikari M, Johansson I. Effect of saliva composition on growth of Candida albicans and Torulopsis glabrata. Oral Microbiol Immunol. 1995;10:233–240. doi: 10.1111/j.1399-302x.1995.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 11.Li Y M, Tan A X, Vlassara H. Antibacterial activity of lysozyme and lactoferrin is inhibited by binding of advanced glycation-modified proteins to a conserved motif. Nature Med. 1995;1:1057–1061. doi: 10.1038/nm1095-1057. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Suarez J V, Rodriguez-Tudela J L. In vitro activities of semisynthetic pneumocandin L-733,560 against fluconazole-resistant and -susceptible Candida albicans isolates. Antimicrob Agents Chemother. 1996;40:1277–1279. doi: 10.1128/aac.40.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masson P L, Heremans J F, Dive C H. An iron-binding protein common to many external secretions. Clin Chim Acta. 1966;14:735–739. [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Tentative standard M27-T. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1995. [Google Scholar]
- 15.Nikawa H, Samaranayake L P, Tenovuo J, Pang K M, Hamada T. The fungicidal effect of human lactoferrin on Candida albicans and Candida krusei. Arch Oral Biol. 1993;38:1057–1063. doi: 10.1016/0003-9969(93)90167-k. [DOI] [PubMed] [Google Scholar]
- 16.Odds F C. Candida and candidosis: a review and bibliography. 2nd ed. London, United Kingdom: Balliere Tindall; 1988. [Google Scholar]
- 17.Odds F C, Cockayne A, Hayward J, Abbott A B. Effects of imidazole- and triazole-derivative antifungal compounds on the growth and morphological development of Candida albicans hyphae. J Gen Microbiol. 1985;131:2581–2589. doi: 10.1099/00221287-131-10-2581. [DOI] [PubMed] [Google Scholar]
- 18.Odds F C, Schmid J, Soll D R. Epidemiology of Candida infections in AIDS. In: Vanden Bossche H, et al., editors. Mycoses in AIDS patients. New York, N.Y: Plenum Press; 1990. pp. 67–74. [Google Scholar]
- 19.Okutomi T, Abe S, Tansho S, Wakabayashi H, Kawase K, Yamaguchi H. Augmented inhibition of growth of Candida albicans by neutrophils in the presence of lactoferrin. FEMS Immunol Med Microbiol. 1997;18:105–112. doi: 10.1111/j.1574-695X.1997.tb01034.x. [DOI] [PubMed] [Google Scholar]
- 20.Prasad R, de Wergifosse P, Goffeau A, Balzi E. Molecular cloning and characterization of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr Genet. 1995;27:320–329. doi: 10.1007/BF00352101. [DOI] [PubMed] [Google Scholar]
- 21.Rex J H, Rinaldi M G, Pfaller M A. Resistance of Candida species to fluconazole. Antimicrob Agents Chemother. 1995;39:1–8. doi: 10.1128/aac.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruhnke M, Schmidt-Westhausen A, Trautmann M. In vitro activities of voriconazole (UK-109,496) against fluconazole-susceptible and -resistant Candida albicans isolates from oral cavities of patients with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1997;41:575–577. doi: 10.1128/aac.41.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valenti P, Visca P, Antonini G, Orsi N. Interaction between lactoferrin and ovotransferrin and Candida cells. FEMS Microbiol Lett. 1986;33:271–275. [Google Scholar]
- 24.Wakabayashi H, Abe S, Okutomi T, Tansho S, Kawase K, Yamaguchi H. Cooperative anti-Candida effects of lactoferrin or its peptides in combination with azole antifungal agents. Microbiol Immunol. 1996;40:821–825. doi: 10.1111/j.1348-0421.1996.tb01147.x. [DOI] [PubMed] [Google Scholar]
- 25.Wakabayashi H, Hiratani T, Uchida K, Yamaguchi H. Antifungal spectrum and fungicidal mechanism of an N-terminal peptide of bovine lactoferrin. J Infect Chemother. 1996;1:185–189. doi: 10.1007/BF02350646. [DOI] [PubMed] [Google Scholar]