Abstract
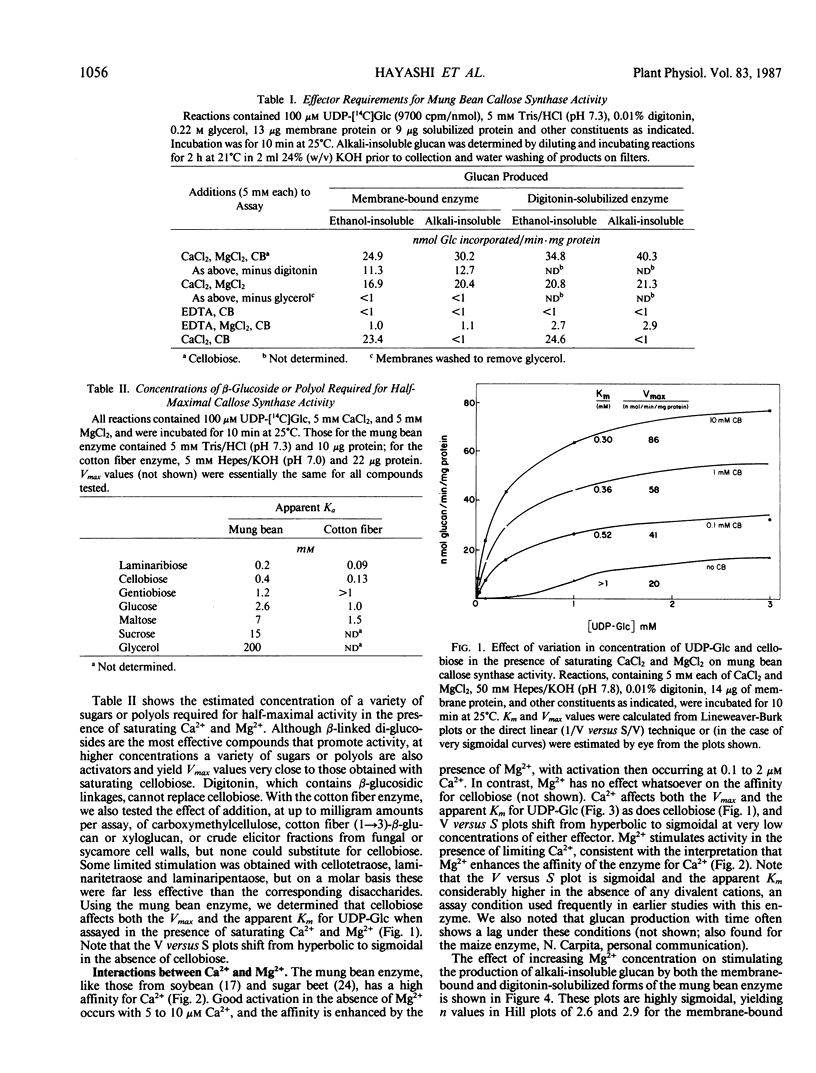
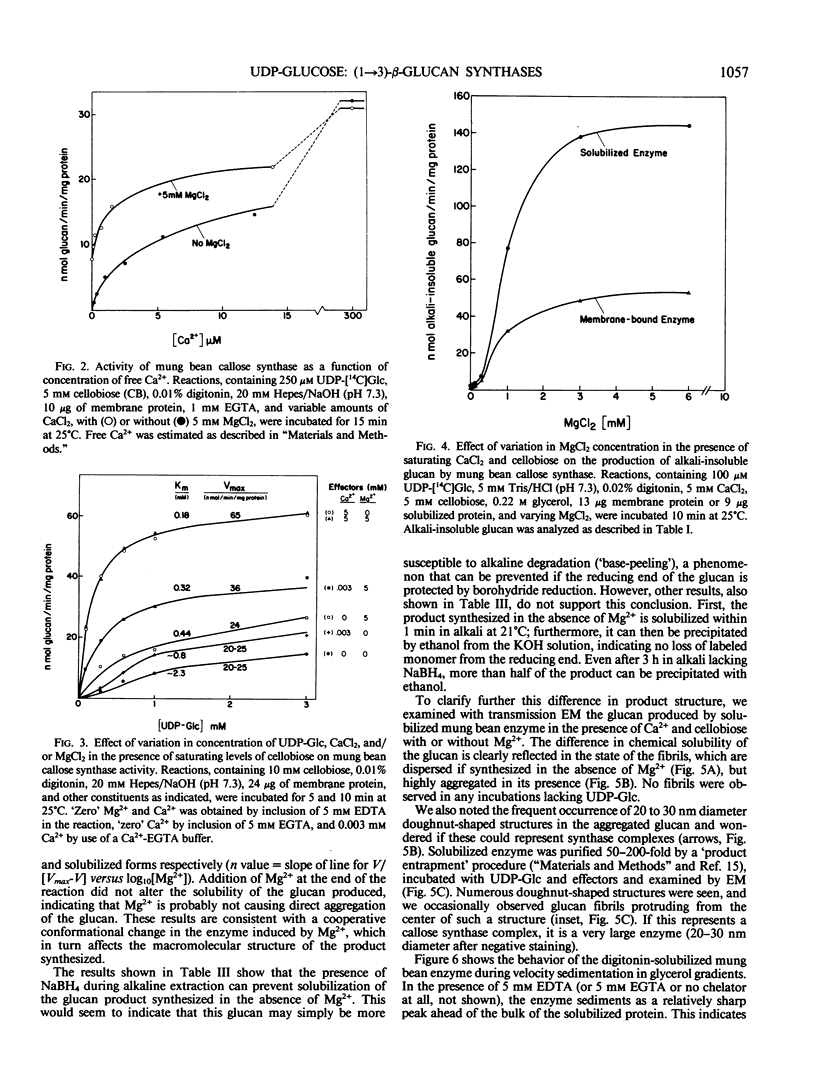
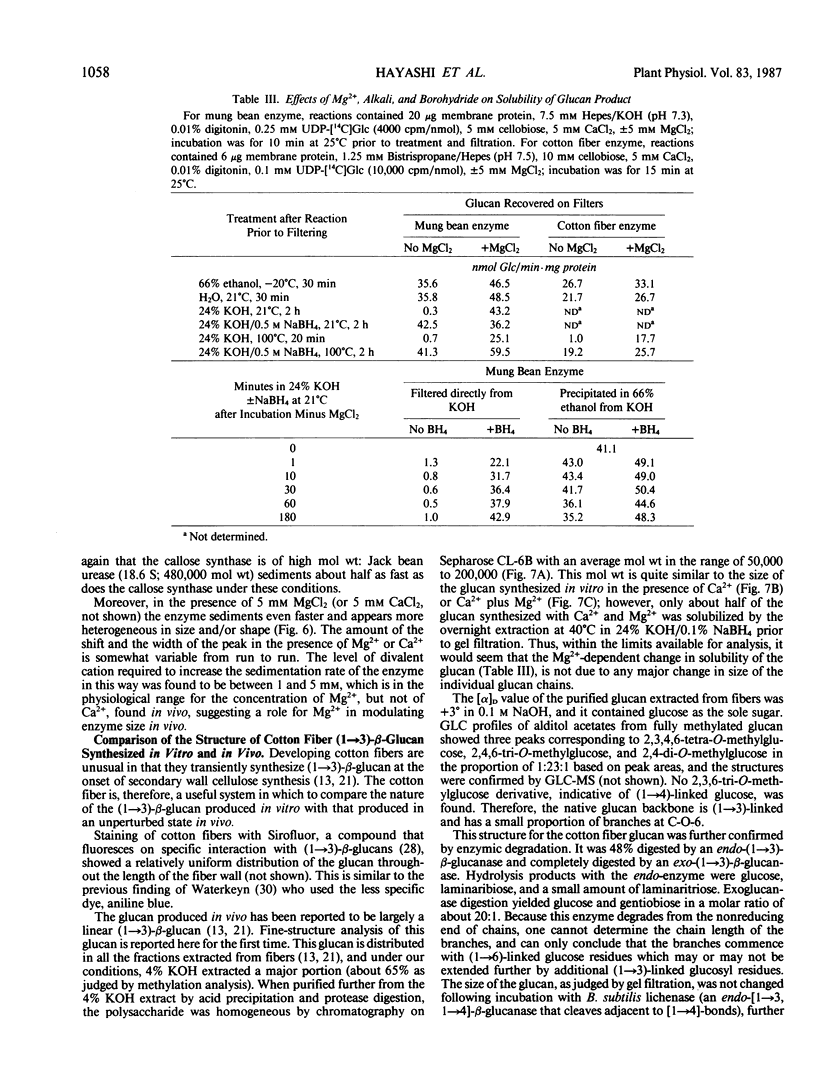
A re-examination of the kinetic properties of UDP-glucose: (1→3)-β-glucan (callose) synthases from mung bean seedlings (Vigna radiata) and cotton fibers (Gossypium hirsutum) shows that these enzymes have a complex interaction with UDP-glucose and various effectors. Stimulation of activity by micromolar concentrations of Ca2+ and millimolar concentrations of β-glucosides or other polyols is highest at low (<100 micromolar) UDP-glucose concentrations. These effectors act both by raising the Vmax of the enzyme, and by lowering the apparent Km for UDP-glucose from >1 millimolar to 0.2 to 0.3 millimolar. Mg2+ markedly enhances the affinity of the mung bean enzyme for Ca2+ but not for β-glucoside; with saturating Ca2+, Mg2+ only slightly stimulates further production of glucan. However, the presence of Mg2+ during synthesis, or NaBH4 treatment after synthesis, changes the nature of the product from dispersed, alkali-soluble fibrils to highly aggregated, alkali-insoluble fibrils. Callose synthesized in vitro by the Ca2+, β-glucoside-activated cotton fiber enzyme, with or without Mg2+, is very similar in size to callose isolated from cotton fibers, but is a linear (1→3)-β-glucan lacking the small amount of branches at C-0-6 found in vivo. We conclude that the high degree of aggregation of the fibrils synthesized with Mg2+in vitro is caused either by an alteration of the glucan at the reducing end or, indirectly, by an effect of Mg2+ on the conformation of the enzyme. Rate-zonal centrifugation of the solubilized mung bean callose synthase confirms that divalent cations can affect the size or conformation of this enzyme.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown R. M., Jr Cellulose microfibril assembly and orientation: recent developments. J Cell Sci Suppl. 1985;2:13–32. doi: 10.1242/jcs.1985.supplement_2.2. [DOI] [PubMed] [Google Scholar]
- Delmer D. P. Biosynthesis of cellulose. Adv Carbohydr Chem Biochem. 1983;41:105–153. doi: 10.1016/s0065-2318(08)60057-8. [DOI] [PubMed] [Google Scholar]
- Delmer D. P., Cooper G., Alexander D., Cooper J., Hayashi T., Nitsche C., Thelen M. New approaches to the study of cellulose biosynthesis. J Cell Sci Suppl. 1985;2:33–50. doi: 10.1242/jcs.1985.supplement_2.3. [DOI] [PubMed] [Google Scholar]
- Delmer D. P., Heiniger U., Kulow C. UDP-glucose: Glucan Synthetase in Developing Cotton Fibers: I. Kinetic and Physiological Properties. Plant Physiol. 1977 Apr;59(4):713–718. doi: 10.1104/pp.59.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P. J., Henry R. J., Blakeney A. B., Stone B. A. An improved procedure for the methylation analysis of oligosaccharides and polysaccharides. Carbohydr Res. 1984 Apr 2;127(1):59–73. doi: 10.1016/0008-6215(84)85106-x. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Maclachlan G. Pea xyloglucan and cellulose : I. Macromolecular organization. Plant Physiol. 1984 Jul;75(3):596–604. doi: 10.1104/pp.75.3.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry R. J., Stone B. A. Factors Influencing beta-Glucan Synthesis by Particulate Enzymes from Suspension-Cultured Lolium multiflorum Endosperm Cells. Plant Physiol. 1982 Mar;69(3):632–636. doi: 10.1104/pp.69.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S. R., Northcote D. H. In vitro glucan synthesis by membranes of celery petioles: the role of the membrane in determining the type of linkage formed. J Cell Sci Suppl. 1985;2:1–11. doi: 10.1242/jcs.1985.supplement_2.1. [DOI] [PubMed] [Google Scholar]
- Kang M. S., Elango N., Mattia E., Au-Young J., Robbins P. W., Cabib E. Isolation of chitin synthetase from Saccharomyces cerevisiae. Purification of an enzyme by entrapment in the reaction product. J Biol Chem. 1984 Dec 10;259(23):14966–14972. [PubMed] [Google Scholar]
- Kauss H. Callose biosynthesis as a Ca2+-regulated process and possible relations to the induction of other metabolic changes. J Cell Sci Suppl. 1985;2:89–103. doi: 10.1242/jcs.1985.supplement_2.5. [DOI] [PubMed] [Google Scholar]
- López-Romero E., Ruiz-Herrera J. Biosynthesis of beta-glucans by cell-free extracts from Saccharomyces cerevisiae. Biochim Biophys Acta. 1977 Dec 22;500(2):372–384. doi: 10.1016/0304-4165(77)90028-9. [DOI] [PubMed] [Google Scholar]
- Maltby D., Carpita N. C., Montezinos D., Kulow C., Delmer D. P. beta-1,3-Glucan in Developing Cotton Fibers: Structure, Localization, and Relationship of Synthesis to That of Secondary Wall Cellulose. Plant Physiol. 1979 Jun;63(6):1158–1164. doi: 10.1104/pp.63.6.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Montezinos D., Brown R. M., Jr Cell wall biogenesis in Oocystis: experimental alteration of microfibril assembly and orientation. Cytobios. 1978;23(90):119–139. [PubMed] [Google Scholar]
- Morrow D. L., Lucas W. J. (1-->3)-beta-d-Glucan Synthase from Sugar Beet : I. Isolation and Solubilization. Plant Physiol. 1986 May;81(1):171–176. doi: 10.1104/pp.81.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen M. P., Delmer D. P. Gel-Electrophoretic Separation, Detection, and Characterization of Plant and Bacterial UDP-Glucose Glucosyltransferases. Plant Physiol. 1986 Jul;81(3):913–918. doi: 10.1104/pp.81.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]