Abstract
We have studied the pharmacokinetics of amphotericin B (AmB) in lung lymph circulation and bronchial-wash fluid after intravenous infusion and inhalation, respectively. For two experiments with awake sheep, we used lung lymph fistulas and tracheotomy. In experiment 1, AmB concentrations in plasma and lung lymph after intravenous infusion of AmB (1 mg/kg of body weight) over 1.5 h were measured. The mean peak in plasma level was 756.0 ± 188.8 ng/ml at 3 h after the start of infusion, and the level then decreased gradually to 194.8 ± 28.9 ng/ml at 24 h. The stable and maximal levels in lung lymph last 5 to 9 h after the start of AmB infusion. The concentrations in lung lymph after 9 h were slightly higher than those in plasma. Thus, the lung lymph-to-plasma ratio of AmB concentrations increased gradually during infusion, and the ratio was more than 1.0 after the end of infusion, suggesting that AmB could be easily moved from plasma to pulmonary interstitium and/or lung lymph circulation. In another experiment, 5 or 30 mg of aerosol AmB was inhaled, and the concentration of AmB in the bronchial-wash fluid was determined by bronchoalveolar lavage. The peak AmB concentration in the fluid was observed at 0.5 h. After that, AmB was slowly eliminated over 24 h. The area under the concentration-time curve for 30 mg of inhaled AmB was higher than that for 5 mg, but maximum concentrations of AmB in serum for 5 and 30 mg were almost similar. These observations identify the pharmacokinetic characteristics of AmB in the lung and may provide a new insight into the strategy for clinical treatment of fungal pneumonia.
Amphotericin B (AmB) remains the standard treatment for most serious fungal infection. Intravenous infusion of AmB has been used successfully for a long time against pulmonary fungal infections (1, 3, 8, 9, 11, 14). It has been known that after intravenous infusion of AmB, roughly 10% of the bioactivity is retained in plasma, strongly bound to plasma proteins (4, 5). The affinity for lipoprotein may influence the tissue distribution and catabolism of the drug. Indeed, lung tissue distribution and/or excretion after intravenous infusion of AmB are not well characterized. Aerosol as well as intravenous AmB has been used in the treatment of pulmonary fungal infections (2, 10, 19). In particular, prophylactic use of aerosol AmB is effective in the prevention of pulmonary aspergillosis in an experimental model (2) and humans (10). However, since little is known about pharmacokinetics of aerosol AmB, optimal dose and regimen for clinical trials remain to be determined (19).
Accordingly, in the present study, we evaluated the pharmacokinetics of AmB in the lung given by two different administrations. First, we studied pharmacokinetics in the lung lymph after AmB infusion to see the movement and/or distribution of AmB to lung lymph circulation and lung interstitium. Second, we performed bronchial washes after aerosol administration and measured the concentration of AmB in the fluid, to determine the elimination rate of the drug in the bronchial epithelium. The experimental animal model used in the study was the sheep, an animal sufficiently large for easy administration of an aerosol and an infusion and repeated sampling.
MATERIALS AND METHODS
Animal preparation.
Adult sheep weighing 28 to 32 kg were used. No animal had previously received AmB or other drugs. Since there were two experimental schedules in the study, two different operations were performed. In one group, lung lymph fistulas were created for the collection of lung lymph fluid by the method by Staub et al. (18). Briefly, sheep was anesthetized with intravenous sodium pentobarbital (12.5 mg/kg of body weight) and then ventilated with 0.5 to 1.0% halothane by positive-pressure ventilation. We inserted catheters into the right carotid artery and extrajugular vein for the collection of blood and for drug infusion, respectively. Through a right thoracotomy in the sixth intercostal space, the efferent lymphatic channel from the caudal mediastinal node was cannulated with a thin silicon tube. This tube was secured and brought to the outside. Through a second thoracotomy in the ninth intercostal space, the tail of the caudal mediastinal node was ligated at the free margin of the inferior pulmonary ligament to eliminate contamination with nonpulmonary lymph. The animals were then allowed to recover with free excess of food and water for at least 7 days before the experiment. In another group, after the cannulation of catheters into the right carotid artery and extrajugular vein, each sheep underwent a tracheotomy for administration of aerosol AmB. The experiment was conducted 2 days after the surgery.
Aerosol generation and delivery system.
Aerosols were generated with an ultrasonic nebulizer (TUR-3200; Nihon Koden Ltd., Tokyo, Japan). The nebulizer produced an aerosol with a mean (and median) aerodynamic diameter of 2 to 6 μm. AmB was dissolved in 5% glucose buffer and aerosolized. The output from the nebulizer was directed into a plastic Y piece attached to the endotracheal tube. The nebulizer was also connected to the inspiratory port of a Harvard respiratory (NSH34RH; Bodine Electronic Co., Chicago, Ill.), and the expiratory port was connected to the other part of the Y piece. The aerosol AmB was delivered at a tidal volume of 500 ml and a rate of 20 ml/min.
Experimental protocols.
AmB was supplied by Bristol Myers Squibb, Tokyo, Japan. Experiments were done with the animals awake and standing. We conducted the following two experiments.
(i) Experiment 1.
Four sheep were prepared for experiment 1. One milligram of AmB per kilogram was dissolved by 5% of 500 ml glucosan and given to the animal by an intravenous drip infusion for 1.5 h via the extrajugular vein. Lung lymph was collected before infusion and 0.75, 1.5, 3, 5, 9, 12, and 24 h after the start of AmB infusion. Blood samples were also drawn from aortic artery at the same time as lung lymph collections.
(ii) Experiment 2.
Two different doses (5 and 30 mg) of AmB were diluted with 5% glucose to a total volume of 15 ml. These drugs were inhaled over 15 min with the nebulizer described above. Wash fluid from the lower respiratory tract was obtained by bronchoalveolar lavage through a fiber-optic bronchoscope (length, 0.9 m; external diameter, 5.6 mm; Olympus Co., Tokyo, Japan) using a 50 ml of normal saline. Bronchoalveolar lavage was performed before and 0.5, 1, 3, 5, 12, and 24 h after the administration of aerosolized AmB. Each bronchoalveolar lavage sample was obtained at a different site. Likewise, blood samples were drawn from an indwelling catheter in the carotid artery. Samples were centrifuged immediately at 4°C and frozen (−80°C) until the time of analysis.
Measurements and analysis of AmB concentrations.
A modular high-performance liquid chromatography system (L-4250, L-6200, AS-4000; Hitachi, Tokyo, Japan) was used for the measurements of AmB. The analyses were performed with Lichrospher RP-18(e) column (4 by 250 mm; Merck). Detection was performed by monitoring fluorescence with excitation and emission set at 405 nm. The mobile phase for quantification of AmB consisted of 10 mM EDTA.2K-methanol (25/75). The range of quantitative detection of AmB was 10 to 5,000 ng/ml. All samples were assayed in duplicate. We calculated the lymph-to-plasma concentration ratio at each time point in experiment 1. Maximum concentration of AmB in serum (Cmax) was determined from the concentrations observed, and the area under the concentration-time curve (AUC) from the initiation of the administration to 24 h after the start of infusion or inhalation was calculated by the trapezoidal method. Data were expressed as means ± standard deviations.
RESULTS
Pharmacokinetics of AmB in plasma and lung lymph.
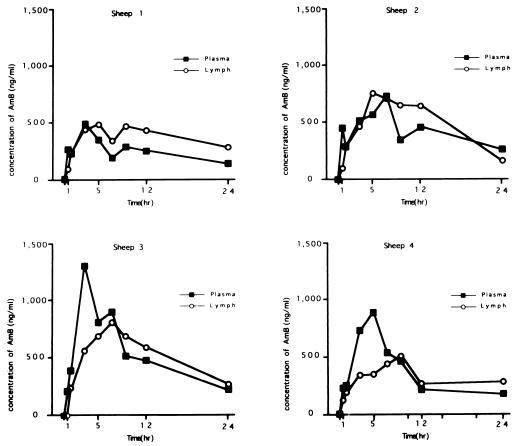
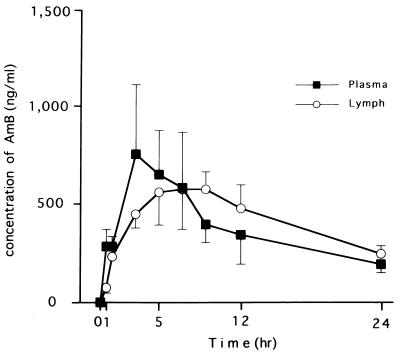
Pharmacodynamics of AmB in plasma and lung lymph after infusion of AmB are summarized in Fig. 1 and 2 and Table 1. The mean peak level in plasma was 756.0 ± 188.8 ng/dl at 3 h after the start of infusion, and the level then decreased gradually to 194.8 ± 28.9 ng/dl at 24 h. The stable and maximal levels in lung lymph last 5 to 9 h after the start of AmB infusion. The concentrations in lung lymph after 9 h were slightly higher than the corresponding concentrations in plasma. Thus, the lung lymph-to-plasma ratio of AmB concentrations increased gradually during infusion, and the ratio was more than 1.0 after the end of infusion. The peak of the ratio was observed at 9 h after the start of AmB infusion. AUCs in plasma and lung lymph were almost the same (9,230 ± 1,280 and 10,000 ± 1,000 ng · h/ml, respectively). These data suggest that AmB could be easily moved from plasma to pulmonary interstitium and/or lung lymph circulation.
FIG. 1.
Time courses of AmB concentrations in plasma and lung lymph after drip infusion.
FIG. 2.
Time course of mean concentrations of AmB in plasma and lung lymph after drip infusion in four sheep. The data are means with standard deviations.
TABLE 1.
Lung lymph-to-plasma AmB concentration ratios after infusion
| h | Mean concn ± SD |
|---|---|
| 0.5 | 0.26 ± 0.20 |
| 1 | 0.85 ± 0.18 |
| 3 | 0.76 ± 0.24 |
| 5 | 0.99 ± 0.40 |
| 7 | 1.12 ± 0.40 |
| 9 | 1.50 ± 0.36 |
| 12 | 1.41 ± 0.20 |
| 24 | 1.38 ± 0.56 |
Pharmacokinetics of aerosol AmB.
The time course of AmB concentrations in bronchial-wash fluid and pharmacokinetic analysis data are summarized in Table 2. The peak AmB concentration in bronchial-wash fluid was observed at 0.5 h. After that, AmB was slowly eliminated over 24 h. The AUC for 30-mg inhaled AmB was higher than that for 5 mg, but Cmax values for 5-mg AmB and 30-mg AmB were almost similar. The drug was undetectable in blood after aerosol administration of 5 and 30 mg.
TABLE 2.
AmB concentrations in bronchial-wash fluid after aerosol administration
| Dose | Concn (ng/ml) at:
|
AUCa (ng · h/ml) | |||||
|---|---|---|---|---|---|---|---|
| 0.5 h | 1 h | 3 h | 5 h | 12 h | 24 h | ||
| 5 mg | |||||||
| Sheep 1 | 74.0 | 62.4 | 45.5 | 87.6 | 25.5 | 25.0 | 240.0 |
| Sheep 2 | 300.0 | 53.3 | 59.7 | 67.1 | 10.5 | 20.5 | 403.1 |
| Sheep 3 | 173.0 | 148.0 | 44.4 | 64.5 | 16.9 | 22.1 | 709.0 |
| Sheep 4 | 388.0 | 78.4 | 59.0 | 20.9 | 20.3 | 20.8 | 575.1 |
| Mean ± SD (n = 4) | 233.8 ± 138.3 | 85.5 ± 42.9 | 45.5 ± 19.2 | 60.0 ± 28.1 | 18.3 ± 6.3 | 22.1 ± 2.0 | 481.8 ± 204.1 |
| 30 mg | |||||||
| Sheep 1 | 242.0 | 199.0 | 165.0 | 115.0 | 43.0 | 42.0 | 1,367.8 |
| Sheep 2 | 156.0 | 63.6 | 82.7 | 172.0 | 26.2 | 24.1 | 1,188.6 |
| Sheep 3 | 255.0 | 102.0 | 161.0 | 67.5 | 45.7 | 23.6 | 1,040.7 |
| Mean ± SD (n = 3) | 217.7 ± 53.8 | 121.5 ± 69.8 | 136.2 ± 46.4 | 118.2 ± 52.3 | 38.3 ± 10.6 | 29.9 ± 10.4 | 1,199.0 ± 163.8 |
From 0 to 24 h.
DISCUSSION
Since the lymph obtained from sheep has been confirmed to be of pulmonary origin, this model has been widely adopted for the studies of lung fluid and protein exchange (6, 12, 13, 15). As previously discussed (6), we believed that determining pharmacokinetics in lung lymph circulation is a useful and encouraging method to evaluate the lung tissue distribution of a drug. Since the pharmacokinetic characteristics of AmB after infusion in sheep are almost similar to those in humans (3, 8, 9, 11), we think that the pharmacological results in lung lymph can be applied to the clinical setting.
It has been known that AmB strongly binds to lipoproteins after intravenous administration (4, 5). The affinity for lipoproteins may affect the distribution and elimination of the drug in the organs and tend to decrease extravascular diffusion. Bindschadler and Bennet reported that concentrations of AmB in cerebrospinal fluid and parotid gland fluid following the administration of a standard dose of AmB were very low (3). In addition, concentrations in urine after intravenous AmB infusion showed a small fraction of the drug in spite of the high concentration in the kidney (3–5, 8, 9, 11). Based on these data, movement and distribution of AmB from blood circulation into body fluid appeared to be poor. However, our present data suggest that AmB was easily transferred from blood to the interstitium space and lung lymph. The high partition of AmB into lung tissue indicated that AmB should have a favorable effect on therapeutic activity against pulmonary fungal infection. Chabot et al. measured AmB concentrations in pleural fluid after continuous infusion of AmB in cancer patients and found them to be 22% of the plasma AmB levels at the same times (8). Furthermore, another study showed that at one time point the concentration of AmB in the blood-free pleural fluid was 1.0 μg/ml while the level in serum was 1.8 μg/ml (3). These findings suggested that AmB was well distributed from blood to pleural space, compared to other body fluids. Production and/or absorption of pleural effusion are partially dependent on lymph circulation (7, 20). Based on the present study, AmB may have a unique pharmacological behavior in the lung. The clearance mechanism for AmB might use the reticuloendothelial system (17). It is likely that the metabolic rate of AmB itself is low in the lung tissue, because the lung has few reticuloendothelial systems.
Aerosol AmB.
We studied the elimination of the drug in the bronchial epithelium after AmB inhalation. To our knowledge, there are no reports of AmB concentrations in bronchial-wash fluid. We did not show the calculated data for concentration in urea. However, the difference in recovery rate between bronchial washes was small (36 to 50%), and bronchial washing was performed at different sites in each experiment. We believe that variabilities and dilution effects in the present data are minimal.
We found that relatively stable levels were maintained during the first 24 h after administration, indicating a slow elimination from bronchial wall. Several investigators have studied the pharmacological characteristics of AmB inhalations, and their findings support our results (2, 16). Niki et al. (16) measured AmB concentrations in the rat lung tissue at different intervals after a single administration of aerosol AmB. They found that the half-life of elimination from the lung tissue was 4.8 days. In addition, they also found that AmB was eliminated more slowly after repeated administrations than after a single dose. Beyer et al. (2) showed substantial remaining activity of radiolabeled AmB in the human lung at 14 h postinhalation using scintigraphy, suggesting a prolonged deposition of AmB. Furthermore, we had a case of pulmonary aspergilloma treated with AmB inhalation. The patient received aerosol AmB at 15 mg/day, and the concentration of AmB was found to be 13.5 ng/ml in plasma at 3 weeks after the initiation of inhalation (unpublished data). Based on these findings and our present data, it is likely that aerosol administration of AmB every day might cause an unexpectedly high accumulation in the lung tissue. The prophylactic dose and schedules of aerosol AmB have not been established yet. It is necessary to analyze the late pharmacokinetics after repeated daily inhalation of AmB in order to evaluate the safety of its clinical applicability.
We studied two different doses of aerosol AmB, 5 and 30 mg. Interestingly, Cmax values in the bronchial-wash fluid at 0.5 h after administration of 5 and 30 mg aerosol AmB were almost the same, although AUC showed a stepwise increase in concentration with the higher dose. Our results suggest that a peak concentration in the bronchus after AmB inhalation might not be always directly proportional to dose. The inhaled dose may be influenced by the duration of inhalation. However, since we chose a sixfold difference in the dose of AmB, our mean peak concentrations results were evaluable and may show pharmacokinetic characteristics of aerosol AmB.
In summary, we have described the lung tissue distribution and penetration of AmB given by intravenous and aerosol administrations. AmB is still the most effective agent currently available for the treatment and prevention of serious fungal infection. More clinical and experimental studies could be needed to determine the best tolerated and effective regimen of AmB.
ACKNOWLEDGMENT
We thank M. Nagasaka for technical assistance and cooperation.
REFERENCES
- 1.Bennett J E. Chemotherapy of systemic mycoses (first of two parts) N Engl J Med. 1974;290:30–32. doi: 10.1056/NEJM197401032900107. [DOI] [PubMed] [Google Scholar]
- 2.Beyer J, Barzen G, Risse R, Weyer C, Miksits K, Dullenkopf K, Huhn D, Siegert W. Aerosol amphotericin B for prevention of invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 1993;37:1367–1369. doi: 10.1128/aac.37.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bindschadler D D, Bennett J E. A pharmacologic guide to the clinical use of amphotericin B. J Infect Dis. 1969;120:427–436. doi: 10.1093/infdis/120.4.427. [DOI] [PubMed] [Google Scholar]
- 4.Block E R, Bennett J E, Livoti L G, Klein W J, MacGregor R R, Henderson L. Flucytosine and amphotericin B; hemodialysis effects on the plasma concentration and clearance. Ann Intern Med. 1974;80:613–617. doi: 10.7326/0003-4819-80-5-613. [DOI] [PubMed] [Google Scholar]
- 5.Brajtburg J, Elberg S, Bolard J, Kobayashi G S, Levi R A, Ostlund R E, Schlessinger D, Medoff G. Interaction of plasma proteins and lipoproteins with amphotericin B. J Infect Dis. 1984;149:986–997. doi: 10.1093/infdis/149.6.986. [DOI] [PubMed] [Google Scholar]
- 6.Brigham K L, Boweres R, Haynes J. Increased sheep lung vascular permeability caused by Escherichia coli endotoxin. Circ Res. 1979;45:292–297. doi: 10.1161/01.res.45.2.292. [DOI] [PubMed] [Google Scholar]
- 7.Broaddus V C, Wiener-Kronish J P, Berthiaume Y, Staub N C. Removal of pleural liquid and protein by lymphatics in awake sheep. J Appl Physiol. 1988;64:384–390. doi: 10.1152/jappl.1988.64.1.384. [DOI] [PubMed] [Google Scholar]
- 8.Chabot G G, Pazdur R, Valeriote F A, Baker L H. Pharmacokinetics and toxicity of continuous infusion amphotericin B in cancer patients. J Pharm Sci. 1989;78:307–310. doi: 10.1002/jps.2600780409. [DOI] [PubMed] [Google Scholar]
- 9.Collette N, van der Auwera P, Lopez A P, Heymans C, Meunier F. Tissue concentrations and bioactivity of amphotericin B in cancer patients treated with amphotericin B-deoxycholate. Antimicrob Agents Chemother. 1989;33:362–368. doi: 10.1128/aac.33.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conneally E, Cafferkey M T, Daly P A, Keane C T, McCann S R. Nebulized amphotericin B as prophylaxis against invasive aspergillosis in granulocytopenic patients. Bone Marrow Transplant. 1990;5:403–406. [PubMed] [Google Scholar]
- 11.Fields B T, Bates J H, Abernathy R S. Amphotericin B serum concentrations during therapy. Appl Microbiol. 1970;19:955–959. doi: 10.1128/am.19.6.955-959.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koizumi T, Kubo K, Kobayashi T, Sekiguchi M. Effects of thromboxane synthase inhibition on tumor necrosis factor-induced lung injury in sheep. J Appl Physiol. 1992;73:618–624. doi: 10.1152/jappl.1992.73.2.618. [DOI] [PubMed] [Google Scholar]
- 13.Koizumi T, Kubo K, Shinozaki S, Koyama S, Amari T, Hayano T, Fujimoto K, Kobayashi T, Sekiguchi M, Sakai R, Ohshima T, Miyamoto K. Pharmacokinetic evaluation of (glycolato-O,O′)diammine platinum(II) in lung lymph in sheep. Jpn J Cancer Res. 1993;84:468–473. doi: 10.1111/j.1349-7006.1993.tb00160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medoff G, Kobayashi G S. Strategies in the treatment of systemic fungal infections. N Engl J Med. 1980;302:145–155. doi: 10.1056/NEJM198001173020304. [DOI] [PubMed] [Google Scholar]
- 15.Newman J H, Butka B J, Parker R E, Roselli R J. Effect of progressive exercise on lung fluid balance in sheep. J Appl Physiol. 1988;64:2125–2131. doi: 10.1152/jappl.1988.64.5.2125. [DOI] [PubMed] [Google Scholar]
- 16.Niki Y, Bernard E M, Schmitt H J, Tong W P, Edwards F F, Armstrong D. Pharmacokinetics of aerosol amphotericin B in rats. Antimicrob Agents Chemother. 1990;34:29–32. doi: 10.1128/aac.34.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paul M, Durand R, Fessi H, Rivollet D, Houin R, Astier A, Deniau M. Activity of a new liposomal formulation of amphotericin B against two strains of Leishmania infantum in a murine model. Antimicrob Agents Chemother. 1997;41:1731–1734. doi: 10.1128/aac.41.8.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Staub N C, Bland R D, Brigham K L, Demling R, Erdman A J, Woolverton W C. Preparation of chronic lung lymph fistulas in sheep. J Surg Res. 1975;19:315–320. doi: 10.1016/0022-4804(75)90056-6. [DOI] [PubMed] [Google Scholar]
- 19.Uzun O, Anaissie E J. Antifungal prophylaxis in patients with hematologic malignancies: a reappraisal. Blood. 1995;86:2063–2072. [PubMed] [Google Scholar]
- 20.Wiener-Kronish J P, Broaddus V C, Albertine K H, Gropper M A, Matthay M A, Staub N C. Relationship of pleural effusions to increased permeability pulmonary edema in anesthetized sheep. J Clin Invest. 1988;82:1422–1429. doi: 10.1172/JCI113747. [DOI] [PMC free article] [PubMed] [Google Scholar]