Abstract
Zidovudine (3′-azido-3′-deoxythymidine [AZT]), an antiviral nucleoside analog effective in the treatment of human immunodeficiency virus infection, is primarily metabolized to an inactive glucuronide form, GAZT, via uridine-5′-diphospho-glucuronosyltransferase (UGT) enzymes. UGT enzymes exist as different isoforms, each exhibiting substrate specificity. Published clinical studies have shown that atovaquone, fluconazole, methadone, and valproic acid decreased GAZT formation, presumably due to UGT inhibition. The effect of these drugs on AZT glucuronidation was assessed in vitro by using human hepatic microsomes to begin understanding in vitro-in vivo correlations for UGT metabolism. The concentrations of each drug studied were equal to those reported with the usual clinical doses and at concentrations at least 10 times higher than would be expected with these doses. High-performance liquid chromatography was used to assess the respective metabolism and formation of AZT and GAZT. All four drugs exhibited concentration-dependent inhibition of AZT glucuronidation. The respective concentrations of atovaquone and methadone which caused 50% inhibition of GAZT were >100 and 8 μg/ml, well above their usual clinical concentrations. Fluconazole and valproic acid exhibited 50% inhibition of GAZT at 50 and 100 μg/ml, which are within the clinical ranges of 10 to 100 and 50 to 100 μg/ml, respectively. These data suggest that inhibition of AZT glucuronidation may be more clinically significant with concomitant fluconazole and valproic acid. Factors such as inter- and intraindividual pharmacokinetic variability and changes in AZT intracellular concentrations should be considered as other mechanisms responsible for changes in AZT pharmacokinetics with concomitant therapies.
Two major enzyme groups responsible for the metabolism of both endogenous and exogenous compounds to more water-soluble conjugates are the cytochrome P-450 (CYP) and the uridine 5′-diphospho-glucuronosyltransferase (UGT) families. Of these, the CYP enzymes have been the most extensively studied. These enzymes are known to exist as several different isoforms. Research over the last several years has yielded valuable information on the use of metabolism data from in vitro experiments to predict in vivo metabolism (14, 16). In fact, CYP in vitro data have been shown to be so predictive that they minimize the need for in vivo data to characterize drug metabolism, modify drug doses, and predict some drug interactions. Furthermore, these data have played an integral role in more rational study designs and drug development for new therapeutic entities (3, 37).
Glucuronidation via UGT to more water-soluble glucuronide forms is another major metabolic pathway for a large number of endogenous and exogenous compounds. As has been demonstrated with CYP enzymes, UGT also has been found to exist in several isoforms, with each isoform exhibiting substrate specificity for metabolism. However, the full characterization of UGT enzymes has yet to occur (4, 5, 11). Specifically, there are no published data which correlate glucuronidation data in vitro with findings in vivo.
This investigation was undertaken to begin to assess the relationship between glucuronidation in vitro and human metabolism in vivo for zidovudine (3′-azido-3′-deoxythymidine [AZT]), a reverse transcriptase inhibitor effective against human immunodeficiency virus (HIV). AZT has been shown to prolong survival, decrease the incidence of opportunistic infections, and increase the quality of life in HIV-infected patients and is effective in preventing perinatal transmission when used in HIV-infected pregnant mothers pre- and peripartum (7, 13). AZT is used in combination with several other drugs in the medical management of HIV-infected patients. However, it has a narrow therapeutic index, with dose-limiting bone marrow toxicities being the most common toxicities reported. Higher concentrations of AZT are associated with higher frequency and greater severity of bone marrow suppression (26).
AZT is primarily metabolized to its inactive glucuronide, GAZT, via UGT, although the specific isoform responsible for this reaction has yet to be determined (4). There are data from numerous studies in vitro and in vivo where the inhibition of AZT glucuronidation has been reported, but there are no data which directly correlate these in vitro data with data obtained from in vivo studies (12, 15, 28–31, 33, 35).
Atovaquone, fluconazole, methadone, and valproic acid are drugs often used in combination with AZT in the medical management of patients infected with HIV. Atovaquone and fluconazole are therapies used in the treatment or prevention of Pneumocystis carinii pneumonia and fungal infections, respectively (10, 34). Methadone may be used chronically as part of a maintenance program for HIV-infected patients with a past history of drug abuse (8). Valproic acid is an anticonvulsant drug which may be used in HIV-infected patients with central nervous system complications (6). Drug interaction studies in vivo have reported that these drugs alter AZT pharmacokinetics via inhibition of formation of the glucuronide metabolite of this compound, GAZT, with subsequent increases in the concentrations of the parent compound, AZT, in serum (18, 23, 24, 32).
We report the results of this in vitro investigation, the purpose of which was to determine if atovaquone, fluconazole, methadone, and valproic acid inhibited AZT glucuronidation in human hepatic microsomes. We correlated these in vitro findings with the published in vivo data to begin to develop a more complete understanding of both the mechanism behind the observed clinical data and the usefulness of metabolic data in vitro to serve as a predictor for pharmacokinetic interactions in vivo.
MATERIALS AND METHODS
Materials.
Atovaquone and fluconazole were obtained from reference stocks of the Food and Drug Administration. All other compounds were obtained from Sigma Chemical Company (St. Louis, Mo.). Human liver samples, medically unsuitable for transplantation, were obtained from the Washington Regional Transplant Consortium (Washington, D.C.). Human liver samples were obtained and immediately sectioned and stored at −70°C. Microsomes were prepared by differential centrifugation as previously described and stored at −70°C until used (17).
Glucuronidation of AZT by human liver microsomes.
Metabolic time curves were performed to determine the optimal incubation time and microsomal protein content and to assess AZT glucuronidation in buffer solution in the presence and absence of bovine serum albumin (BSA). GAZT was not produced in the absence of uridine-5′-diphosphoglucuronic acid (UDPGA). All incubations were of 0.5- to 1-ml mixtures which contained 20 μM AZT, 1 mM UDPGA, 2.25% BSA in 5 mM MgCl2, 0.1 M NaPO4, 1 mM EDTA (pH 7.4), and 1 mg of protein per ml from a mixture of liver microsomes from three human donors and the individual inhibitors. The inhibitors and the concentrations used included atovaquone at 40 and 400 μg/ml; fluconazole at 10 and 100 μg/ml; ketoprofen at 0.5 mM; methadone at 0.1, 1.0, 10, and 100 μg/ml; miconazole at 0.5 mM; probenecid at 0.5 mM; and valproic acid at 100 and 1,000 μg/ml. Atovaquone was dissolved in 0.1 N NaOH, and an aliquot was added to each sample, which contained a molar equivalent of HCl to neutralize any effect of the NaOH. Fluconazole was dissolved in 0.01 M HCl. Miconazole was dissolved in ethanol, 100 μl was added to the sample tube, and the ethanol was evaporated before any other additions were made to the sample tube. Probenecid was dissolved in 3% NaHCO3, and 25 μl was added to the sample tube. There were no vehicle effects from the solvents used to dissolve the test compounds. All other compounds were initially dissolved in water. AZT samples were incubated for 60 min in a 37°C shaking water bath. Each 1-ml reaction was stopped with an equal volume of acetonitrile, and an additional 1 ml of acetonitrile was added to a 200-μl aliquot of this mixture. The samples were then centrifuged at 14,000 × g for 3 min, and the resultant supernatants were dried under vacuum and used for analysis of AZT and GAZT concentrations. All experiments were run in triplicate, and the results were confirmed by running each set of experiments on 2 separate days.
Analysis of AZT and GAZT.
AZT and GAZT were analyzed by high-performance liquid chromatography. The dried sample extract was reconstituted with 100 μl of the mobile phase consisting of 9% acetonitrile, 0.1% trifluoroacetic acid, 0.15% triethylamine pH 2 to 3. The separation of AZT and GAZT was accomplished under isocratic conditions by using a Zorbax 300SB C8 column (4.6 by 250 mm) (Mac-Mod, Intl., Chadds Fords, Pa.), with a similar guard column, at a flow rate of 1 ml/min. Under these conditions, the retention times for GAZT and AZT were 7.3 and 9.3 min, respectively. Confirmation of GAZT was made with a reference standard, and spectral confirmation was done with a diode array detection system. The percentage of AZT metabolism was determined from each sample by dividing the area of the GAZT peak by the sum of the areas of the AZT and GAZT peaks. The coefficient of variation for the assay was 6%, and the limit of sensitivity was 0.4 μM.
RESULTS
Glucuronidation of AZT by human liver microsomes.
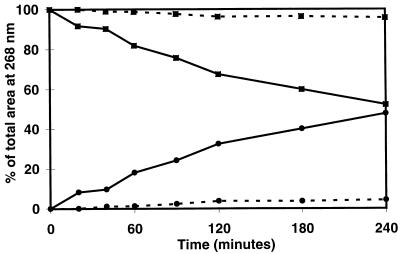
The rate of AZT glucuronidation by human liver microsomes was substantially increased by the addition of 2.25% BSA (Fig. 1). Under these conditions, AZT was readily metabolized for up to 240 min when human liver microsomes containing 1 mg of microsomal protein per ml were used. For all subsequent reactions, we chose to incubate all of our reaction mixtures for 60 min, since the reaction rate remained linear over that time period, and we observed approximately 20% conversion of AZT to its glucuronide metabolite at that time point.
FIG. 1.
Rate of AZT (▪) glucuronidation to its metabolite, GAZT (•), in the presence (solid lines) and absence (dashed lines) of BSA after 60 min of incubation.
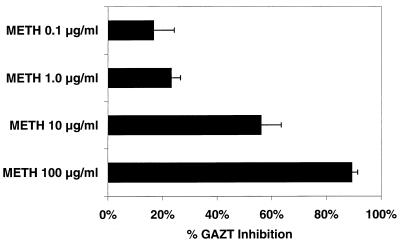
The effects of methadone on the glucuronidation of AZT are shown in Fig. 2. Methadone inhibited the glucuronidation of AZT in a concentration-dependent manner, as determined by the percentage of GAZT inhibition compared to that in control reactions. Methadone at 0.1 μg/ml showed 17% ± 8% GAZT inhibition, while methadone at 100 μg/ml showed almost total inhibition of the metabolism of AZT to its glucuronide metabolite.
FIG. 2.
Effects of increasing concentrations of methadone (METH) on the glucuronidation of AZT after 60 min of incubation as determined by the percentage of GAZT inhibition compared to that in controls (mean ± standard deviation).
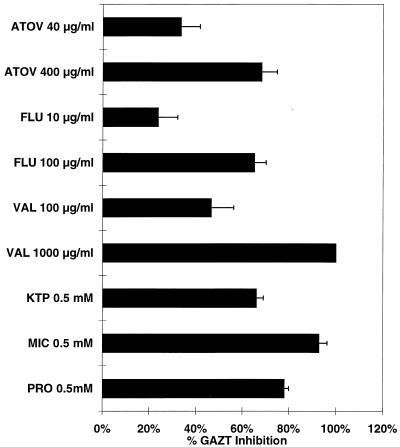
Figure 3 shows the effects of the other inhibitors used in this study on AZT glucuronidation, determined in a manner similar to that described above with the methadone incubations. The three control compounds, ketoprofen, miconazole, and probenecid, all inhibited the glucuronidation of AZT. These three compounds are known to inhibit AZT glucuronidation in vitro (12, 25, 31). Probenecid has been shown to inhibit AZT glucuronidation in vivo, although it is not frequently used in the treatment of patients with HIV infection (22). Atovaquone, fluconazole, and valproic acid inhibited AZT glucuronidation in a concentration-dependent fashion. Approximately 30% inhibition of AZT glucuronidation was seen with the lower concentrations of atovaquone and fluconazole, whereas the higher concentrations of both of these inhibitors produced 70% inhibition of AZT glucuronidation. Valproic acid at 100 μg/ml produced 50% inhibition of AZT glucuronidation, while complete inhibition was seen when a concentration of 1,000 μg of valproic acid per ml was used.
FIG. 3.
Effects of other inhibitors on glucuronidation of AZT after 60 min of incubation as determined by the percentage of GAZT inhibition compared to that in controls (mean ± standard deviation). ATOV, atovaquone; FLU, fluconazole; VAL, valproic acid; KTP, ketoprofen; MIC, miconazole; PRO, probenicid.
DISCUSSION
The characterization and understanding of the uridine-5′-glucuronosyltransferase enzyme family has become a more recent focus of metabolism research. The UGT enzymes have been divided into two distinct groups, the UGT1 and UGT2 families, based on different amino acid sequences. These two families are further divided into several different isoforms, based on gene sequencing and cDNA cloning techniques. Like the CYP enzymes, the UGT isoenzymes exhibit substrate specificity. For example, UGT1*1 is known to be the major isoform that metabolizes bilirubin, while many of the isoforms of the UGT2 family metabolize both endogenous and exogenous steroid molecules. There are many isoforms of UGT that are yet to be identified; in fact, the isoform which is responsible for the glucuronidation of AZT has not yet been elucidated (4, 5, 11).
It has been established that the binding site for UGT is on the luminal side of the endoplasmic reticulum in the microsomal preparation; UGT is latent until the endoplasmic reticulum membrane is disrupted to expose the enzyme’s active site. Compounds which disrupt these membranes increase the enzymatic activity of UGT up to 20-fold (5, 11). It is also known that these compounds may act to solubilize UGT, rendering the enzyme more active (5). In our experiments, the rate of AZT metabolism to its glucuronide metabolite was substantially increased when BSA was included in the incubation mixtures; there was a 15-fold increase in the amount of GAZT formed after 60 min of incubation (Fig. 1). Fenoldopam glucuronidation showed a similar effect with BSA (21). We believe that BSA was necessary for these experiments, by acting to disrupt the membrane of the endoplasmic reticulum. This, then, allows UGT’s active site(s) to be available to catalyze the glucuronidation reaction.
Atovaquone’s effect on zidovudine metabolism was evaluated in a clinical drug interaction study by Lee and colleagues (23). They reported a 33% increase in the area under the concentration-time curve (AUC) for AZT, with a corresponding decrease in the AUC of GAZT of 6% at atovaquone concentrations of 17 μg/ml. However, the true effects of atovaquone on AZT pharmacokinetics are difficult to assess in this clinical study because of the large standard deviations reported for the AZT and GAZT data (23). The atovaquone steady-state maximum concentration in plasma was reported to be 24 μg/ml when an atovaquone suspension of 750 mg two times daily was given to five HIV-infected volunteers (9). In our in vitro experiments, atovaquone concentrations of 40 and 400 μg/ml caused decreases in GAZT formation of 33% ± 8% and 68 ± 7% (mean ± standard deviation), respectively. Our results show that atovaquone at concentrations of ≥40 μg/ml inhibits AZT glucuronidation. The lower concentrations of this drug in plasma seen in patients lessen the clinical relevance of this inhibition in vivo.
Fluconazole levels in plasma at conventional doses of 200 mg have been reported to be 10 μg/ml, or 100 μg/ml when fluconazole was administered at doses of 2 g daily (1, 36). Our study showed concentration-dependent inhibition of AZT glucuronidation at these concentrations of 24% ± 8% and 65% ± 5%, respectively. Sahai et al. (32) reported an increase in both AZT maximum concentration in plasma and AUC, with a corresponding decrease in the oral clearance of this drug in 12 HIV-infected men receiving 400 mg of fluconazole daily. The average peak concentration of fluconazole in plasma in these subjects was 23.8 μg/ml. The authors hypothesized that fluconazole was directly inhibiting the glucuronidation of AZT; our in vitro data support this hypothesis as a likely mechanism to explain these clinical observations.
Methadone is a narcotic analgesic used for pain relief as well as in patients enrolled in maintenance programs for purposes of drug rehabilitation. Methadone concentrations in plasma in patients range from the low value of 0.03 μg/ml for pain relief up to 0.6 μg/ml in patients enrolled in methadone maintenance programs (2, 19, 20). In a more recent study of the pharmacokinetics of oral and intravenous AZT in patients enrolled in a methadone maintenance program, Jatlow and colleagues found a 30% decrease in GAZT formation with methadone therapy; methadone concentrations ranged from 0.19 to 0.38 μg/ml (18). Our in vitro data are consistent with these clinical results. As shown in Fig. 2, at concentrations of 0.1 and 1 μg/ml, methadone exerted approximately a 20% inhibition of the conversion of AZT to its glucuronide metabolite. The quantitative differences between these in vitro and in vivo data are due, perhaps, to our incomplete understanding of the contribution of all factors, including drug concentration, on these findings. However, in this case, dose-limiting methadone toxicities make the inhibition of AZT glucuronidation at higher methadone concentrations (i.e., >1 μg/ml) irrelevant from a clinical perspective.
Valproic acid is an anticonvulsant whose levels in plasma are titrated to concentrations of between 50 and 100 μg/ml, although lower or higher concentrations can be used, based on the success of seizure control (6). Lertora et al. (24) reported inhibition of AZT glucuronidation measured by a 50% decrease in urinary excretion of GAZT in six HIV-infected patients receiving valproic acid at doses of 250 mg every 8 h in a concentration-dependent manner. Increases in the AUC for AZT in plasma were found to be linearly correlated to trough concentrations of valproic acid in plasma of between 32 and 68 μg/ml (24). Our in vitro data show 47% ± 10% GAZT inhibition at a concentration of 100 μg/ml and complete inhibition of this reaction at a concentration of 1,000 μg/ml.
We chose microsomal drug concentrations in vitro equal to concentrations of these drugs in plasma reported when each is used clinically, as well as concentrations 10-fold higher. In addition, for methadone, we evaluated concentrations 100 and 1,000 times above the usual clinical concentrations. It is not known, however, if microsomal concentrations are higher than, lower than, or similar to plasma drug concentrations. At concentrations equal to the usual clinical plasma drug concentrations, all four drugs tested proved to be inhibitors of AZT glucuronidation to some degree, with the inhibition being greater at the higher concentrations studied.
However, it is not clear how to interpret the results obtained from the experiments both in vitro and in vivo with respect to their clinical relevance for patients receiving AZT and these other therapies. Table 1 shows the comparison of the usual clinical concentrations of these drugs in plasma compared to the concentration in vitro which caused 50% inhibition of GAZT formation. The clinical concentrations achieved with atovaquone and methadone compared to the concentrations in vitro needed for 50% inhibition of GAZT formation make it less likely that UGT inhibition by these two drugs would be relevant at the usual clinical plasma drug concentrations, whereas it may be more significant with fluconazole and valproic acid. Furthermore, as shown in Fig. 2 and 3, complete inhibition of AZT glucuronidation in vitro occurred only with concentrations of the inhibiting drugs well above the plasma drug concentrations observed with the usual clinical doses of these drugs (1, 2, 6, 9, 19, 20, 36). None of the in vivo interaction studies included any pharmacodynamic assessments to accompany the pharmacokinetic results (18, 23, 24, 32). Finally, there is significant inter- and intrapatient variability in the pharmacokinetics of AZT (27). The variability in the AZT concentrations reported in the clinical studies could, to some degree, be due to the inherent pharmacokinetic variability of the drug. Therefore, while inhibition of glucuronidation may indeed result in higher AZT concentrations in vivo, it is important to remember all of the factors that in toto, could affect the clinical and toxicity profiles observed in patients receiving AZT therapy.
TABLE 1.
Clinical concentrations of atovaquone, fluconazole, methadone, and valproic acid versus in vitro concentration causing 50% inhibition of GAZT formationa
| Drug | In vitro concn causing 50% inhibition of GAZT formation (μg/ml) | Clinical concn (μg/ml) | References |
|---|---|---|---|
| Atovaquone | >100 | ≥10 | 9, 23, 34 |
| Fluconazole | 50 | 10–100 | 1, 10, 32, 36 |
| Methadone | 8 | <0.6 | 2, 8, 18–20 |
| Valproic Acid | 100 | 50–100 | 6, 24 |
Compared with that in controls.
REFERENCES
- 1.Anaissie E J, Kontoyiannia D P, Huls C, Vartivarian S E, Karl C, Prince R A, Bosso J, Bodey G P. Safety, plasma concentrations, and efficacy of high-dose fluconazole in invasive mold infections. J Infect Dis. 1995;172:599–602. doi: 10.1093/infdis/172.2.599. [DOI] [PubMed] [Google Scholar]
- 2.Bell J, Bowron P, Lewis J, Batey R. Serum levels of methadone in maintenance clients who persist in illicit drug use. Br J Addict. 1990;85:1599–1602. doi: 10.1111/j.1360-0443.1990.tb01648.x. [DOI] [PubMed] [Google Scholar]
- 3.Bertz R J, Granneman G R. Use of in vitro and in vivo data to estimate the likelihood of metabolic pharmacokinetic interactions. Clin Pharmacokinet. 1997;32:210–258. doi: 10.2165/00003088-199732030-00004. [DOI] [PubMed] [Google Scholar]
- 4.Burchell B, Brierley C H, Rance D. Specificity of human UDP-glucuronosyltransferases and xenobiotic glucuronidation. Life Sci. 1995;57:1819–1831. doi: 10.1016/0024-3205(95)02073-r. [DOI] [PubMed] [Google Scholar]
- 5.Clarke D J, Burchell B. The uridine diphosphate glucuronosyltransferase multigene family: function and regulation. In: Kauffman F C, editor. Handbook of experimental pharmacology. Conjugation-deconjugation reactions in drug metabolism and toxicity. Berlin, Germany: Springer-Verlag; 1994. pp. 3–43. [Google Scholar]
- 6.Davis R, Peters D H, McTavish D. Valproic acid: a reappraisal of its pharmacological properties and clinical efficacy in epilepsy. Drugs. 1994;2:332–372. [PubMed] [Google Scholar]
- 7.De Santis M, Noia G, Caruso A, Mancuso S. Guidelines for the use of zidovudine in pregnant women with HIV infection. Drugs. 1995;50:43–47. doi: 10.2165/00003495-199550010-00004. [DOI] [PubMed] [Google Scholar]
- 8.Farrell M, Ward J, Mattick R, Hall W, Stimson G V, des Jarlais D, Gossop M, Strang J. Methadone maintenance treatment in opiate dependence: a review. Br Med J. 1994;309:997–1001. doi: 10.1136/bmj.309.6960.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glaxo Wellcome, Inc. Mepron (atovaquone) suspension product monograph. Triangle Park, N.C: Glaxo Wellcome, Inc., Research; 1997. [Google Scholar]
- 10.Goa K L, Barradell L B. Fluconazole: an update of its pharmacodynamic and pharmacokinetic properties and therapeutic use in major superficial and systemic mycoses in immunocompromised patients. Drugs. 1995;50:658–690. doi: 10.2165/00003495-199550040-00007. [DOI] [PubMed] [Google Scholar]
- 11.Green M D, Tephly T R. Glucuronidation of amines and hydroxylated xenobiotics and endobiotics catalyzed by expressed human UGT1.4 protein. Drug Metab Dispos. 1996;24:356–363. [PubMed] [Google Scholar]
- 12.Herber R, Magdalou J, Haumont M, Bidault R, van Es H, Siest G. Glucuronidation of 3′-azido-3′-deoxythymidine in human liver microsomes: enzyme inhibition by drugs and steroid hormones. Biochim Biophys Acta. 1992;1139:20–24. doi: 10.1016/0925-4439(92)90077-z. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch M S, D’Aquila R T. Therapy for human immunodeficiency virus infection. N Engl J Med. 1993;328:1686–1695. doi: 10.1056/NEJM199306103282307. [DOI] [PubMed] [Google Scholar]
- 14.Hoener B A. Predicting the hepatic clearance of xenobiotics in humans from in vitro data. Biopharm Drug Dispos. 1994;15:295–304. doi: 10.1002/bdd.2510150404. [DOI] [PubMed] [Google Scholar]
- 15.Hoggard P G, Veal G J, Wild M J, Barry M G, Back D J. Drug interactions with zidovudine phosphorylation in vitro. Antimicrob Agents Chemother. 1995;39:1376–1378. doi: 10.1128/aac.39.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houston J B. Utility of in vitro drug metabolism data in predicting in vivo metabolic clearance. Biochem Pharmacol. 1994;47:1469–1479. doi: 10.1016/0006-2952(94)90520-7. [DOI] [PubMed] [Google Scholar]
- 17.Jamis-Dow C A, Klecker R W, Katki A G, Collins J M. Metabolism of taxol by human and rat liver in vitro: a screen for drug interactions and interspecies differences. Cancer Chemother Pharmacol. 1995;36:107–114. doi: 10.1007/BF00689193. [DOI] [PubMed] [Google Scholar]
- 18.Jatlow P, McCance E F, Rainey P M, Trapnell C B, Friedland G. Program and abstracts of the 3rd Conference on Retroviruses and Opportunistic Infections, Washington, D.C. 1996. Methadone increases zidovudine exposure in HIV-infected injection drug users; p. 129. [Google Scholar]
- 19.Kell M J. Utilization of plasma and urine methadone concentrations to optimize treatment in maintenance clinics. I. Measurement techniques for a clinical setting. J Addict Dis. 1994;13:5–26. doi: 10.1300/J069v13n01_02. [DOI] [PubMed] [Google Scholar]
- 20.Kell M J. Utilization of plasma and urine methadone concentration measurements to limit narcotics use in methadone maintenance patients. II. Generation of plasma concentration response curves. J Addict Dis. 1995;14:85–108. doi: 10.1300/J069v14n01_09. [DOI] [PubMed] [Google Scholar]
- 21.Klecker R W, Collins J M. Stereo-selective metabolism of fenoldopam and its metabolites in human liver microsomes, cytosol and slices. J Cardiovasc Pharmacol. 1997;30:69–74. doi: 10.1097/00005344-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Kornhauser D M, Petty B G, Hendrix C W, Woods A S, Nerhood L J, Bartlett J G, Lietman P S. Probenecid and zidovudine metabolism. Lancet. 1989;ii:473–475. doi: 10.1016/s0140-6736(89)92087-4. [DOI] [PubMed] [Google Scholar]
- 23.Lee B L, Täuber M G, Sadler B, Goldstein D, Chambers H F. Atovaquone inhibits the glucuronidation and increases the plasma concentrations of zidovudine. Clin Pharmacol Ther. 1996;59:14–21. doi: 10.1016/S0009-9236(96)90019-3. [DOI] [PubMed] [Google Scholar]
- 24.Lertora J J L, Rege A B, Greenspan D L, Akula S, George W J, Hyslop N E, Agrawal K C. Pharmacokinetic interaction between zidovudine and valproic acid in patients infected with human immunodeficiency virus. Clin Pharmacol Ther. 1994;56:272–278. doi: 10.1038/clpt.1994.137. [DOI] [PubMed] [Google Scholar]
- 25.Macleod R, Eagling V A, Sim S M, Back D J. In vitro inhibition studies of the glucuronidation of 3′-azido-3′-deoxythymidine catalysed by human liver UDP-glucuronosyl transferase. Biochem Pharmacol. 1992;43:382–386. doi: 10.1016/0006-2952(92)90303-z. [DOI] [PubMed] [Google Scholar]
- 26.McLeod G X, Hammer S M. Zidovudine: five years later. Ann Intern Med. 1992;117:487–501. doi: 10.7326/0003-4819-117-6-487. [DOI] [PubMed] [Google Scholar]
- 27.Mentré F, Escolano S, Diquet B, Golmard J L, Mallet A. Clinical pharmacokinetics of zidovudine: inter- and intraindividual variability and relationship to long term efficacy and toxicity. Eur J Clin Pharmacol. 1993;45:397–407. doi: 10.1007/BF00315509. [DOI] [PubMed] [Google Scholar]
- 28.Rajaonarison J F, Lacarelle B, Catalin J, Placidi M, Rahmani R. 3′-Azido-3′-deoxythymidine drug interactions: screening for inhibitors in human liver microsomes. Drug Metab Dispos. 1992;20:578–584. [PubMed] [Google Scholar]
- 29.Rajaonarison J F, Lacarelle B, De Sousa G, Catalin J, Rahmani R. In vitro glucuronidation of 3′-azido-3′-deoxythymidine by human liver: role of UDP-glucuronosyltransferase 2 form. Drug Metab Dispos. 1991;19:809–815. [PubMed] [Google Scholar]
- 30.Rajaonarison J F, Lacerelle B, Catalin J, Durand A, Cano J P. Effect of anticancer drugs on the glucuronidation of 3′-azido-3′-deoxythymidine in human liver microsomes. Drug Metab Dispos. 1993;21:823–829. [PubMed] [Google Scholar]
- 31.Resetar A, Minick D, Spector T. Glucuronidation of 3′-azido-3′-deoxythymidine catalyzed by human liver UDP-glucuronosyltransferase. Biochem Pharmacol. 1991;43:559–568. doi: 10.1016/0006-2952(91)90319-z. [DOI] [PubMed] [Google Scholar]
- 32.Sahai J, Gallicano K, Pakuts A, Camerson D W. Effect of fluconazole on zidovudine pharmacokinetics in patients infected with human immunodeficiency virus. J Infect Dis. 1994;169:1103–1107. doi: 10.1093/infdis/169.5.1103. [DOI] [PubMed] [Google Scholar]
- 33.Sim S M. The effect of various drugs on the glucuronidation of zidovudine (azidothymidine; AZT) by human liver microsomes. Br J Clin Pharmacol. 1991;32:17–21. doi: 10.1111/j.1365-2125.1991.tb05607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spencer C M, Goa K L. Atovaquone. A review of its pharmacological properties and therapeutic efficacy in opportunistic infections. Drugs. 1995;50:176–196. doi: 10.2165/00003495-199550010-00011. [DOI] [PubMed] [Google Scholar]
- 35.Taburet A-M, Singlas E. Drug interactions with antiviral drugs. Clin Pharmacokinet. 1996;30:385–401. doi: 10.2165/00003088-199630050-00005. [DOI] [PubMed] [Google Scholar]
- 36.Trapnell C B, Narang P K, Li R, Lavelle J P. Increased plasma rifabutin levels with concomitant fluconazole therapy in HIV-infected patients. Ann Intern Med. 1996;124:573–576. doi: 10.7326/0003-4819-124-6-199603150-00006. [DOI] [PubMed] [Google Scholar]
- 37.Wrighton S A, Ring B J, VandenBranden M. The use of in vitro metabolism techniques in the planning and interpretation of drug safety studies. Toxicol Pathol. 1995;23:199–208. doi: 10.1177/019262339502300214. [DOI] [PubMed] [Google Scholar]