Key Clinical Message
The aortic chordae tendineae strands (ACTS) is a rare complication that can induce aortic regurgitation. Reported cases of ACTS are very few, and this is the first case reported in Iran. Patients with unexplained aortic regurgitation should be carefully evaluated for ACTS, which can be easily observed by TEE; a decision regarding aortic valve surgery should be made based on the severity of AR. Herein we reported A 64‐year‐old male was admitted to our hospital for dyspnea on exertion. In transthoracic Echocardiography a fibrous band‐like chordae in the aortic root attached to the noncoronary cusp of the aorta was seen, which caused retraction of the noncoronary cusp, mal‐coaptation of the aortic valves, and severe eccentric jet posterior directed aortic regurgitation. As a result of the ACTS, the patient was diagnosed with severe aortic regurgitation (AR); due to the symptomatic severe AR, the patient underwent aortic valve surgical replacement.
Keywords: abnormal fibrous strand, aortic chordae tendineae strands, aortic regurgitation, aortic valve, echocardiography
1. INTRODUCTION
The aortic chordae tendineae strands (ACTS), also known as “fibrous strands” in the literature, are believed to be embryonic remnants of the process of cusp formation. Rarely have ACTS on bicuspid and tricuspid aortic valves (AV) caused spontaneous aortic regurgitation (AR). 1 , 2 In this study, we present the case of a patient with tricuspid AV with normal thickening and ACTS. The patient had a smoking history and suffered from dyspnea and severe AR due to ACTS attached to a noncoronary cusp (NCC).
2. CASE REPORT
A 64‐year‐old male was admitted to Farshchian Cardiovascular Hospital for dyspnea on exertion (functional class II). The patient reported a smoking history but had no cardiovascular disease history. Physical examination revealed a systolic murmur III/VI and early to mid‐diastolic murmur in the right parasternal. The individuals' electrocardiogram and lung sounds were normal. No fever or chills were observed, and laboratory findings (complete blood count, serum inflammatory markers, and blood cultures samples) were within normal range. On the chest x‐ray, the trachea was observed in the midline, the cardiothoracic ratio was normal, the ascending aorta was dilated, and there were mild prominent bilateral hila and pulmonary vascular markings.
Coronary and Aortic Computed tomography angiography (CTA) was also performed and revealed patent coronary arteries and normal‐sized ascending aorta, root, and descending aorta without intimal flap and complex plaque. Furthermore, transthoracic (TTE) and transesophageal (TEE) echocardiography performed in our hospital indicated dilatation of the aortic root (diameter at the sinus of Valsalva: 44 mm) and ascending aorta (aortic annulus 24 mm, ascending aorta 40 mm), mild left ventricle enlargement (Left‐Ventricular‐End‐Systolic‐Volume:60CC/m2) with mild systolic dysfunction, (LVEF: 45%–50%), and normal right ventricle size and systolic function. No significant abnormality was observed on the mitral valve except for mild mitral regurgitation. Moreover, AV were tricuspid with normal thickening; however, a fibrous band‐like chordae in the aortic root attached to the NCC of the aorta caused retraction of the NCC, malcoaptation of the AV, and a severe eccentric jet posteriorly directed AR (Figure 1A, and supplementary file). Mild pulmonary arterial hypertension (systolic pulmonary pressure: 40 mmHg, tricuspid regurgitation peak gradient (TRPG) was 35 mmHg and right atrial pressure was 5 mmHg) was also detected.
FIGURE 1.
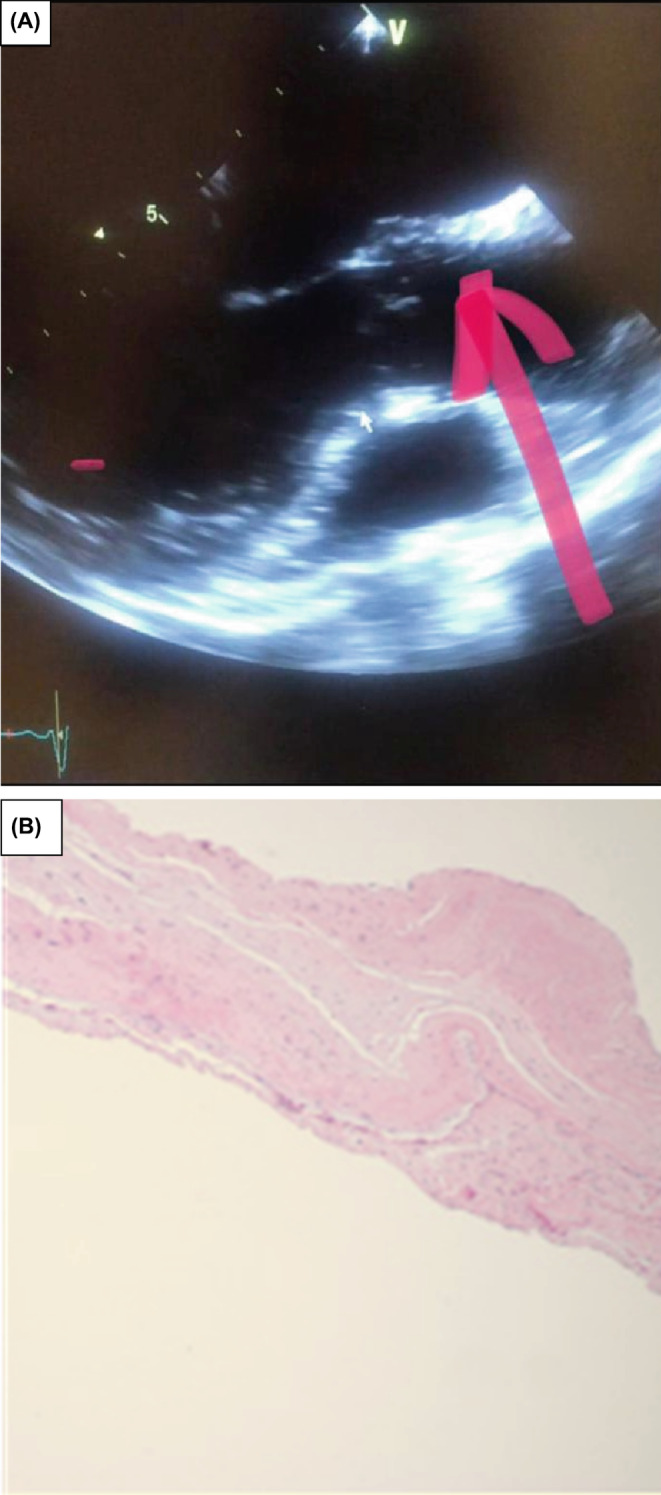
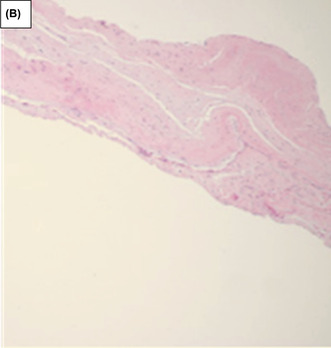
(A) ACTS (pink arrow) attached to the NCC. (B) Pathological examination showed fibrous tissue of AV.
Consequently, the patient was diagnosed with severe AR due to the ACTS, and AV surgical replacement was performed due to the symptomatic severe AR. The aortic annulus was normal, the left ventricle was enlarged, and the ACTS was attached to NCC during the intraoperative examination (Figure 1B), a mechanical valve was implanted and the patient was discharged in good condition after 4 days, with a prescription for warfarin.
3. DISCUSSION AND CONCLUSION
AR caused by the ACTS is rare; it was previously believed to be associated with bicuspid AV and infrequently with tricuspid AV 3 ; however, several reports have recently documented tricuspid AV cases. 4 Most ACTS in the right coronary cusps or NCC, although patterns of ACTS attachment varied in each case 5 ; however, most patients with ACTS have been from Japan and other Asian countries, indicating that heredity may play a role in the formation of ACTS. 4 ACTS was coupled to NCC in our patient.
If the ACTS efficiently maintains balance, no valve dysfunction with severe regurgitation will develop; however, two mechanisms have been proposed to cause AR in patients with ACTS: first, the ACTS rupture between the aortic cusp and aortic wall, and second, the ACTS' restricted closure of the AV. 1 , 6 Our case may be an example of the second mechanism's involvement; however, most reported cases are associated with the first mechanism.
Patients with unexplained AR should undergo a thorough evaluation for ACTS, which TEE more easily observes than TTE. 1 Additionally, three‐dimensional TEE plays a significant role in making a diagnosis. 7
In our patient both TTE and TEE echocardiograms were performed, and both revealed abnormal lines in the aortic valve. ACTS should be distinguished from Lambl's excrescences and infectious and noninfectious neoplasms when abnormal lines are detected at the AV.
Thin, filiform, or papillary structures called Lambl's excrescences can be found on the outer edges of valves in elderly individuals. These structures are more commonly seen on the MV near its closure line, rather than on the AV. Despite their presence, Lambl's excrescences rarely cause valve regurgitation, therefore this diagnosis is not supported by our case. 7
Infectious neoplasms are those caused by bacteria, fungi, or other microorganisms. Platelets, fibrin, red blood cells, white blood cells, and pathogens are the most prevalent pathological changes in the heart valve. Echocardiography facilitates the diagnosis by revealing rough‐edged and shaped growths on one or more leaflets. Given the patient's negative blood culture and normal CBC, the infectious neoplasm was not a suitable diagnosis. 8
Typically, patients with rheumatic endocarditis, antiphospholipid syndrome, extramedullary proliferative disease, and solid tumors have noninfectious neoplasms. Autoimmune diseases manifest as thickened valves or tendons, with or without valve dysfunction. However, the medical history of our patient and the normality of inflammatory factors did not support the diagnosis of a noninfectious neoplasm. 1
Most patients with severe AR will need aortic valve replacement (AVR). An aortic valve repair is only an option for patients whose anatomy is suitable (aortic dilation without a thickened, deformed, or calcified valve). AVR is, therefore, the primary treatment for AR caused by ACTS. 1
Only 19 cases of ACTS have been reported since 1984, 15 of which were in men and four in women. The average age of reported cases was 55.85 ± 17.58 years, and the majority of patients presented with dyspnea. TEE revealed a single bicuspid aortic valve; additionally, TCVS was connected to RCC in five cases, LCC in five cases, NCC in six cases, and all cusps in four cases. AR occurred in 10 patients due to TCVS rupture (Table 1).
TABLE 1.
Summary of case reports on ACTS.
| First Author(ref) | Year | Race | Age (years), sex | Type of AV | Location of ASTS | Raptured cusp | AR | Sign and symptom | Treatment (Type of valve) |
|---|---|---|---|---|---|---|---|---|---|
| R Hashimoto 9 | 1984 | Japanese | 10, male | Tricuspid | RCC | None | Not reported | Asymptomatic | AVR (Mechanical valve) |
| S Yavuz 10 | 1999 | Turk | 46, male | Tricuspid | NCC | None | Severe | Not reported | AVR (Mechanical valve) |
| M Nakajima 11 | 2002 | Japanese | 52, male | Tricuspid | all cusps | RCC | Severe | Not reported | AVR (Mechanical valve) |
| T Mishima 12 | 2010 | Japanese | 18, male | Bicuspid | all cusps | None | Severe | Fatigue | AVR (Mechanical valve) |
| H Minami 13 | 2011 | Japanese | 63, female | Tricuspid | NCC | RCC | Severe | Fatigue | AVR (Bioprosthetic valve) |
| K Akasaka 14 | 2012 | French | 74, male | Tricuspid | all cusps | None | Severe | Dyspnea | AVR (Bioprosthetic valve) |
| AA Bouchachi 2 | 2012 | Japanese | 56, female | Tricuspid | LCC | LCC | Severe | Not reported | AVR (Mechanical valve) |
| A Ishige 15 | 2012 | Japanese | 56, female | Tricuspid | LCC | LCC | Severe | Heart failure | AVR (Mechanical valve) |
| Y Irisawa 4 | 2014 | Japanese | 76, male | Tricuspid | all cusps | LCC | Severe | Dyspnea | AVR (Bioprosthetic valve) |
| I Esteve‐Ruiz 6 | 2015 | Spanish | 70, male | Tricuspid | LCC | None | Severe | Not reported | Not reported |
| MM Abdelaziz 16 | 2016 | Englishman | 66, male | Tricuspid | RCC | RCC | Severe | Not reported | CABG |
| S Ogawa 17 | 2016 | Japanese | 67, male | Tricuspid | NCC | NCC | Moderate | Chest and back pain | AVR (Mechanical valve) |
| S Matsukuma 18 | 2017 | Japanese | 60, male | Tricuspid | LCC | None | Severe | Chest pain | Aortic root remodeling |
| H Nishida 19 | 2018 | Japanese | 53, male | Tricuspid | RCC | RCC | Severe | Dyspnea | AVR (Mechanical valve) |
| D Geindreau 20 | 2018 | United Kingdom | 35, male | Not reported | NCC | None | Moderate | Not | Not reported |
| S Yuan 1 | 2021 | Chinese | 70, male | Tricuspid | RCC&NCC | RCC | Severe | Dyspnea and chest tightness | AVR (Bioprosthetic valve) |
| X Wei 21 | 2021 | Chinese | 56, female | Tricuspid | LCC | None | Severe | Dyspnea and chest pain | AVR |
| B Ophuis 22 | 2021 | Netherlands | 73, male | Tricuspid | RCC | RCC | Severe | Circulatory shock | AVR (Mechanical valve) |
| Our case | 2022 | Iran | 63, male | Tricuspid | NCC | None | Severe | Dyspnea | AVR (Mechanical valve) |
AUTHOR CONTRIBUTIONS
Nakisa Khansari: Conceptualization; data curation; formal analysis; investigation; methodology. Amir Mohammad Salehi: Conceptualization; formal analysis; funding acquisition; investigation; methodology.
FUNDING INFORMATION
This research received no specific funding from any public, commercial, or not‐for‐profit funding agency.
CONFLICT OF INTEREST STATEMENT
The author declares no conflict of interest.
ETHICS STATEMENT
This study was approved by the Ethics Committee of Hamadan University of Medical Science.
CONSENT
The patient has given his written permission for the publication of this report and the accompanying images. A manuscript of the informed consent form is available by contacting the corresponding author. This study was conducted in accordance with the Declaration of Helsinki.
Supporting information
Data S1: Patient’s echocardiography video
ACKNOWLEDGMENTS
Not Applicable.
Khansari N, Salehi AM. The extremely rare case of aortic chordae tendineae strands: A case report with literature review. Clin Case Rep. 2023;11:e8033. doi: 10.1002/ccr3.8033
Contributor Information
Nakisa Khansari, Email: n_kh_80@yahoo.com.
Amir Mohammad Salehi, Email: amirchsalehi19171917@gmail.com.
DATA AVAILABILITY STATEMENT
Access to data is permitted with the author's permission.
REFERENCES
- 1. Yuan S, Mou R, Sun X, Mou Y. Aortic chordae tendineae strands with significant aortic regurgitation a case report and review of the literature. Int Heart J. 2021;62(5):1160‐1163. [DOI] [PubMed] [Google Scholar]
- 2. Bouchachi A‐A, Folliguet T, Hébert J‐L, et al. A severe restrictive aortic regurgitation resulting from valve tenting by unusual aortic chordae tendineae strands. Circulation. 2012;126(10):e139‐e141. [DOI] [PubMed] [Google Scholar]
- 3. Misawa Y, Hasegawa T, Oyama H, Sudo H, Hasegawa N, Kamisawa O. Congenital bicuspid aortic valve with regurgitation—a rare case showing a fibrous band between the conjoined cusp and the ascending aorta. Nihon Kyobu Geka Gakkai. 1993;41(10):2156‐2159. [PubMed] [Google Scholar]
- 4. Irisawa Y, Itatani K, Kitamura T, et al. Aortic regurgitation due to fibrous strand rupture in the fenestrated left coronary cusp of the tricuspid aortic valve. Int Heart J. 2014;55(6):550‐551. [DOI] [PubMed] [Google Scholar]
- 5. Akiyama K, Hirota J, Taniyasu N, Maisawa K, Kobayashi Y, Tsuda M. Pathogenetic significance of myxomatous degeneration in fenestration‐related massive aortic regurgitation. Circ J. 2004;68(5):439‐443. [DOI] [PubMed] [Google Scholar]
- 6. Esteve‐Ruiz I, López‐Pardo F, Lagos‐Degrande Ó, López‐Haldón JE, Urbano‐Moral JÁ. Severe restrictive aortic regurgitation due to aortic fibrous strand. Eur Heart J Cardio Imag. 2015;16(5):465. [DOI] [PubMed] [Google Scholar]
- 7. Chu A, Aung TT, Sahalon H, Choksi V, Feiz H. Lambl's excrescence associated with cryptogenic stroke: a case report and literature review. Am J Case Rep. 2015;16:876‐881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435‐1486. [DOI] [PubMed] [Google Scholar]
- 9. Hashimoto R, Miyamura H, Eguchi S. Congenital aortic regurgitation in a child with a tricuspid non‐stenotic aortic valve. Heart. 1984;51(3):358‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yavuz S, Türk T, Celkan MA, Koca V, Ata Y, Ozdemir IA. Congenital aortic insufficiency due to aortic cusp stretching:'kite anomaly'. J Heart Valve Dis. 1999;8(3):284‐286. [PubMed] [Google Scholar]
- 11. Nakajima M, Tsuchiya K, Naito Y, Hibino N, Inoue H. Aortic regurgitation caused by rupture of a well‐balanced fibrous strand suspending a degenerative tricuspid aortic valve. J Thorac Cardiovasc Surg. 2002;124(4):843‐844. [DOI] [PubMed] [Google Scholar]
- 12. Mishima T, Yamamoto K, Sugimoto T, Sakakibara K, Uehara A, Yoshii S. Severe aortic regurgitation resulting from a downward displacement of anterior aortic annulus and fibrous strands in the bicuspid aortic valve. Ann Thorac Cardiovasc Surg. 2010;16(1):57‐59. [PubMed] [Google Scholar]
- 13. Minami H, Asada T, Gan K, Yamada A, Sato M. Aortic regurgitation caused by rupture of the abnormal fibrous band between the aortic valve and aortic wall. Gen Thorac Cardiovasc Surg. 2011;59(7):488‐490. [DOI] [PubMed] [Google Scholar]
- 14. Akasaka K, Saito E, Higuchi T, et al. Aortic regurgitation caused by fibrous strand rupture in a fenestrated aortic valve. J Echocardiography. 2012;10(4):151‐153. [DOI] [PubMed] [Google Scholar]
- 15. Ishige A, Uejima T, Kanmatsuse K, Endo M. Giant fenestration and fibrous strand rupture of aortic valve without massive regurgitation. J Cardiol Cases. 2012;5(3):e163‐e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abdelaziz MM, Martinelli G, Luckraz H. Chordae tendineae of the aortic valve. J Card Surg. 2016;31(5):328‐329. [DOI] [PubMed] [Google Scholar]
- 17. Ogawa S, Sawada K, Goto Y, Koyama Y, Okawa Y. Abnormal mobile structure in the aortic valve due to fibrous strand rupture. Asian Cardiovasc Thora Ann. 2017;25(4):304‐306. [DOI] [PubMed] [Google Scholar]
- 18. Matsukuma S, Inoue T, Tanigawa K, et al. Valve‐sparing aortic root re‐implantation for commissural detachment with fibrous strand. Gen Thorac Cardiovasc Surg. 2018;66(1):54‐56. [DOI] [PubMed] [Google Scholar]
- 19. Nishida H, Suenaga E, Ishii K. Acute aortic regurgitation following fibrous strand rupture of aortic valve successfully diagnosed by transesophageal echocardiography. Echocardiography. 2018;35(5):753‐754. [DOI] [PubMed] [Google Scholar]
- 20. Geindreau D, Pabari P, Cole G, Anderson J, Gopalan D, Ariff B. Aortic valve chordae tendineae causing aortic insufficiency and mimicking an aortic root dissection flap. J Cardiovasc Comput Tomogr. 2018;12(4):e11‐e12. [DOI] [PubMed] [Google Scholar]
- 21. Wei X, Zhou W, Feng Y, Chen M. Special aortic chordae tendineae Strand causing severe aortic regurgitation treated by transcatheter aortic valve replacement. Cardiovasc Interv. 2021;14(19):e267‐e269. [DOI] [PubMed] [Google Scholar]
- 22. Ophuis B, Lexis CP, Wieringa WG, et al. A rare cause of cardiogenic shock: a case report of aortic regurgitation due to rupture of a fibrous Strand suspending a tricuspid aortic valve. CASE. 2021;5(5):335‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: Patient’s echocardiography video
Data Availability Statement
Access to data is permitted with the author's permission.


