Abstract
Objective
To evaluate the impact of mobile vaccination units on COVID-19 vaccine uptake of the first dose, the percentage of vaccinated people among the total eligible population. We further investigate whether such an effect differed by deprivation, ethnicity and age.
Design
Synthetic control analysis.
Setting
The population registered with general practices (GPs) in nine local authority areas in Cheshire and Merseyside in Northwest England, UK.
Intervention
Mobile vaccination units that visited 37 sites on 54 occasions between 12 April 2021 and 28 June 2021. We defined intervention neighbourhoods as having their population weighted centroid located within 1 km of mobile vaccination sites (338 006 individuals). A weighted combination of neighbourhoods that had not received the intervention (1 495 582 individuals) was used to construct a synthetic control group.
Outcome
The weekly number of first-dose vaccines received among people aged 18 years and over as a proportion of the population.
Results
The introduction of a mobile vaccination unit into a neighbourhood increased the number of first vaccinations conducted in the neighbourhood by 25% (95% CI 21% to 28%) within 3 weeks after the first visit to a neighbourhood, compared with the synthetic control group. Interaction analyses showed smaller or no effect among older age groups, Asian and black ethnic groups, and the most socioeconomically deprived populations.
Conclusions
Mobile vaccination units are effective interventions for increasing vaccination uptake, at least in the short term. While mobile units can be geographically targeted to reduce inequalities, we found evidence that they may increase inequalities in vaccine uptake within targeted areas, as the intervention was less effective among groups that tended to have lower vaccination uptake. Mobile vaccination units should be used in combination with activities to maximise outreach with black and Asian communities and socioeconomically disadvantaged groups.
Keywords: COVID-19, Public health, Health policy
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The synthetic control method for microdata offers a rigorous method for identifying control areas that experienced similar levels of COVID-19 vaccine uptake as the intervention areas prior to the introduction of mobile vaccination units, supporting a possible causal interpretation of the finding of increased uptake in areas with mobile vaccination visits following their introduction.
The use of individual-level data enabled us to construct weighted Poisson regression models to offer robust estimation of the observed effect, with interaction analysis to reveal whether this effect varied by level of deprivation, age groups and ethnicity.
We were not able to include individuals who had not been registered with the general practic (GP) at time of our study, which could lead to the underestimation of the effect given that the main vaccination programme was provided through GPs.
As our analysis covers a relatively short follow-up period, the results may not reflect the sustained impact of the intervention over longer periods of time.
There may also be other differences between places being visited by the mobile vaccination units and those that were not and differences in individual characteristics, beyond those included in this study, which led to the differences in the observed effect.
Introduction
Vaccination is one of the most effective public health interventions for improving health and saving lives.1 Following the national roll-out of the COVID-19 vaccine programme on 8 December 2020 in the UK, relatively high vaccine uptake has helped reduce the risk of hospitalisation and mortality from COVID-19.2 Nevertheless, vaccine uptake was lower among young people, socioeconomically disadvantaged groups and ethnic minorities for a combination of factors including vaccine eligibility (online supplemental appendix 1 presents the timeline for age eligibility of the first dose of the COVID-19 vaccine in England) and hesitancy, health disparities and inequalities, convenience and access, language and cultural barriers, and trust in healthcare systems.3–6 Some of these groups have also often experienced disproportionately greater levels of infections, hospitalisations and deaths during the pandemic. With successive SARS-CoV-2 variants, additional booster vaccinations were needed to give sufficient protection. Inequalities in uptake of these subsequent doses were greater than with the initial doses,7 potentially undermining responses to new variants that rely largely on increased uptake of booster vaccinations. The UK government invested £22.5 million to help areas increase uptake among hard-to-reach groups in December 2021, as tackling health inequalities is a core government priority.8 A central part of this strategy was to use mobile vaccination units such as pop-up sites and vaccine buses—‘taking the vaccines into the hearts of local communities’.9
bmjopen-2023-071852supp001.pdf (3MB, pdf)
Mobile vaccination units have been used in many countries to increase uptake in disadvantaged communities.10 These generally involve vaccination clinics based in large vehicles such as buses or temporary pop-up clinics in community settings. As well as bringing vaccines into targeted communities, they usually involve outreach programmes (eg, leaflet campaigns to advertise vaccination units and explain the benefits of vaccination). The use of mobile vaccination units to increase uptake is based on the premise that geographic accessibility and convenience of vaccination services are potentially important determinants of uptake and that they support uptake from people who have difficulty in accessing existing sites due to information or travel barriers, childcare or healthcare responsibilities, or being excluded from the official healthcare system.11
Although former studies found mobile units as useful tools to reduce health inequalities in administering other forms of preventative care such as cancer screening,12–15 there has been limited research evaluating interventions that aim to increase COVID-19 vaccine uptake. One randomised controlled trial (RCT) shows that provision of information on personal benefit reduces hesitancy,16 with another RCT indicating that text message reminders lead to small increases in uptake.17 One study of a community-based strategy in an underserved Latinx population in San Francisco found that mobile units reached their intended recipients, but the study was unable to estimate the impact on vaccination uptake.18 Another study found that users of the COVID-19 mobile vaccination units tended to be younger, non-white race and Hispanic ethnicity compared with the general vaccinated populations in the Greater Boston area, suggesting the potential benefits of mobile vaccination units without estimating the average treatment effect.19 A few studies investigated the impact of interventions aiming to increase influenza and pneumococcal vaccination, including one systematic review,20 five RCTs21–25 and two cluster RCTs.26 27 Of these, three were published in the UK,21 22 26 three in the USA20 23 24 and two in Hong Kong.25 27 They all show that sending out reminders, telephone calls and educational outreach tend to increase uptake. We found no studies that quantified the effect of introducing mobile vaccination units on COVID-19 vaccine uptake.
It also remains largely uncertain how effective mobile vaccination units are at increasing uptake among disadvantaged groups, having been deployed to improve vaccine access for those communities.2 18 19 The lack of existing evidence is concerning, considering their central role in the strategies to reduce vaccine uptake inequalities. We, therefore, used a synthetic control approach to investigate the impact of mobile vaccination units in the Northwest of England and how this varied by age, socioeconomic status and ethnicity.
Methods
Data
We used anonymised electronic health records (EHR) on all people aged 18 and over, registered with a general practice (GP) in Cheshire and Merseyside, England, between 22 February 2021 and 19 July 2021.28 These data included vaccination status, vaccination date, age, sex, ethnicity, chronic health conditions diagnosed in primary care (chronic obstructive pulmonary disease, chronic heart disease, diabetes, chronic kidney disease asthma, cancer, obesity, depression and stroke/transient ischaemic attack), whether people were in contact with social care, whether they were a paid carer, travel time by car to the nearest conventional static vaccination site requiring booking in advance and the lower super output areas (LSOA) of residence. LSOAs are small geographical areas of England, with approximately 1500 residents each. Ethnicity was based on information of primary care records. Where unavailable, ethnicity was taken from hospital, community or social care records if available in these datasets. Ethnicity was categorised in these datasets using the 17 standard Office for National Statistics Categories. These were then recoded to five categories for analysis (white/white British, Asian/Asian British, black/black British, mixed and other).
We linked the EHR data to LSOA-level data, including 2019’s indices of multiple deprivation (IMD)—a composite measure of deprivation across seven domains (income, employment, education, health, crime, barriers to housing and services, and living environment), an overcrowded housing measure (the proportion of households with at least one-bedroom fewer than they need) based on 2011’s Census, and population density using 2019’s mid-year population estimates from the Office for National Statistics. The public health teams of the nine local authorities in the Cheshire and Merseyside region were asked to provide a list of locations and dates of mobile vaccination units in their areas between May and November 2021. Six local authorities provided this information and two local authorities reported that they had had no mobile vaccination units. Analysis was, therefore, limited to these eight local authorities.
Intervention
Six local authorities operated mobile vaccination units, that visited 37 sites on 54 occasions, between 12 April 2021 and 28 June 2021. This included 52 visits from vaccine buses and 2 from two pop-up static clinics (different from conventional static vaccination sites, these two pop-up sites offered walk-in services without the need to book in advance). Six sites were visited more than once during our study period, with repeated visits scheduled either consecutively or at least 1 week apart. Vaccines were offered typically from 9:00 to 16:30 hours or 17:00 hours on the day of most visits, with four visits operating from 8:00 to 18:00, 9:00 to 15:00, 9.15 to 14.30 and 10:00 to 14:00 hours, respectively. We chose to treat all sites uniformly in our analysis to avoid potentially overemphasising the impact of sites with multiple visits and minimise the potential influence of site-specific factors. This simplifying assumption offers us a more conservative approach and allows us to focus on the overall effect of the intervention rather than site-specific variations, thus enabling clearer interpretation and generalisability of the results. The deployment of the mobile vaccination units also involved outreach programmes to advertise vaccination units and explain the benefits of vaccination for each visit. Units were primarily focused on offering first-dose COVID-19 vaccinations to those that had not received a vaccine at any of the city’s existing conventional static vaccination sites and were eligible for vaccination at the time.29 Vaccines were offered on a drop-in basis with no appointment needed. The number of vaccinations received at these mobile units was unavailable for one local authority (Warrington). The total number of first dose vaccinations for the remaining five local authorities that had deployed the mobile vaccination units was 3824. Sites visited by mobile vaccination units were identified by local public health and health service teams based on their knowledge of vaccine uptake (ie, the percentage of adults who have received the first dose of the COVID-19 vaccine among the total eligible adult population in our study), practicalities of having space and permissions to locate the vaccination unit, and relationships with local community groups.
We defined intervention neighbourhoods as having their population weighted centroid located within 1 km of mobile vaccination sites and identified 216 LSOAs that were either hosts for mobile vaccination units or had population weighted centroids within a 1 km radius of mobile vaccination units. We calculated the start time of the intervention as the week a mobile vaccination unit first visited. This left 1112 non-intervention LSOAs within the eight participating local authorities that did not have population weighted centroids within 1 km of a mobile unit. We then applied a ‘placebo’ start time to each of these non-intervention LSOAs, generated uniformly at random in the same weekly proportion as the distribution of intervention start dates for the intervention LSOAs.
Statistical analysis
We aggregated the individual-level data to construct a panel of weekly measures at the LSOA level from 7 weeks (22 February 2021) before the first mobile vaccination unit visit on 12 April 2021 to 3 weeks (19 July 2021) after the last mobile vaccination unit visit on 28 June 2021. We chose 7 weeks as our preintervention period after evaluating trends of the accumulated uptake, overall and by ethnic and socioeconomic groups, with consideration of the roll-out of the vaccine prioritised by age groups (online supplemental appendices 1 and 2). A 7-week preintervention period allowed us to capture trends for most people eligible for the vaccine and focus on the intervention at a relatively stable stage of the pandemic. We then chose a 3-week follow-up period due to the policy change in July 2021 to prioritise administrating the second doses. A 3-week period avoided spill-over effect of this prioritisation and allowed us to estimate the effect of repeated visits to some sites and the accompanying outreach programmes. We, therefore, aimed to investigate the short-term effect of the intervention here. The weekly panel data included LSOA-level measures of total GP registered population size, the proportion of black/black British people, the proportion of Asian/Asian British people, the proportion of people of mixed ethnicity, mean age of residents, population density, the proportion of women, the IMD score, the proportion of households living in overcrowded housing, the average travel time by car to the nearest conventional static vaccine site, the cumulative number of first dose administered at the start of the preintervention period, and the number of new first dose vaccinations administered per week (online supplemental appendix 3 compares these measures between the intervention and the rest of Cheshire and Merseyside).
We apply the synthetic control method for microdata developed by Robbins et al to estimate the effect of mobile vaccination units on vaccine uptake. Our outcome variable, weekly vaccine uptake, is the percentage of adults who have received the first dose of the COVID-19 vaccine during each week among the total eligible adult population.30 31 The synthetic control method is a generalisation of difference-in-difference methods.32 An untreated version of the intervention areas (ie, a synthetic control) is created using a weighted combination of areas that were unexposed to the intervention. The intervention effect is estimated by comparing the trend in outcomes in the intervention areas to that in the synthetic control areas following the intervention.33
The weights were calculated using the raking method34 so that the weighted averages for all the variables outlined above in the synthetic control group were the same as for the intervention. This included the cumulative number of first vaccine doses administered prior to the preintervention period, the number of first dose vaccines administered in each of the 7 weeks prior to the intervention (online supplemental appendix 4) and each local area characteristic, outlined above.30 Online supplemental appendix 5 presents the geographical distribution of these weights and illustrates how the synthetic control group is constructed. The estimated effect of the mobile vaccination unit on the weekly number of first doses, was calculated as the difference between the intervention and the (weighted) synthetic control cohorts in the weekly number of vaccines received over a 3-week period after the intervention. To estimate the sampling distribution of the treatment effect, and the permuted p values and 95% CIs, we applied a permutation procedure outlined by Robbins et al by repeating the analysis through 250 placebo permutations randomly allocating non-intervention LSOAs to the intervention group.31
As our area-based analysis could be biased by insufficiently accounting for confounders at the individual level, we additionally conducted analysis using individual-level data on all adults registered with the GP and living in the intervention and non-intervention LSOAs (as defined above), who had been unvaccinated before the mobile vaccination unit first visited their neighbourhood. We used a weighted Poisson regression model with robust sandwich variance estimators, which has been shown to be a valid alternative to the logistic regression model for the analysis of binary outcomes,35 36 to estimate the relative risk (RR) of vaccination by comparing the vaccine rates between these two groups in the 3 weeks following the intervention. Using a weighted Poisson regression model to estimate this effect without considering the differences between the intervention and non-intervention areas on the aggregate level would potentially underestimate standard errors and p values, as this would not account for complex aspects of the process used to generate the synthetic control weights for LSOAs. We, therefore, weighted this regression using the synthetic control weights as highlighted above to account for systematic LSOA-level differences, after additionally controlling for the following individual-level potential confounders: age, sex, ethnicity, health conditions (as defined above), whether people were in contact with social care, whether they were paid carers and the travel time by car from each person’s address to the nearest conventional static vaccination centre. Overall, 18.62% and 0.01% of ethnicity and sex were missing, respectively, leaving 233 278 records for the main complete case analysis.
To explore whether the mobile vaccination unit effect differed by deprivation, ethnicity and age, we fitted another weighted Poisson model including interaction terms between the intervention indicator (mobile vaccination unit) and IMD tercile within Cheshire and Merseyside, ethnic group, and age group, respectively (see online supplemental appendices 6 and 7 for detailed results). Apart from the sensitivity test of excluding ethnicity (online supplemental appendix 8) and the two pop-up sites (online supplemental appendix 9) from the analysis to assess the potential impact of the missing data and two pop-up static sites on our results, respectively, we have additionally conducted a series of sensitivity tests on the dose effect of distance threshold used to construct the intervention and non-intervention areas (online supplemental appendix 10), the potential spatial spill-over effect of the intervention (online supplemental appendix 11), and survival analyses (online supplemental appendix 12) to further check the robustness of our results.
Patient and public involvement
The local authorities delivering the intervention engaged with various community groups in promoting the intervention and identifying intervention sites. Patients and the public were, however, not directly involved in designing this analysis.
Results
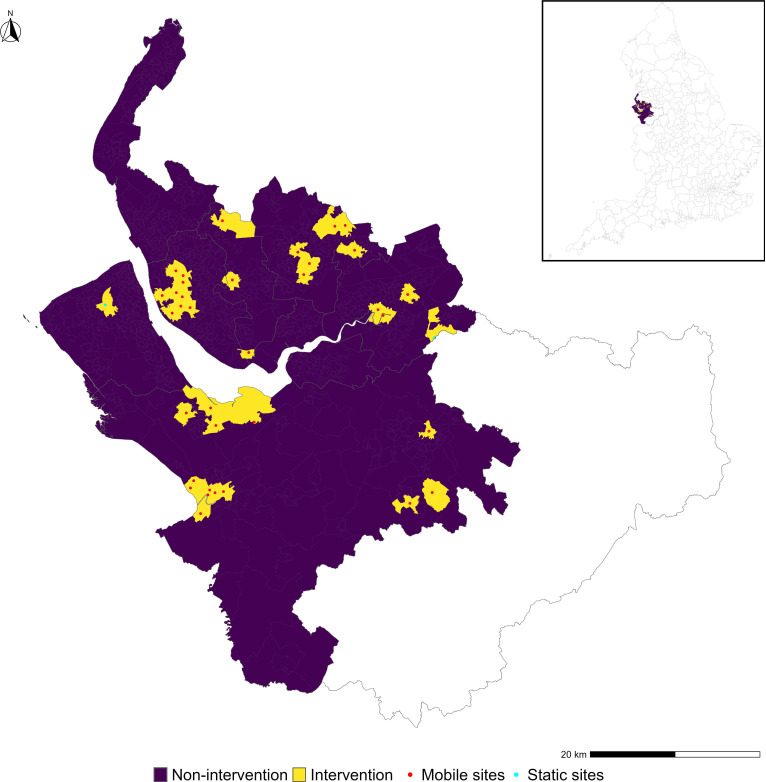
Figure 1 shows the intervention (yellow) and the non-intervention areas (purple). The red and cyan dots represent the mobile and pop-up static vaccination sites respectively. The map in online supplemental appendix 5 shows the weights that were applied to the non-intervention areas to construct the synthetic control.
Figure 1.
Location of the 35 eligible mobile vaccination units (red dots), the two static pop-up sites (cyan dots) and the non-intervention (purple) and intervention (yellow) areas across Cheshire and Merseyside. The submap in the box of the top right shows the location of Cheshire and Merseyside in England.
Table 1 presents summary statistics for the intervention and non-intervention areas (see online supplemental appendix 3 for the comparison between the intervention and the rest of Cheshire and Merseyside). The average vaccination rate of the first dose was much higher in the non-intervention areas before the introduction of the intervention. On average, residents of the non-intervention areas had to travel slightly longer to get to the nearest conventional static vaccine site. The intervention areas were younger and more deprived, and had a higher proportion of overcrowded households, higher proportions of Asian/Asian British and black/black British ethnic minority groups, and higher population density. There were no differences in terms of the proportion of mixed ethnicity. As the matching algorithm achieved an exact match, the weighted average of each variable in table 1 was identical in the synthetic control to those in the intervention areas.
Table 1.
The summary statistics for the intervention and non-intervention areas at the intervention onset in the 7 weeks prior to the introduction of the mobile vaccination unit
| Non-intervention areas | Intervention areas | |
| Total population | 1 495 582 | 338 006 |
| % women | 50.84 | 48.92 |
| Population density—people per hectare | 38.84 | 65.11 |
| Mean age | 42.71 | 38.03 |
| % Asian/Asian British | 1.10 | 3.28 |
| % Black/Black British | 0.52 | 2.37 |
| % Mixed people | 1.48 | 1.87 |
| IMD score* | 28.71 | 40.38 |
| % households with at least one-bedroom fewer than they need | 2.69 | 4.77 |
| Average travel time by car to the nearest conventional static vaccine site—minutes | 4.14 | 2.83 |
| First dose vaccine uptake among adults (the percentage of adults who have received the first dose of COVID-19 vaccine among the total eligible adult population prior to preintervention) (%) | 69.28 | 52.65 |
| Average weekly first dose vaccination rate among adults in the 7 weeks prior to intervention (%) | 1.85 | 1.51 |
| No of LSOAs | 1112 | 216 |
*We primarily used the IMD score in our main models 1 and 2 in the following analysis. We then sorted the IMD score in ascending order and divided it into three equal parts to facilitate our subgroup analysis by level of deprivation.
IMD, index of multiple deprivation; LSOAs, lower super output areas.
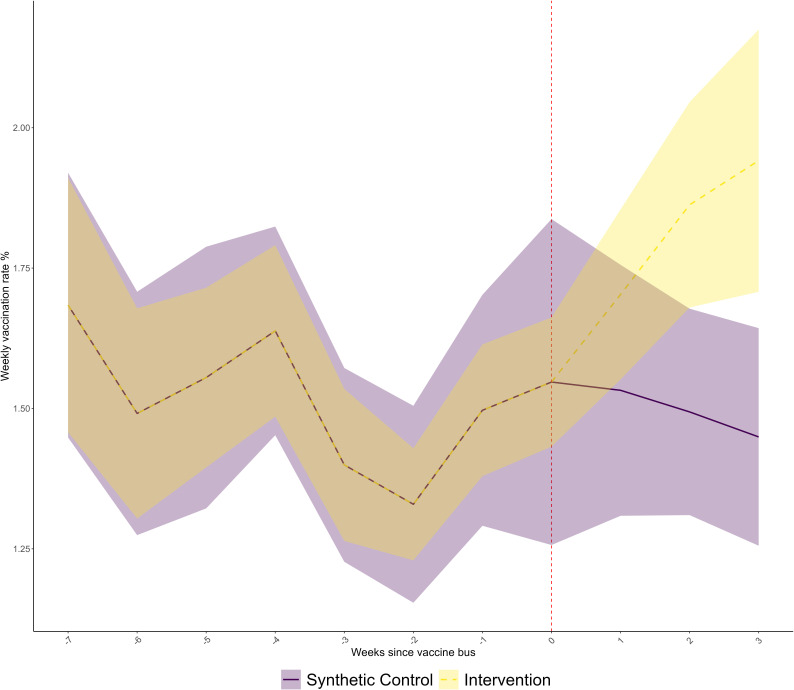
Figure 2 shows the trend in weekly vaccination rate in the intervention and synthetic control areas, during an 11-week period (7 weeks before and 3 weeks after the introduction of the mobile vaccination unit). For the preintervention period, trends were indistinguishable, as the synthetic control algorithm has achieved an exact match by successfully calibrating the weights. From the time point of the intervention being introduced, weekly vaccination rates increased from 1.5% to 1.9% in the intervention areas. Trends in the matched synthetic control areas that were not visited by a mobile vaccination unit remained fairly flat, with a small decrease in the postintervention period. From 2 weeks postintervention, 95% CIs stopped overlapping.
Figure 2.
The trend in the weekly vaccination rate with their 95% CIs in the intervention and synthetic control areas.
Table 2 shows the estimated effect of the mobile vaccination units on weekly vaccination rate, the percentage of adults who have received the first dose of the COVID-19 vaccine in each week among the total eligible adult population, using two models based on area-level and individual-level data, respectively. The two analyses show similar results. The area-based analysis indicates that vaccination rates in the neighbourhoods visited by mobile vaccination units were 23% higher in the 3 weeks after the first visit, compared with what would have been the case without the mobile vaccination units’ visits (RR 1.23, 95% CI 1.11 to 1.36). The RRs adjusted for individual-level characteristics is slightly higher than that of the area-based model (RR 1.25, 95% CI 1.21 to 1.28). This overall effect size estimates 3723 additional vaccinations over 3 weeks of follow-up across the study area, similar to the actual number of people vaccinated in the mobile units (n=3824). Sensitivity tests excluding ethnicity and the two pop-up sites showed similar results with those of model 2 (see online supplemental appendices 8 and 9, respectively). While sensitivity tests on the distance threshold demonstrated the spatially sensitive nature of our analysis (online supplemental appendix 10), our results are relatively robust against the spatial spill-over effect once an appropriate distance threshold was chosen (online supplemental appendix 11).
Table 2.
Estimated effect of mobile vaccination units on weekly vaccination rate (the percentage of adults who have received the first dose of the COVID-19 vaccine in each week among the total eligible adult population)
| RR | 95% CI | P value | ||
| LCL | UCL | |||
| Model 1. LSOA level synthetic control analysis | 1.23 | 1.11 | 1.36 | <0.001 |
| Model 2. Individual level weighted Poisson regression analysis | 1.25 | 1.21 | 1.28 | <0.001 |
The table shows the relative risks indicating the estimated ratio of vaccine rates in the intervention group compared with the synthetic control group in the 3 weeks following intervention. Model 1 uses LSOA level data, accounting for area-based differences between intervention and non-intervention areas, with permuted p values and confidence intervals, while model 2 additionally controls for individual differences between intervention and non-intervention groups.
LCL and UCL, lower and upper CI; RR, relative risk.
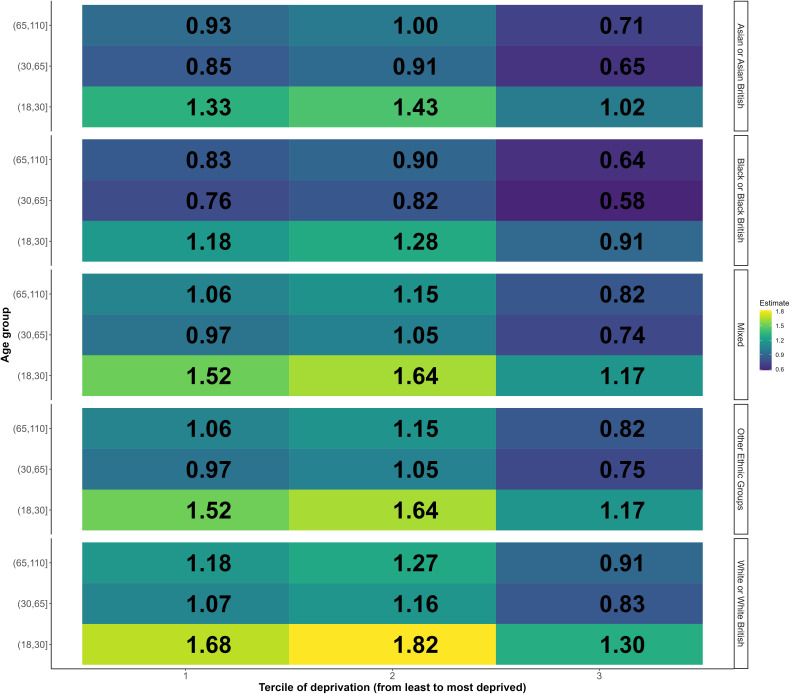
We used interaction analyses to investigate if the intervention effect was modified by deprivation, ethnicity and age (see online supplemental appendix 6). We found a significant interaction between deprivation and the intervention indicating reduced effectiveness with increased deprivation (p<0.001), a significant interaction with ethnicity indicating reduced effectiveness for Asian/Asian British (p=0.006), black/black British (p=0.005) or other ethnic groups (p=0.010), compared with white/white British people, and a significant interaction with age indicating reduced effectiveness in older age groups. The combination of these interaction effects means that in many groups defined by age, ethnicity and deprivation there was no evidence of effectiveness (see figure 3).
Figure 3.
Heatmap of the estimated impact of the mobile vaccination units on Weekly number of first dose COVID-19 vaccines administered among all the subgroups based on interaction analysis.
Figure 3 shows estimated effect of mobile vaccination units on weekly vaccine uptake for all the subgroups using the combination of the effect in the reference group (18–30 years of white ethnicity living in the least deprived neighbourhoods that were visited by the mobile vaccination unit) and the interaction terms for deprivation, ethnicity and age group. The relative effect of the intervention for the reference group is shown in the bottom left cell of figure 3, indicating that the intervention increased vaccine uptake by 68% (RR 1.68) for 18–30 years of white ethnicity living in the least deprived neighbourhoods and having received the intervention relative to people of the same age group, ethnicity, living in neighbourhoods of the same deprivation level but without the intervention. For people of same ethnic and age group but living in intermediately deprived areas, the effect was slightly larger (RR 1.82, 82% increase; calculated as the combination of the effect on the reference group and the interaction term for intermediate deprivation with mobile vaccination units: 1.683×1.081=1.82; see online supplemental appendix 6), while for those living in the most deprived areas it was lower (RR 1.30, 30% increase; calculated as 1.683×0.770 in the same manner as above). Effects of other subgroups could be interpreted in the same way. Due to the small sample sizes of these 45 subgroups (see online supplemental appendix 7), these results should be treated with caution. However, they indicate the likely pattern of effects of the intervention across these groups. Overall, white, 18–30 years living in neighbourhoods of intermediated deprivation appeared to benefit the most from visits of the mobile vaccination unit (figure 3). Effects were lowest for older age groups in the most deprived areas. Although the effect of the mobile vaccination unit may be lower for the most deprived population and for people from black, Asian and other ethnic minority groups, the mobile vaccination unit does still appear to have increased uptake in these groups for younger people, while we find no evidence of a positive effect for most older age groups from black, Asian and other ethnic minority groups, particularly in deprived areas. The survival analysis showed similar results overall (online supplemental appendix 12).
Discussion
Our study presents much-needed empirical evidence of the effectiveness of mobile vaccination units in promoting COVID-19 vaccination, indicating that they can increase uptake in their targeted neighbourhoods. The effect size estimated in our analysis closely corresponds to the actual number, indicating that a significant proportion of vaccinations conducted in the mobile units were additional. Put simply, our findings suggest that among individuals using the mobile vaccination units, there was a minimal number who would have otherwise sought their vaccinations at conventional static centres. This implies that the mobile vaccination units effectively reached individuals who may have faced barriers in accessing conventional static vaccination sites. Our study, therefore, also lends support to previous studies on other forms of preventative care that mobile units are useful tools in improving geographical accessibility and convenience of services for patients.12–15
Within those neighbourhoods, however, the intervention tended to increase uptake most among younger people, white people and less socioeconomically deprived areas. However, the targeted neighbourhoods were the most deprived. Our analysis indicated that the intervention was similarly effective in the least deprived and intermediate levels of deprivation within these targeted neighbourhoods. It is only in areas with IMD score of over 53, the lower bound of the most deprived, that the effectiveness declined. However, 53 is at the 95th percentile of the national distribution of IMD scores, representing very deprived areas nationwide. Our findings indicate, therefore, that the intervention was effective at increasing uptake among young people in relatively deprived areas (although not in extremely deprived areas) and across young people from all ethnic groups in these areas.
Our study has limitations. We only had data on individuals who had been registered with the GP at time of our study. The GP registered population is larger than that of the 2021 Census (2.7 million vs 2.5 million people) due to known data issues (eg, delays in people updating addresses when move home, different definitions of resident population). Although unregistered people attending for mobile units were encouraged and can register with a GP in their attendance, there may have been people vaccinated who did not wish to be registered, such as undocumented immigrants, homeless populations, travellers and displaced people. We were unable to estimate the impact of the intervention in these groups. The mobile vaccination unit could have increased uptake due to improved accessibility through the mobile vaccination unit itself and/or the awareness raising and publicity associated with visits of these units. Our analysis cannot distinguish between these effects.
Our analysis covers a relatively short period of follow-up. We are, therefore, unable to determine whether the observed effect is due to people being vaccinated earlier than they would otherwise have been or if they would have never taken up vaccine without the intervention. We could link approximate but not exact time/date visits of the mobile units. Further research should look to evaluate if mobile units had different effects at different stages of a pandemic (eg, initial roll-out compared with reopening of society or once most people have been vaccinated by traditional means). We used a measure of average distance to construct the intervention and non-intervention group, which would have made our effect estimate more conservative (see online supplemental appendix 11) and more sensitive to the choice of threshold (online supplemental appendix 10). Selecting an appropriate distance threshold is difficult, suggesting the need to evaluate how distance might impact the delivery and impact of mobile interventions. This not only reflects the geographical nature of the intervention but indicates the potential benefit of improving the data quality for the distance measurement in future research. Although we have accounted for observed differences between places visited by the mobile vaccination units and those not and differences in individual characteristics, it is still possible that unmeasured confounding could bias the results.
Conclusion
Our study has implications for strategies aiming to increase vaccine uptake. By improving geographical accessibility and convenience of services, the mobile vaccination unit likely promoted uptake of the first dose of the COVID-19 vaccine in our study population. The evidence indicating greater effects in less deprived areas and in the white/white British population raises concerns that the intervention could however lead to an increase in inequalities in uptake within targeted areas. It is important to ensure the mobile vaccination units are effectively targeted to the communities with low uptake and combined with comprehensive engagement and outreach with black and Asian ethnic groups and with more socioeconomically disadvantaged communities. With successive SARS-CoV-2 variants and the need for multiple vaccine doses to achieve sufficient immunity, rapidly increasing uptake and reducing inequalities in uptake was of critical public health importance. Deployment of mobile units alongside other effective approaches to increase uptake in disadvantaged groups can contribute to this goal.
Supplementary Material
Footnotes
Contributors: BB is the guarantor and accepts full responsibility for the finished work and the conduct of the study. BB conceived the original idea, devised the project, the main conceptual ideas and proof outline. XZ contributed to the literature review, collected the data and carried out the data analysis. RA conducted the initial literature review. XZ drafted the manuscript and designed the figures with support from BB and MAG, JSPT, SK and PP assisted with the data access and commented on the manuscript. RP assisted in coding. MG-F and MAG aided in commenting on the manuscript. IEB aided in supervising the project and commented on the manuscript. SB helped to improve the language and visualisation. All authors provided critical feedback and helped to shape the research manuscript.
Funding: This research was funded by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Gastrointestinal Infections (ref NIHR200910), the NIHR Policy Research Programme (RESTORE; Award ID, NIHR202484), the NIHR Applied Research Collaboration Northwest Coast (NWC ARC) and The Pandemic Institute (formed of seven founding partners: The University of Liverpool, Liverpool School of Tropical Medicine, Liverpool John Moores University, Liverpool City Council, Liverpool City Region Combined Authority, Liverpool University Hospital Foundation Trust, and Knowledge Quarter Liverpool).
Disclaimer: The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health and Social Care, Public Health England, or The Pandemic Institute.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. The individual-level data are sensitive and could not be shared. But statistical codes are available from https://github.com/civicdatacoop/mobileVaxUnit.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
University Research Ethics Committee review is not required by the University of Liverpool for the secondary analysis of data which have been anonymised by an external party and are provided to the research team in a fully anonymised format.
References
- 1.WHO . Vaccination greatly reduces disease, disability, death and inequity worldwide. WHO. Available: https://www.who.int/bulletin/volumes/86/2/07-040089/en/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baraniuk C. Covid-19: how the UK vaccine rollout delivered success, so far. BMJ 2021;372:n421. 10.1136/bmj.n421 [DOI] [PubMed] [Google Scholar]
- 3.Campos-Matos I, Mandal S, Yates J, et al. Maximising benefit, reducing inequalities and ensuring deliverability: prioritisation of COVID-19 vaccination in the UK. Lancet Reg Health Eur 2021;2:100021. 10.1016/j.lanepe.2020.100021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Razai MS, Osama T, McKechnie DGJ, et al. Covid-19 vaccine hesitancy among ethnic minority groups. BMJ 2021;372:n513. 10.1136/bmj.n513 [DOI] [PubMed] [Google Scholar]
- 5.Osama T, Razai MS, Majeed A. COVID-19 vaccine allocation: addressing the United Kingdom’s colour-blind strategy. J R Soc Med 2021;114:240–3. 10.1177/01410768211001581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collaborative TO, MacKenna B, Curtis HJ, et al. Trends, regional variation, and clinical characteristics of COVID-19 vaccine recipients: a retrospective cohort study in 23.4 million patients using Opensafely. 10.1101/2021.01.25.21250356 [DOI]
- 7.Vaccine coverage - third/booster doses. OpenSAFELY: Reports. Available: http://reports.opensafely.org/reports/vaccine-coverage-thirdbooster-doses/ [Google Scholar]
- 8.£22.5m of funding announced in new community push to get nation boosted now. Gov.uk. Available: https://www.gov.uk/government/news/225m-of-funding-announced-in-new-community-push-to-get-nation-boosted-now [Google Scholar]
- 9.Final report on progress to address COVID-19 health inequalities. Gov.uk, Available: https://www.gov.uk/government/publications/final-report-on-progress-to-address-covid-19-health-inequalities/final-report-on-progress-to-address-covid-19-health-inequalities [Google Scholar]
- 10.ECDC . Facilitating vaccination acceptance and uptake in the EU/EEA. Stockholm: ECDC; 2021. Available: https://www.ecdc.europa.eu/en/publications-data/facilitating-covid-19-vaccination-acceptance-and-uptake [Google Scholar]
- 11.Armocida B, Formenti B, Missoni E, et al. Challenges in the equitable access to COVID-19 vaccines for migrant populations in Europe. Lancet Reg Health Eur 2021;6:100147. 10.1016/j.lanepe.2021.100147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams EM, Vessey MP. Randomised trial of two strategies offering women mobile screening for breast cancer. BMJ 1989;299:158–9. 10.1136/bmj.299.6692.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenwald ZR, El-Zein M, Bouten S, et al. Mobile screening units for the early detection of cancer: a systematic review. Cancer Epidemiol Biomarkers Prev 2017;26:1679–94. 10.1158/1055-9965.EPI-17-0454 [DOI] [PubMed] [Google Scholar]
- 14.Reuben DB, Bassett LW, Hirsch SH, et al. A randomized clinical trial to assess the benefit of offering on-site mobile mammography in addition to health education for older women. Am J Roentgenol 2002;179:1509–14. 10.2214/ajr.179.6.1791509 [DOI] [PubMed] [Google Scholar]
- 15.Shima A, Tanaka H, Okamura T, et al. Offering on-site mammography in workplaces improved screening rates: cluster randomized controlled trial. J Occup Health 2023;65:e12389. 10.1002/1348-9585.12389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman D, Loe BS, Yu L-M, et al. Effects of different types of written vaccination information on COVID-19 vaccine hesitancy in the UK (OCEANS-III): a single-blind, parallel-group, randomised controlled trial. Lancet Public Health 2021;6:e416–27. 10.1016/S2468-2667(21)00096-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai H, Saccardo S, Han MA, et al. Behavioural nudges increase COVID-19 vaccinations. Nature 2021;597:404–9. 10.1038/s41586-021-03843-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marquez C, Kerkhoff AD, Naso J, et al. A multi-component, community-based strategy to facilitate COVID-19 vaccine uptake among Latinx populations: from theory to practice. PLOS ONE 2021;16:e0257111. 10.1371/journal.pone.0257111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta PS, Mohareb AM, Valdes C, et al. Expanding COVID-19 vaccine access to underserved populations through implementation of mobile vaccination units. Prev Med 2022;163:107226. 10.1016/j.ypmed.2022.107226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ecarnot F, Maggi S, Michel J-P. Strategies to improve vaccine uptake throughout adulthood. Vaccines Older Adults Curr Pract Future Oppor 2020;43:234–48. 10.1159/isbn.978-3-318-06678-4 [DOI] [PubMed] [Google Scholar]
- 21.Arthur AJ, Matthews RJ, Jagger C, et al. Improving uptake of influenza vaccination among older people: a randomised controlled trial. Br J Gen Pract 2002;52:717–8, [PMC free article] [PubMed] [Google Scholar]
- 22.Hull S, Hagdrup N, Hart B, et al. Boosting uptake of influenza immunisation: a randomised controlled trial of telephone appointing in general practice. Br J Gen Pract 2002;52:712–6. [PMC free article] [PubMed] [Google Scholar]
- 23.Humiston SG, Bennett NM, Long C, et al. Increasing inner-city adult influenza vaccination rates: a randomized controlled trial. Public Health Rep 2011;126 Suppl 2(Suppl 2):39–47. 10.1177/00333549111260S206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krieger JW, Castorina JS, Walls ML, et al. Increasing influenza and pneumococcal immunization rates: a randomized controlled study of a senior center–based intervention. Am J Prev Med 2000;18:123–31. 10.1016/s0749-3797(99)00134-8 [DOI] [PubMed] [Google Scholar]
- 25.Leung KC, Mui C, Chiu WY, et al. Impact of patient education on influenza vaccine uptake among community-dwelling elderly: a randomized controlled trial. Health Educ Res 2017;32:455–64. 10.1093/her/cyx053 [DOI] [PubMed] [Google Scholar]
- 26.Siriwardena AN, Rashid A, Johnson MRD, et al. Cluster randomised controlled trial of an educational outreach visit to improve influenza and pneumococcal immunisation rates in primary care. Br J Gen Pract 2002;52:735–40. [PMC free article] [PubMed] [Google Scholar]
- 27.Chan SSC, Leung DYP, Leung AYM, et al. A nurse-delivered brief health education intervention to improve pneumococcal vaccination rate among older patients with chronic diseases: a cluster randomized controlled trial. Int J Nurs Stud 2015;52:317–24. 10.1016/j.ijnurstu.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CIPHA . Available: https://www.cipha.nhs.uk/
- 29.Best J. How the JCVI sets who gets a COVID-19 vaccine and when. BMJ 2021;373:n820. 10.1136/bmj.n820 [DOI] [PubMed] [Google Scholar]
- 30.Robbins MW, Davenport S. Microsynth: synthetic control methods for disaggregated and micro-level data in R. J Stat Softw;Vol 1. [Google Scholar]
- 31.Robbins MW, Saunders J, Kilmer B. A framework for synthetic control methods with high-dimensional, micro-level data: evaluating a neighborhood-specific crime intervention. Journal of the American Statistical Association 2017;112:109–26. 10.1080/01621459.2016.1213634 [DOI] [Google Scholar]
- 32.Barr B, Zhang X, Green M, et al. A blueprint for synthetic control methodology: a causal inference tool for evaluating natural experiments in population health. BMJ 2022;379:o2712. 10.1136/bmj.o2712 [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Owen G, Green M, et al. Evaluating the impacts of tiered restrictions introduced in England, during October and December 2020 on COVID-19 cases: a synthetic control study. Rochester, NY: Social Science Research Network, 2021. 10.2139/ssrn.3805859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raking. In: Encyclopedia of Survey Research Methods. California, United States of America: Sage Publications, Inc, 2008. 10.4135/9781412963947 [DOI] [Google Scholar]
- 35.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–6. 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 36.Greenland S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol 2004;160:301–5. 10.1093/aje/kwh221 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-071852supp001.pdf (3MB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. The individual-level data are sensitive and could not be shared. But statistical codes are available from https://github.com/civicdatacoop/mobileVaxUnit.