ABSTRACT
Perivascular fibroblasts (PVFs) are a fibroblast-like cell type that reside on large-diameter blood vessels in the adult meninges and central nervous system (CNS). PVFs contribute to fibrosis following injury but their homeostatic functions are not defined. PVFs were previously shown to be absent from most brain regions at birth and are only detected postnatally within the cerebral cortex. However, the origin, timing and cellular mechanisms of PVF development are not known. We used Col1a1-GFP and Col1a2-CreERT2 transgenic mice to track PVF development postnatally. Using lineage tracing and in vivo imaging we show that brain PVFs originate from the meninges and are first seen on parenchymal cerebrovasculature at postnatal day (P) 5. After P5, PVF coverage of the cerebrovasculature expands via local cell proliferation and migration from the meninges. Finally, we show that PVFs and perivascular macrophages develop concurrently. These findings provide the first complete timeline for PVF development in the brain, enabling future work into how PVF development is coordinated with cell types and structures in and around the perivascular spaces to support normal CNS vascular function.
Keywords: Neurovascular unit, Meninges, Cell migration, Perivascular spaces, Perivascular macrophages, Cerebral vasculature, Mice
Highlighted Article: Brain perivascular fibroblasts migrate from their origin in the meninges and proliferate locally to fully cover penetrating vessels during postnatal mouse development.
INTRODUCTION
The central nervous system (CNS) requires continuous delivery of blood containing oxygen and nutrients. This is ensured by a robust vascular network made up of different cell types – endothelial cells, pericytes, vascular smooth muscle cells (vSMCs), astrocytes, immune cells and perivascular fibroblasts (PVFs) – which come together to form the specialized neurovascular unit. The endothelium, pericytes, vSMCs and astrocytes have all been well studied in the context of development of the neurovascular unit and their developmental origins are known (Coelho-Santos and Shih, 2020; Paredes et al., 2018). In contrast, much less is known about how PVFs develop, integrate and function in the neurovasculature.
PVFs are a fibroblast-like cell type located on the outside of the vSMC layer of large-diameter arterioles and venules, but not capillaries, in the adult mouse brain (Bonney et al., 2022; Soderblom et al., 2013; Vanlandewijck et al., 2018) and in adult human brain (Garcia et al., 2022; Yang et al., 2022). PVFs express collagen 1 and are distinct from other mural cells (pericytes and vSMCs) in their expression of both platelet derived growth factor (PDGF) receptors α and β and absence of mural cell markers including desmin and NG2 (Fernández-Klett et al., 2013; Kelly et al., 2016; Riew et al., 2020; Soderblom et al., 2013; Vanlandewijck et al., 2018). Given their position within the neurovascular unit, PVFs are uniquely positioned to provide support to vascular structures and cell types, potentially via secretion or modification of extracellular matrix proteins such as type I and III collagens (Hannocks et al., 2018; Vanlandewijck et al., 2018; Yang et al., 2022). Most research on PVFs has focused on their robust CNS injury and disease response. Our lab and others have shown that, following acute CNS injuries such as stroke and traumatic brain injury, PVFs detach from the vasculature, proliferate and contribute to fibrotic scar formation (Fernández-Klett et al., 2013; Kelly et al., 2016; Soderblom et al., 2013). In addition, there is evidence that PVFs contribute to pathologies seen in neurodegeneration and neuroinflammation (Dorrier et al., 2021; Månberg et al., 2021). Despite what is known about the role of PVFs in CNS injury and disease, there have been few insights into the cellular mechanisms of their development and how this relates to other neurovascular cell types. Previous work from our lab has shown that PVFs are absent from the brain vasculature at birth and only appear postnatally (Kelly et al., 2016), days after other cell types begin to assemble a functional neurovascular unit. An important step towards understanding the homeostatic and disease-response functions of PVFs is to identify the cellular mechanisms that underlie PVF development, and how PVF development relates to other cell types in the neurovascular unit.
The collagen 1a1 (Col1a1)-GFP and collagen 1a2 (Col1a2)-CreERT2 mouse lines are established tools for labeling and identifying PVFs in the mouse brain, as they are expressed in all CNS fibroblasts (Bonney et al., 2022; Dorrier et al., 2021; Soderblom et al., 2013). Here, we use these tools to investigate PVF development in the cerebral cortex during postnatal development. We show that Col1a1-GFP+ PVFs appear on leptomeningeal vessels embryonically, but do not start to cover vessels in the cerebral cortex until postnatal day (P)5 and reach full coverage by P14. We also show that timing of brain PVF appearance aligns with the timing of another CNS perivascular cell type, perivascular border-associated macrophages (PVMs), with PVF and PVM coverage of cerebral vessels occurring in tandem. Lineage tracing using the Col1a2-CreERT2 allele along with tdTomato-flox and Brainbow-flox reporters shows that PVFs in the brain originate from the meninges and have distinct clonal boundaries on cerebral vessels, suggesting specific temporal and regional contributions of PVFs to vessel coverage. Further, in vivo two-photon imaging and proliferation assays reveal that both migration and proliferation drive PVF coverage of vessels. From these data emerge the first comprehensive timeline of PVF development in the postnatal cerebral cortex, enabling a more complete model of postnatal cerebrovascular development and insight into how PVF development may be coordinated with other perivascular cell types and structures.
RESULTS
PVFs emerge on cerebral vessels between P5 and P14 in the mouse cerebral cortex
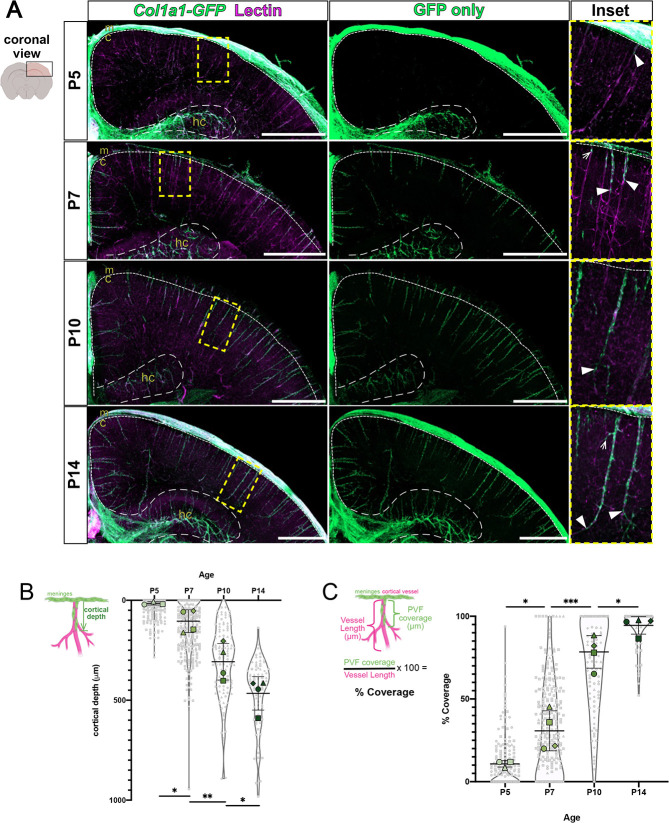
To create a timeline of PVF emergence in the postnatal mouse cerebral cortex, we used the Col1a1-GFP transgenic mouse line, in which all CNS fibroblasts including PVFs are labeled, along with CUBIC tissue clearing of 2 mm brain slices to observe PVF locations at different time points. We used a fluorescent lectin to label vasculature in cleared brains, confirmed by co-localization with antibody labeling for vascular marker PECAM (Fig. S1A). In the adult mouse brain, PVFs are present on large-diameter vessels across all brain regions (Bonney et al., 2022; Kelly et al., 2016). We analyzed non-capillary penetrating vessels in the cerebral cortex, as it has been established that PVFs localize to penetrating arterioles and ascending venules but not capillaries in mice (Bonney et al., 2022; Vanlandewijck et al., 2018). At P5, Col1a1-GFP+ fibroblasts were abundant in the meninges and along the hippocampal vasculature but were rare in the cortex (Fig. 1A). At this age, ∼15% of all penetrating vessels in the cortex had some coverage by Col1a1-GFP+ PVFs (Fig. S1B) and the PVFs present were located close to the meningeal surface (Fig. 1A). At P7 and P10, GFP+ PVFs continued emerging and covering vessels (Fig. 1A). The percent of penetrating vessels with some amount of PVF coverage at P7 and P10 increased significantly, from ∼15% at P5 to 60% at P7 and to 99% at P10 (Fig. S1B). By P14 GFP+ PVFs reached the terminus of vessels (Fig. 1A, P14 inset, arrowheads) and all cerebral penetrating vessels contained PVFs (Fig. S1B). These results are consistent with our previous work using collagen 1 protein expression to observe appearance of fibroblasts between P0 and P21 in the mouse brain (Kelly et al., 2016).
Fig. 1.
Col1a1-GFP+ PVFs increase in depth in the cerebral cortex to cover large-diameter vessels between P5 and P14. (A) Maximum projection images of coronal sections (z-volume: 500-1000 µm) of CUBIC-cleared P5-P14 Col1a1-GFP brains showing fibroblasts (GFP, green) and vessels (lectin, magenta). Arrowheads mark furthest-migrated GFP+ PVFs on vessels. Arrow in P7 inset indicates PVF emerging on vessel. Arrow in P14 inset indicates PVF extending on vessel branch. Decreased GFP fluorescence above cortex in P7 and P10 images was due to meninges damage during processing. Dashed lines indicate borders between the meninges and cortex (small dashes), and hippocampus and cortex (large dashes). c, cortex; hc, hippocampus; m, meninges. Scale bars: 1 mm. (B,C) Graphs depicting maximum depth of the furthest-migrated PVF on vessels (B) and percent coverage of vessels at P5-P14 (C). The mean for each biological replicate (n=3-4 for each age) is represented by large colored shapes and the corresponding technical replicates (n=15-255 vessels per animal) in small gray matching shapes. Data are mean±s.d. *P<0.05, **P<0.01, ***P<0.001. One-way ANOVA with multiple comparisons indicates statistically significant differences in cerebral depth and coverage between each time point: (B) P5 versus P7, P=0.0468; P7 versus P10, P=0.0094; P10 versus P14, P=0.0434; (C) P5 versus P7, P=0.0393; P7 versus P10, P=0.0009; P10 versus P14, P=0.0289.
To investigate the dynamics of PVF appearance, we first measured the cortical depth of the furthest-traveled PVF on individual vessels from the meningeal surface at P5, P7, P10 and P14 (Fig. 1B). At P5, PVFs remained close to the meningeal surface, with a mean depth of ∼11 µm (Fig. 1B). PVF depth on vessels increased significantly from P5 to P7 (P7 mean=99.3 µm) and from P7 to P10 (P10 mean=300.4 µm) (Fig. 1B). By P14, PVFs reached a mean depth of ∼457 µm, a significant increase from P10 (Fig. 1B). We also conducted analysis of the extent of each vessel covered by PVFs at each time point by measuring the length of PVF coverage on individual vessels and dividing this measurement by the total vessel length to yield percent coverage (Fig. 1C). Consistent with our depth analysis, at P5, PVFs remained close to the surface of the cortex, with the mean vessel coverage at 3% (Fig. 1C). This increased significantly from P5 to P7, with the mean percent coverage increasing to 25% (Fig. 1C). Between P7 and P10, PVFs significantly increased their coverage of vessels from 25% to 77% (Fig. 1C); this corresponds with the timespan in which there was the greatest increase in the depth of PVFs on vessels (Fig. 1B, P7 to P10). Between P10 and P14, PVF coverage again significantly increased from 77% to 94%. We measured the density of PVFs within the covered regions on vessels and found no significant changes in cell density across time points (Fig. S1C), indicating that PVFs on cerebral vessels increase their overall numbers to cover a greater length of vessel. It is important to note we did not distinguish between arterioles and venules in our analysis. Previous work on PVFs in adult mice show that venules tend to have lower PVF coverage than arterioles (Bonney et al., 2022), this is likely reflected by data points of PVFs in Fig. 1B,C that had less than average depth or coverage. Taken together, these data show that PVF appearance between P5 and P14 corresponds with an increase in the depth of PVFs on vessels and an increase of coverage of cerebral vessels without any change in PVF density.
Lineage tracing reveals a meningeal origin for cortical PVFs
PVFs are absent from the brain before P5 but are present in the meninges directly above the cortical vessels they will eventually populate. This sets up the question; do brain PVFs originate from the meninges? To test this, we conducted lineage tracing using the inducible Col1a2-CreERT2 transgenic mouse line that is expressed by fibroblasts in the CNS but not other perivascular cell types like vSMCs (Bonney et al., 2022; Dorrier et al., 2021; Zheng et al., 2002). We combined the Col1a2-CreERT2 line with an Ai14(tdTomato)-flox reporter to achieve fibroblast-specific expression of tdTomato only upon introduction of tamoxifen (Fig. S2). We injected Col1a2-CreERT2;tdTomato-flox pups with a saturating dose of tamoxifen (e.g. a high enough dose to cause recombination in most fibroblasts) at P1 and collected brains at P3, P7 and P14 (Fig. 2). At P3, tdTomato+ fibroblasts were observed in the meninges and around hippocampal vasculature but were completely absent from vessels in the cortex (Fig. 2, P3 inset). This is consistent with what we saw in the Col1a1-GFP mouse line at P5, with GFP+ fibroblasts almost exclusively localized to the meninges and the hippocampal vasculature and not yet in the cortex (Fig. 1A). At P7, we saw tdTomato+ PVFs appear on vessels in the cortex at depths similar to what we saw in the Col1a1-GFP line at the same time point (Fig. 2, P7 inset, arrowheads), and by P14 we saw tdTomato+ PVFs extensively covering the cerebral vessels in addition to labeling cells in the meninges, mirroring what we observed with the Col1a1-GFP line at P14 (Fig. 2, P14 inset, and Fig. 1A). Thus, PVFs in the brain developmentally originate from fibroblasts in the meninges.
Fig. 2.
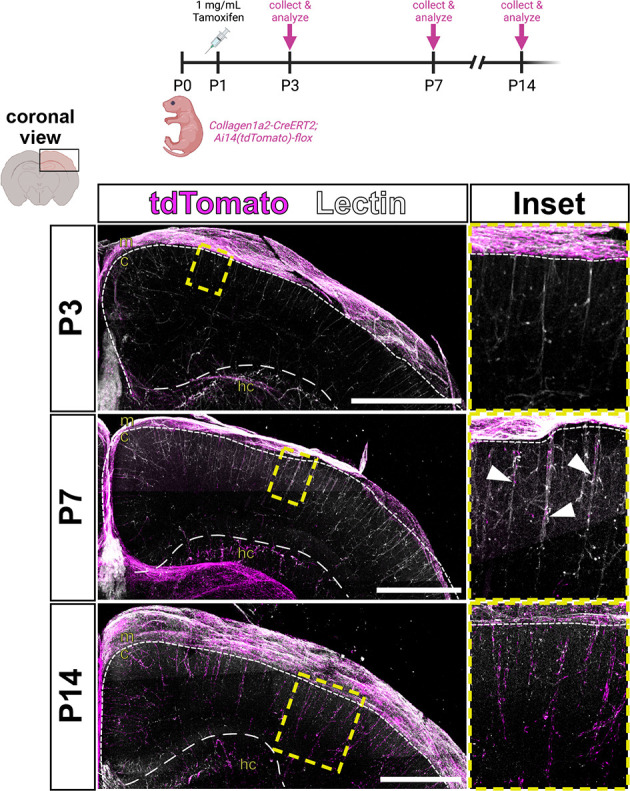
Parenchymal PVFs originate in the meninges. Schematic showing experimental procedure, and maximum-projection images of coronal sections (z-volume: 500-1000 µm) of CUBIC-cleared Col1a2-CreERT2;Ai14-flox brains showing vessels (lectin, white) and fibroblasts recombined with tamoxifen at P1 (tdTomato, magenta), followed at P3, P7 and P14. Arrowheads in P7 inset indicate furthest-migrated tdTomato+ PVFs on each vessel. Dashed lines indicate the borders between the meninges and cortex (small dashes), and hippocampus and cortex (large dashes). Yellow box indicates area magnified in insets. c, cortex; hc, hippocampus; m, meninges. Scale bars: 1 mm.
PVFs first appear in the leptomeninges of embryonic and early postnatal mouse brains
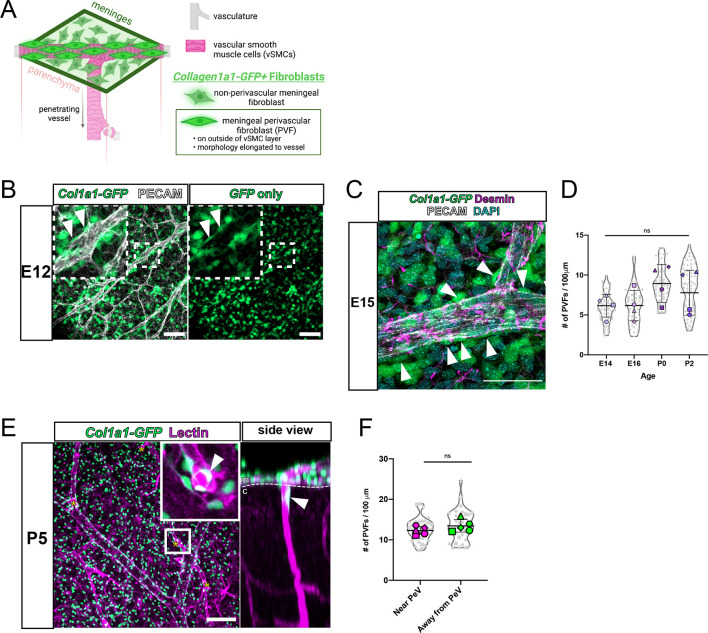
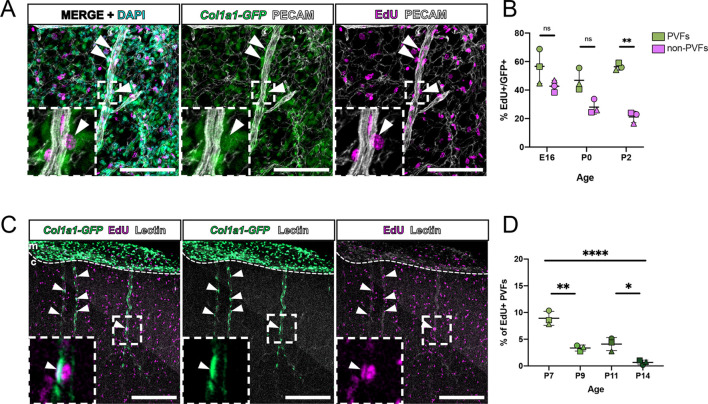
Col1a1-GFP+ PVFs do not appear on cerebral vessels until P5 but are abundant in the meninges at earlier ages (Figs 1A and 2). To better understand the origins of brain PVFs, we investigated PVFs in the meninges before P5. The pial layer of the leptomeninges contains an extensive vascular network that gives rise to the penetrating vessels of the cortex, with non-perivascular and perivascular meningeal fibroblasts distinguished based on localization and morphology (Fig. 3A). We first sought to understand when PVFs begin appearing in the leptomeninges; to investigate this we prepared flat-mounts of the leptomeninges at embryonic day (E)12 (Fig. 3B). At this time, the meninges are a meshwork of Col1a1-GFP+ fibroblasts surrounding the CNS but have not begun to specify into molecularly distinct meningeal layers (DeSisto et al., 2020). Though the fibroblasts at E12 remained mostly undifferentiated, we observed meningeal PVFs with characteristic elongated morphology along vessels at this time point (Fig. 3B inset, arrowheads). As meningeal layer-specific markers emerge around E14-E15 (DeSisto et al., 2020), PVFs continued associating with meningeal vessels and did not express the mural cell marker desmin (Fig. 3C, arrowheads). Staining for protein expression of layer-specific markers S100A6 (pia-specific) and RALDH2 (arachnoid-specific) in E15 flat-mounts revealed that meningeal PVFs heterogeneously express both markers (Fig. S3A,B, arrowheads). We measured the density of Col1a1-GFP+ PVFs on meningeal vessels at E14, E16, P0 and P2 and found that their density did not significantly increase from E14 through to P0 (Fig. 3D). Together, these results suggest that, during embryonic development, meningeal PVFs associate with the meningeal vasculature and reflect a morphologically and spatially distinct population from non-perivascular meningeal fibroblasts and other perivascular cell types, in particular mural cells.
Fig. 3.
PVFs are present on cerebral leptomeningeal vessels embryonically before initiating coverage of parenchymal cerebral vessels at P5. (A) Model depicting location and characteristics of meningeal PVFs before development on parenchymal vessels. (B) Flat-mount of E12 meninges showing fibroblasts (GFP, green) and vasculature (PECAM, white). Arrowheads in inset (magnification of white box) mark meningeal PVFs. (C) Flat-mount of E15 meninges showing fibroblasts (GFP, green), vasculature (PECAM, white), pericytes/vSMCs (desmin, magenta) and nuclei (DAPI, cyan). Arrowheads mark meningeal PVFs. (D) Graph depicts density (number of cells per 100 µm of vessel length) of PVFs on meningeal vessels at E14-P2, with means of each biological replicate (n=4 for each age) represented by large colored shapes and corresponding technical replicates (n=5-28 vessels per animal) in small gray matching shapes. One-way ANOVA with multiple comparisons revealed no significant changes across time points (ns). (E) En-face maximum projection of a 200 µm sub-stack of the meninges in a CUBIC-cleared P5 Col1a1-GFP cortex, showing fibroblasts (GFP, green) and vasculature (lectin, magenta). Yellow asterisks mark vessels penetrating the cortex containing parenchymal ‘initating’ PVFs (inset, arrowhead). Side view shows the yz-plane of the inset showing penetrating vessel and PVF (arrowhead). c, cortex; m, meninges. (F) Graph depicts density of PVFs on meningeal vessels at P5 near and away from a PeV with means of each biological replicate (n=5) represented by large colored shapes and corresponding technical replicates (n=8 PeV areas and n=8 non-PeV areas per biological replicate) in small gray matching shapes. An unpaired t-test between groups revealed no significant changes across time points (ns). Data are mean±s.d. Scale bars: 100 µm (B,E); 50 µm (C).
Blood vessels in the meninges are directly connected to the penetrating vessels of the cortex (Fig. 3A). Our data show that up until P5, PVFs are exclusively associated with meningeal vessels. To investigate the localization of PVFs at P5, we visualized the meningeal vasculature and cerebral penetrating vessels concurrently using whole-cortex clearing and imaging (Fig. 3E). We observed penetrating vessels branching from meningeal vessels (Fig. 3E, asterisks) with some penetrating vessels wrapped with a Col1a1-GFP+ PVF proximal to the meningeal surface (Fig. 3E, inset and side view, arrowheads). We referred to PVFs on penetrating vessels proximal to the meningeal surface as ‘initiating PVFs’, as they are the first PVFs to appear on cerebral penetrating vessels (PeVs) and are initiating coverage of vessels at this time point. We wondered whether, to ‘prepare’ for initiation of coverage, the density of PVFs near a penetrating vessel branching off a meningeal vessel (PeV junction point) would be higher. To address this, we measured the density of PVFs at P5 near and away from PeV junction points and found no significant difference (Fig. 3F). This suggests that initiating PVFs do not increase in number surrounding PeV points to prepare for coverage of cortical vessels, but instead may be opportunistic in their entry into the brain. Combining information from our embryonic meningeal (Fig. 3A-C) and postnatal analyses (Fig. 1), P5 appears to be a crucial time point in PVF development in which meningeal PVFs initiate coverage of cerebral vessels.
PVFs appear on cortical vessels after vSMCs and astrocytic endfeet have been established
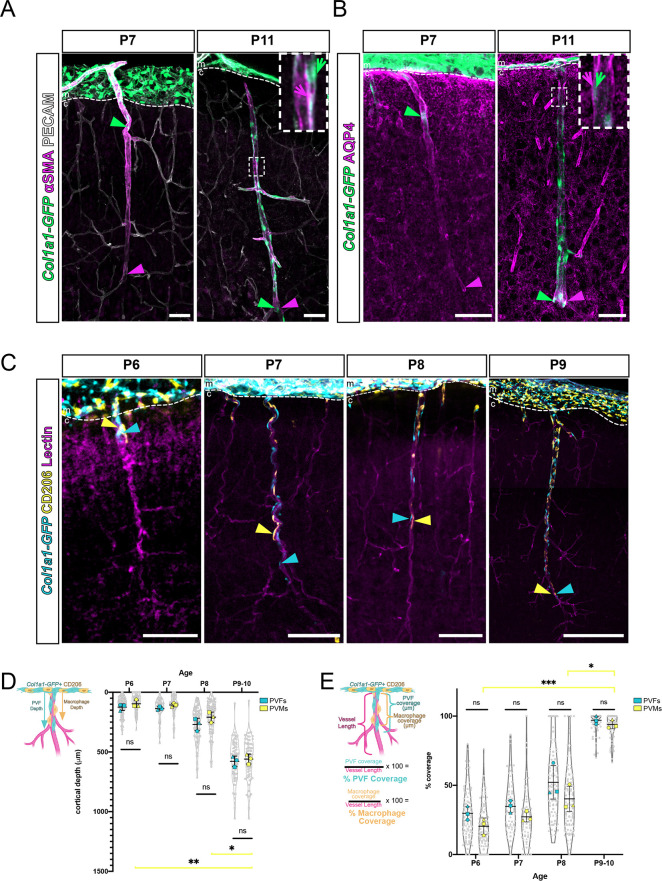
Col1a1-GFP+ PVFs established coverage of cortical vessels between P5 and P14 (Fig. 1), across time points in which the cerebral vasculature is in place and is undergoing remodeling (Coelho-Santos and Shih, 2020). We next sought to understand how PVF appearance at P5-P14 corresponds with the development and appearance of other cell types within the perivascular niche, namely vSMCs and astrocytes. vSMCs are mural cells labeled by αSMA and found abundantly on cortical penetrating arterioles. We labeled sections of Col1a1-GFP+ brains at P7 and P11 with αSMA to determine the relative location and depth of PVFs compared with vSMCs at these two time points (Fig. 4A). At P7, we observed PECAM-labeled cortical vessels covered to their terminus with αSMA+ vSMCs, whereas PVFs were observed on the outside of the αSMA+ layer and more proximal to the meninges (Fig. 4A). By P11, PVFs had extended their coverage on top of the αSMA+ vSMC layer to the terminus of vessels (Fig. 4A). These findings show that vSMCs are in place on vessels before the appearance of PVFs.
Fig. 4.
Col1a1-GFP+ PVFs appear after vSMCs and AQP4+ endfeet coverage of vessels and concurrently with CD206+ PVMs on cerebral vessels postnatally. (A,B) Maximum projection images from 100 µm-thick slices of Col1a1-GFP brains at P7 and P11 showing fibroblasts (GFP, green), vSMCs (αSMA, magenta) and vessels (PECAM, white) (A), and fibroblasts (GFP, green) and astrocytic endfeet (AQP4, magenta) (B). Arrowheads mark positions of furthest-migrated GFP+ PVFs (green arrowheads) and extent of coverage of αSMA or AQP4 (magenta arrowheads) on vessels. Arrows in insets (magnification of white box) indicate positioning of PVFs (green arrow) relative to αSMA or AQP4 labeling (magenta arrow). (C) Maximum projection images (z-volume: 50-100 µm) of CUBIC-cleared Col1a1-GFP brains at P6-P9 showing fibroblasts (GFP, cyan) and PVMs (CD206, yellow) on vessels (lectin, magenta). Arrowheads mark positions of furthest-migrated GFP+ PVFs (cyan arrowhead) and CD206+ PVM (yellow arrowhead). Dashed lines indicate the border between the meninges and cortex. (D,E) Graphs depicting the maximum depth of the furthest-migrated PVF and PVM on vessels (D) and percent coverage of vessels by each cell type (E) at P6-P9/P10. Means of each biological replicate (n=3 for each age) are represented by large colored shapes (PVFs, cyan; PVMs, yellow) and corresponding technical replicates (n=10-50 vessels per animal) in small gray matching shapes. Data are mean±s.d. *P<0.05, **P<0.01, ***P<0.001. One-way ANOVA with multiple comparisons of PVF and PVM groups at each age reveal no statistical significance in depth or coverage measurements between cell types from P6 to P9/P10, but revealed statistical significance between PVM depth and coverage across time points: (C) P6 versus P9/P10, P=0.0097; P8 versus P9/P10, P=0.0354; (D) P6 versus P9/P10, P=0.0009; P8 versus P9/P10, P=0.0353. c, cortex; m, meninges. Scale bars: 100 µm.
Astrocytes form endfeet processes along the entire brain vascular network, and polarization of the water transporter protein aquaporin 4 (AQP4) is required for endfoot functionality in perivascular ‘glymphatic’ fluid flow (Iliff et al., 2012; Mestre et al., 2018). Previous work has shown that AQP4 detection in endfeet increases between P1 and P7 (Munk et al., 2019) and a recent preprint showed that astrocyte endfeet maturation occurs between P0 and P21 in the mouse cortex, with partial endfeet coverage of vessels by P7 (Freitas-Andrade et al., 2023). To understand how timing of AQP4 endfeet localization corresponds to appearance of PVFs, we labeled sections of Col1a1-GFP+ brains at P7 and P11 with astrocyte endfoot marker AQP4. At P7, AQP4 was detected along penetrating vessels to their terminus, whereas PVFs were observed more proximal to the meninges on penetrating vessels (Fig. 4B). By P11, both PVFs and AQP4 labeling extended the entire length of penetrating vessels (Fig. 4B). This shows that AQP4 polarization to endfeet occurs before PVF coverage of vessels. AQP4 labeling appears to be covering the outside of PVFs positioned on vessels (Fig. 4B, inset, arrows). Taken together with labeling of αSMA, this suggests that PVFs cover vessels after vSMCs and astrocyte endfeet are established, and PVFs localize between these two cell types within the perivascular niche.
PVFs and PVMs develop concurrently in the postnatal mouse brain
Recent work has shown that a subset of CNS border-associated macrophages – PVMs, defined by expression of CD206 and their location in perivascular spaces of large diameter vessels – arise from CD206+ leptomeningeal macrophages and appear postnatally (P5-P21) on vessels in the mouse brain (Karam et al., 2022; Masuda et al., 2022). Given the similar timing to PVF development, we analyzed the dynamics of PVF and PVM progression onto cerebral vessels.
First, we determined the relative location, depth and density of CD206+ PVMs and Col1a1-GFP+ PVFs on cerebral vessels at P5, P6, P7, P8 and P9 (Fig. 4C-E). Similar to PVFs, PVMs initiated coverage of penetrating vessels at P5, though their density proximal to and away from PeV points in the meninges was not significantly different (Fig. S4A,B). At P6 and P7, both PVMs and PVFs localized to vessels (Fig. 4C), with most vessels containing both cell types (Fig. S4C; P6, both=81.82%; P7, both=91.30%). PVFs were observed slightly deeper along vessels compared with PVMs (Fig. 4C, P6 and P7, arrowheads) and our analysis showed that most vessels at P6 and P7 time points had a PVF located deeper than a PVM (Fig. S4D; P6, PVF deepest=67.05%; P7, PVF deepest=66.67%). Further, a greater percentage of cerebral vessels had only a PVF at P6 compared with P7 (Fig. S4C; P6, PVF only=15.91%, PVM only=2.27%; P7, PVF only=7.25%, PVM only=1.45%). Like PVFs, PVMs followed a similar pattern with respect to depth on vessels across time points, with PVMs significantly increasing their depth on vessels from P6 to P9/P10 (Fig. 4D); however, the average depth of PVFs compared with PVMs on all cerebral vessels analyzed at P6, P7, P8 or P9/P10 was not significantly different (Fig. 4D). By P9/P10, all cerebral vessels analyzed contained both PVFs and PVMs (Fig. S4C), but PVFs were more often observed slightly deeper than PVMs (Fig. S4D). These data point to a model in which PVFs initiate coverage of cerebral vessels first and are quickly followed by PVMs.
To quantify the dynamics of PVF and PVM coverage, we analyzed the percent coverage of PVFs and PVMs on individual vessels at P6, P7, P8 and P9/P10, and found similar trends in the two cell types (Fig. 4E). Like PVFs, PVMs significantly increased coverage of vessels from P6 to P9/P10, with a mean of 93% PVM coverage by P9/P10 (Fig. 4E). PVFs had a slightly higher average percent coverage at each age; however, there were no statistical differences between the percent coverage of PVFs and PVMs on vessels at each time point (Fig. 4E). We compared density of PVFs and PVMs on vessels within covered regions and found that PVFs and PVMs had approximately the same density across time points, suggesting they were present in a roughly 1-to-1 ratio during postnatal development (Fig. S4F). Similar to PVFs, PVMs did not change in density on vessels from P6-P9/P10 yet they increased in percent coverage over these time points, meaning PVMs increased in number to cover a greater vessel length (Fig. S4F). This aligns with previous work showing clonal expansion of PVMs along vessels from P1 to P14, suggesting that they proliferate locally to cover vessels during this developmental window (Masuda et al., 2022). Taken together, these data show that two different perivascular cell types, macrophages and fibroblasts, develop in perivascular spaces largely in tandem in the postnatal brain.
Lineage tracing reveals clonal domain patterns of brain PVFs on cortical blood vessels
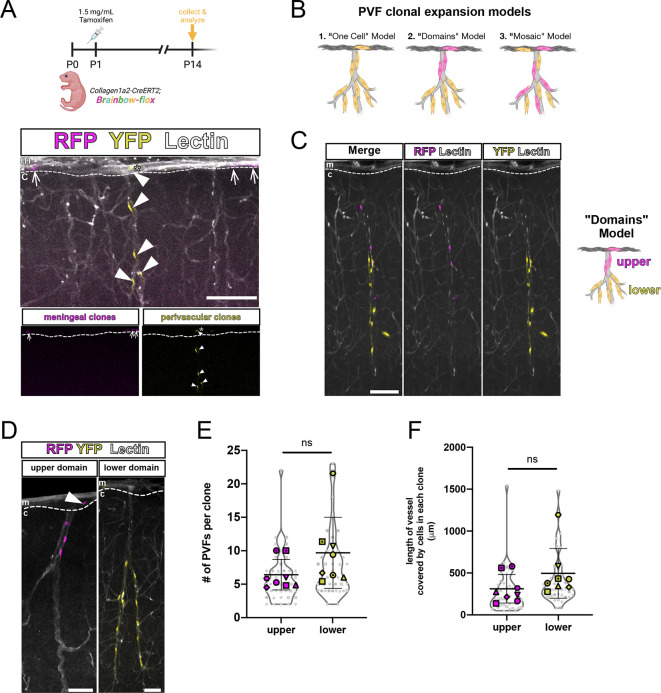
PVFs located on vessels in the brain after P5 appear to emerge from a population of PVFs positioned along the meningeal vasculature during embryonic development and before P5 (Figs 2 and 3). We next wanted to understand the clonal lineage of cerebral PVFs and determine whether there were specific patterns to PVF expansion on vessels. We predicted that meningeal PVFs directly produce brain PVFs on nearby penetrating vessels. To test this, we used the Col1a2-CreERT2 line along with the R26R-Confetti(Brainbow)-flox reporter line, which labels recombined cells stochastically with one of four fluorescent proteins. We determined a tamoxifen dosage at which the Col1a2-CreERT would induce sparse recombination in CNS fibroblasts. This allowed us to identify spatially disparate groups of cells, or clones, that arose from a single recombined cell. We injected Col1a2-CreERT2;Brainbow-flox pups at P1 with a sparse labeling dose of tamoxifen and collected brains at P14 (Fig. 5A). In P14 brains, we observed a population of recombined cells exclusively localized in the meninges, such as the RFP+ cells in the representative image shown (Fig. 5A, meningeal clones, arrows). These labeled cells were likely recombined non-perivascular meningeal fibroblasts and were never observed with corresponding clones on adjacent cerebral vessels. We also observed recombined brain PVFs covering vessels (Fig. 5A, YFP+ perivascular clones, arrowheads) that share a clonal origin with a recombined cell in the leptomeninges located directly above the cerebral vessel (Fig. 5A, asterisk). Taken together, our lineage tracing using the tdTomato-flox and Brainbow-flox reporter lines showed that brain PVFs developmentally originate from meningeal-located fibroblasts located proximal to the junction between the meningeal vessel and corresponding cerebral penetrating vessel.
Fig. 5.
Parenchymal PVFs originate in the meninges and undergo regional clonal expansion on vessels in the brain. (A-D) Maximum-projection images (z-volume: 50-100 µm) of CUBIC-cleared Col1a2-CreERT2;Brainbow-flox brains at P14 showing sparsely-labeled fibroblast clones (RFP+, magenta and YFP+, yellow) and vessels (Lectin, white). (A) Schematic showing experimental procedure, and representative images. Arrows mark RFP+ meningeal fibroblast clones, arrowheads mark YFP+ brain PVF with a meningeal-localized clone (asterisk), insets show RFP+ and YFP+ clones only. (B) Potential models of PVF coverage of parenchymal vessels: (1) ‘One-Cell’ model; (2) ‘Domains’ model; (3) ‘Mosaic’ model. (C) Image shows individual vessel containing two PVF clones of upper (RFP+, magenta) and lower (YFP+, yellow) domains. (D) Representative images of upper (RFP+, magenta) and lower (YFP+, yellow) domain clones. Arrowhead marks an RFP+ meningeal cell belonging to the ‘upper’ domain clone. c, cortex; m, meninges. Dashed lines indicate the border between the meninges and cortex. (E,F) Graphs depicting number of PVFs in each clone (E) and length of vessel covered by a single clone (F), categorized by domain type and with means of each biological replicate (n=8 animals) represented by large colored shapes and corresponding technical replicates (n=5-22 clones per animal) in small gray matching shapes. Data are mean±s.d. Unpaired t-tests between each group revealed no significant differences between domain types (ns). Scale bars: 100 µm.
In Col1a2-CreERT2;Brainbow-flox brains, we observed brain PVFs from the same clonal origin covering vessels (Fig. 5A); however, we noted that labeled clones never covered the entire length of the penetrating vessel. We know from the Col1a1-GFP and Col1a2-CreERT2;tdTomato-flox experiments that all penetrating cerebral vessels were nearly fully covered with PVFs at P14 (Fig. 1A,C, Fig. 2). Thus, there must be unlabeled clone(s) covering the rest of the vessels we observed. This made us speculate on whether there were certain patterns to how PVFs tile on cerebral vessels, so we generated models to explain how this might occur (Fig. 5B). The ‘One-Cell’ model’ dictates that all brain PVFs on a singular vessel share a clonal origin with one meningeal PVF (Fig. 5B, 1), though we can rule out this model because of empty spaces containing unlabeled cells on vessels. Thus, two or more PVF clones must occupy an individual vessel to achieve full coverage. The ‘Domains’ model suggests that PVF clones cover vessels in a patchwork fashion, occupying non-overlapping domains (Fig. 5B, 2), whereas in the ‘Mosaic’ model PVF clones cover vessels in stochastic patterns (Fig. 5B, 3). In brains from Col1a2-CreERT2;Brainbow-flox pups injected with tamoxifen at P1 and collected at P14, we found that PVF clones occupied space as described by the ‘Domains’ model (Fig. 5C,D). These clones covered regionally-restricted stretches of vessels at a density comparable with that seen of Col1a1-GFP+ PVFs at the same time point (Fig. S5). We can categorize recombined PVFs of a single clonal origin into ‘upper’ or ‘lower’ clone types, based on their location on the upper or lower part of the vessel (Fig. 5C,D). In rare examples, we observed vessels with multiple PVF clones that were spatially separate (Fig. 5C). ‘Upper’ domain clones were always observed with a meningeal clone directly adjacent to the vessel, whereas ‘lower’ domain clones often did not have an observable corresponding meningeal clone within the imaging area (Fig. 5D). We speculate that this reflects initiating PVFs that originate in the meninges, but between P5 and P14 migrate deeper on vessels and proliferate to give rise to other PVFs locally. To further characterize ‘upper’ and ‘lower’ PVF clones, we counted the number of cells per clone and the length of vessel covered by each clone (Fig. 5E,F). ‘Lower’ clones tended to contain more cells (mean=10 PVFs) compared with ‘upper’ clones (mean=6 PVFs), and ‘lower’ clones tended to cover more vessel length (mean=497.8 µm) compared with ‘upper’ clones (mean=312.7 µm), though neither of these differences were statistically significant (Fig. 5E,F). To summarize, PVFs located on vessels in the cortex developmentally arise from meningeal fibroblasts near junctions with penetrating vessels, and multiple meningeal cells are required to give rise to all PVFs that cover each vessel. Brain PVFs of the same clonal origin cluster on vessels in distinct non-overlapping domains, and domain location potentially reflects differences in the timing of emergence and migration of individual PVFs.
PVFs migrate and proliferate to achieve full coverage of cerebral vessels
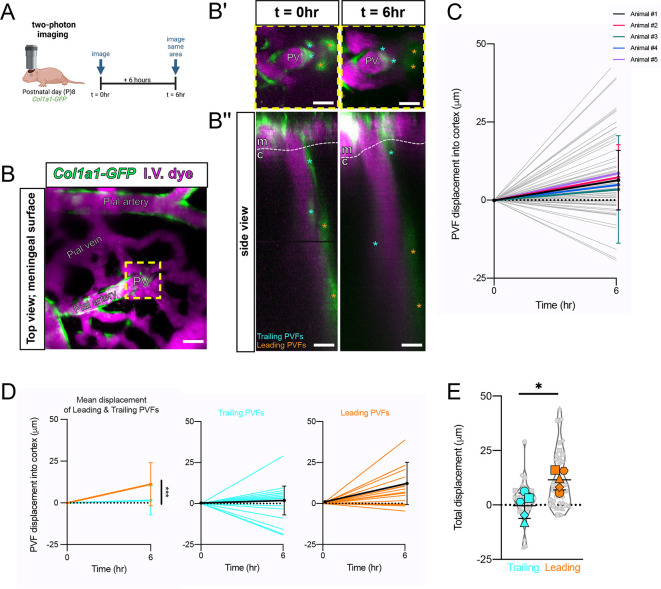
We next investigated migration and proliferation of PVFs to understand how these cellular mechanisms fit in to their developmental progression. We used in vivo two-photon imaging of Col1a1-GFP+ mice at P8 to look for evidence of PVF migration during postnatal development (Fig. 6A). We selected P8 because it falls within the temporal range (P7-P10) where we see the greatest increases in cerebral depth and percent coverage of vessels (Fig. 1B,C). A thinned-skull cranial window was created in Col1a1-GFP+ pups at P7, then pups were imaged twice on P8 at intervals 6 h apart. Intravenous fluorescent dye (2 MDa Alexa-680) was used to visualize blood vessels (Fig. 6A,B). From large overview scans of the meningeal surface, we identified and imaged penetrating vessels containing PVFs and annotated locations of PVFs at each time point (Fig. 6B-B″). We then calculated the displacement of individual PVFs on vessels and found that most PVFs displace positively, meaning they move downward on vessels into the cortex (Fig. 6C). This can be compared with in vivo measurements of PVFs in adult mouse brains showing that they never displace more than 5-10 µm over weeks (Bonney et al., 2022). We noted that PVFs located deeper on vessels make more dynamic movements compared with PVFs located closer to the meningeal surface, which appeared to make more minor movements. We classified deeper, more dynamic PVFs as ‘leading PVFs’ and more superficial PVFs as ‘trailing PVFs’ (Fig. 6B′,B″, asterisks). When we binned displacement values for PVFs based on whether they are leading or trailing, we found that leading PVFs had a significantly higher mean displacement of 11.2 µm compared with trailing PVFs with a mean displacement of 1.5 µm over the 6-h interval (Fig. 6D). We calculated total displacement over time of leading and trailing populations and found that leading PVFs traveled greater total distances from t=0 h to 6 h compared with trailing PVFs (Fig. 6E). Collectively, these data support the suggestion that PVFs migrate to achieve coverage of penetrating cerebral vessels, and there are differences in migratory dynamics based on the position of a PVF on the vessel (trailing versus leading).
Fig. 6.
Col1a1-GFP+ PVFs migrate along vessels in P8 mice in vivo. (A) Schematic of imaging paradigm; Col1a1-GFP+ P8 mice installed with cranial windows imaged using two-photon microscopy at two time points 6 h apart (t=0 h, t=6 h). (B) Overview image of the meninges showing fibroblasts (GFP, green) and vessels (IV dye, magenta). (B′,B″) High-magnification zoom (B′) and side view projection (B″) of area in yellow box in A, with leading PVFs (orange asterisks) and trailing PVFs (cyan asterisks) annotated at t=0 h and 6 h. c, cortex; m, meninges; PV, penetrating vessel. Scale bars: 25 µm. (C-E) Graphs showing displacement of PVFs on vessels into the cortex between t=0 h and t=6 h. (C) Summary of all PVFs measured (gray lines, n=8-22 PVFs per animal) and means for each biological replicate (colored lines, n=5 animals). Two-way ANOVA with multiple comparisons shows no significant differences between animal replicates over time. (D) Displacement data split by leading PVFs (right; orange, black line shows mean) and trailing PVFs (middle; cyan, black line shows mean). Two-way ANOVA of trailing and leading PVFs revealed a statistically significant difference in displacement between trailing and leading populations over time (left; ***P<0.001). (E) Graph showing total displacement (µm) from t=0 h to 6 h of leading (orange) and trailing (cyan) PVF populations in Col1a1-GFP+ mice at P8. Means of each biological replicate (n=5 animals) represented by large colored shapes and corresponding technical replicates (n=8-22 PVFs per animal) in small gray matching shapes. Unpaired t-test analysis reveals a statistically significant difference in the mean displacement over time of these two groups (leading versus trailing, *P<0.05). Data are mean±s.d.
Migration partially accounts for PVF coverage of vessels; however, we also observed expansion of PVF clones (Fig. 5) and that PVFs maintained an equal density while increasing in coverage (Fig. S1B), thus, PVFs must undergo cell division during their development. To determine the timing of PVF proliferation during development, we performed 2-h EdU pulse-chase assays in Col1a1-GFP+ mice at embryonic and postnatal time points (Fig. 7). We quantified proliferation of leptomeningeal PVFs and non-PVFs using leptomeningeal flat-mounts from Col1a1-GFP+ mice injected with EdU 2 h before collection at ages E16, P0 and P2, and counted the percentage of GFP+/EdU+ PVFs on leptomeningeal vessels as well as GFP+/EdU+ non-perivascular fibroblasts (Fig. 7A,B – A inset and arrowheads). The average percent of GFP+/EdU+ PVFs between E16 and P2 was 48-57%, and this did not significantly change across time points (Fig. 7B). Possibly, meningeal PVFs are ‘primed’ for emergence onto cerebral vessels by proliferating during embryonic and early postnatal time points, when the meningeal vasculature is growing and remodeling. This concept is supported by comparing the percentage of GFP+/EdU+ PVFs with non-PVFs; at P2, the percentage of GFP+/EdU+ leptomeningeal PVFs was significantly higher than the percentage of GFP+/EdU+ non-PVFs (Fig. 7B). We then performed the same experiment on Col1a1-GFP+ pups at P7, P9, P11 and P14 and collected brains for CUBIC-clearing and counting of GFP+/EdU+ PVFs on penetrating vessels (Fig. 7C,D). We found a significantly higher percentage of GFP+/EdU+ PVFs on vessels in the brain at P7 compared with later time points, suggesting that P7 is a proliferative period for PVFs (Fig. 7D). In general, brain PVFs were less proliferative between P7 and P14 than meningeal PVFs at earlier time points (Fig. 7B). These data suggest that meningeal PVFs increase in number to prepare for migration and then, once on cerebral vessels, undergo low levels of proliferation over the span of a week to achieve vessel coverage. Interestingly, the P7 proliferative window for PVFs is just before when we observe in vivo migration of PVFs, thus we speculate that brain PVFs migrate and proliferate concurrently. Collectively, this data feeds into a mechanism in which PVFs enter the brain via penetrating vessels at P5, migrate and proliferate locally on vessels between P7 and P10, then further refine their positioning and extend on to branches between P10 and P14 (Fig. 8).
Fig. 7.
Col1a1-GFP+ PVFs proliferate along vessels in the meninges and brain during embryonic and postnatal development. (A) Flat-mount preparation of P5 meninges showing fibroblasts (GFP, green), vasculature (PECAM, white), EdU+ nuclei (magenta) and nuclei (DAPI, cyan). Insets (magnification of white box) and arrowheads show EdU+ meningeal PVFs. (B) Graph showing the percentage of EdU+/GFP+ PVFs (green) compared with EdU+/GFP+ non-perivascular meningeal fibroblasts (pink) at E16-P2. **P<0.01. Two-way ANOVA with multiple comparisons detects no statistically significant differences in the percentage of EdU+/GFP+ PVFs and non-PVFs at E16 and P0, but a significant difference between EdU+/GFP+ PVFs and non-PVFs at P2 (P=0.0075). Experiments were performed with biological replicates of n=3-5 for each age, and for each animal replicate n=96-1230 PVFs were counted. (C) Maximum-projection images of CUBIC-cleared Col1a1-GFP brains (z-volume: 500 µm) at P8 showing fibroblasts (GFP, green), vessels (lectin, white) and EdU+ nuclei (magenta). Insets (magnification of white box) and arrowheads show PVFs that are EdU+. (D) Graph showing the percentage of EdU+/GFP+ brain PVFs at ages P7-P14. *P<0.05, **P<0.01, ****P<0.0001. One-way ANOVA with multiple comparisons revealed statistically significant differences between time points (P7 versus P9, P=0.0013; P7 versus P14, P<0.0001; P11 versus P14, P=0.0103). Experiments performed with biological replicates of n=3 for each age, and for each animal replicate n=70-734 PVFs were counted. Data are mean±s.d. Scale bars: 100 µm (A); 200 µm (C).
Fig. 8.
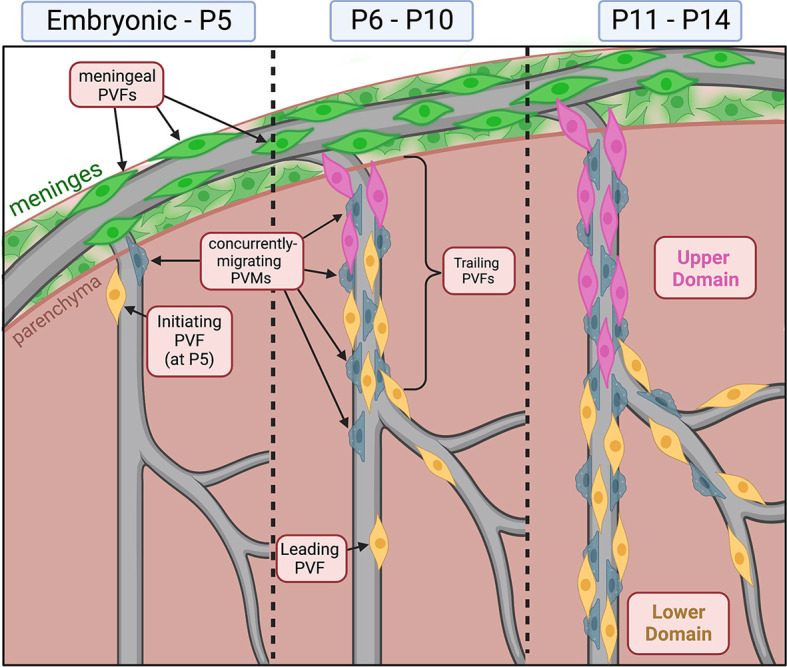
Summary of brain perivascular fibroblast development. PVF development occurs between P5 and P14 in the postnatal mouse brain. This occurs concurrently with PVM development on vessels. Before P5, PVFs are absent from the brain and are located exclusively on meningeal vessels. At P5, initiating PVFs begin migrating on vessels. Between P6 and P10, PVFs migrate and proliferate on vessels. At P8, we find distinct subsets of leading and trailing PVFs based on their average displacement and velocity. Between P11 and P14, PVFs continue to migrate and proliferate to fully cover the vessel. PVF coverage can be separated into ‘upper’ and ‘lower’ domains based on clonal origin.
DISCUSSION
Here, we define PVF development between P5 and P14 in the cerebral cortex and identify the cellular mechanisms by which PVFs cover vessels; from this, we can build a comprehensive timeline of PVF development during postnatal development (Fig. 8). At P5, PVFs initiate coverage of penetrating vessels, between P6 and 10 they migrate and proliferate to cover vessels, and by P14 they fully cover the main branches and extend on to secondary branches (Fig. 8). We show that PVF coverage of vessels occurs after αSMA+ vSMCs and AQP4+ astrocyte endfeet are in place. We also show that PVMs follow a similar developmental trajectory to PVFs (Fig. 8). Analysis of leptomeningeal flat-mounts combined with lineage tracing using Col1a2-CreERT2 mice supports a meningeal origin for brain PVFs. We also find that PVFs expand clonally on vessels and occupy non-overlapping domains, and the locations of these domains may correlate with differential cell migratory dynamics and timing of emergence on vessels, as shown by in vivo observation (Fig. 8). Altogether, this work sheds light on a poorly understood developmental process and may provide us with clues about the functions of PVFs within the neurovascular unit.
The entirely postnatal timing of PVF appearance on vessels in the cerebral cortex is particularly striking, given that the ‘scaffold’ of the neurovascular unit (endothelium, pericytes/vSMCs) upon part of which PVFs migrate is established days earlier during prenatal development (Paredes et al., 2018). PVF coverage of cerebral vessels occurs in parallel with the appearance of PVMs, and we show that both cell types have nearly identical progressive coverage of cerebral vessels. Further, PVFs and CD206+ leptomeningeal macrophages, which give rise to CD206+ PVFs (Masuda et al., 2022), are in high abundance in the leptomeninges for days before their appearance on cerebral vessels. What events occurring in the cerebral vasculature could account for the timing of PVFs and PVMs moving onto cerebral vessels? Possibly, it could relate to the postnatal appearance of perivascular spaces, a gap that forms around large diameter vessels between the vSMC-covered endothelium and astrocyte endfeet that contains interstitial fluid and is continuous with the subarachnoid space of the meninges (Wardlaw et al., 2020; Zhang et al., 1990). Perivascular spaces play a crucial role in waste clearance and fluid homeostasis as part of the CNS ‘glymphatic’ system (Plog and Nedergaard, 2018; Smith et al., 2017), and studies in humans and rodents indicate that perivascular space (PVS) function is necessary for maintaining brain health (Francis et al., 2019; Iliff et al., 2012). Interestingly, astrocyte endfeet polarization of AQP4, and presence of interstitial fluid in perivascular spaces, a key characteristic of the glymphatic system, emerges between P7 and P14 in the mouse cortex (Munk et al., 2019). We show that polarization of AQP4 to endfeet occurs before PVF emergence, suggesting perivascular space establishment may serve as a trigger for PVF migration. Recent work on PVM development in mice reported that perivascular spaces near and distal from the pial surface widen between P4 and P9 (Masuda et al., 2022). These observations support the postnatal appearance of perivascular spaces into which PVFs and PVMs migrate. In addition, extensive expansion of the cerebral capillary network occurs between P8 and P12 (Coelho-Santos et al., 2021). Possibly, ongoing cerebrovascular development to support rapid increases in metabolic demand of the postnatal brain may create permissive signals to attract PVFs onto the cerebral vasculature. Further work is required to detail how the development of PVFs corresponds with emergence of perivascular spaces and the postnatal maturation of the neurovascular unit.
Our lineage tracing data and in vivo imaging of developing PVFs identified specific spatiotemporal patterns of PVF emergence. ‘Lower’ domain PVF clones, seen covering the lower branches of vessels, may correspond to ‘leading’ PVFs seen displacing further and migrating faster to reach vessel termini and expand locally, whereas ‘upper’ PVF clones localized more proximal to the meningeal surface may correspond to ‘trailing’ PVFs that have a lower average displacement. We speculate that the distinction between these two PVF subtypes relates to their proximity to junctions between meningeal and penetrating vessels before P5; meningeal PVFs close to these junctions will be the first to initiate coverage at P5, and remaining PVFs on the meningeal vessel will follow behind. Whether ‘leading’ and ‘trailing’ PVF populations have distinct molecular and functional characteristics is unknown. In models of neural crest and cancer cell collective migration, leader cell populations have distinct morphology and cytoskeleton organization (Qin et al., 2021), and in trunk neural crest, ablating leader cells results in failure of follower cell migration (Richardson et al., 2016). Potentially, ‘leading’ and ‘trailing’ PVFs have differential propensities to respond to molecular signals, explaining their differential migratory capacity.
PVF response to CNS injury, which includes both enhanced extracellular matrix (ECM) and signaling molecule production, provides some insight into the functional significance of postnatal PVF development. In spinal cord injury and stroke, PVF numbers expand dramatically, leading to the deposition of ECM proteins that form the fibrotic scar (Fernández-Klett et al., 2013; Kelly et al., 2016; Soderblom et al., 2013). In healthy tissues, fibroblasts of many organs function in producing ECM; it is therefore possible that PVFs may function in secreting, maintaining and modifying ECM during development and homeostasis. This potential function of PVFs may also be tied to development of PVMs. During postnatal development, brain PVMs rely on integrin-dependent mechanisms to populate parenchymal vessels (Masuda et al., 2022). Potentially, PVFs function to contribute to local ECM for integrin-dependent migration of PVMs. This may also explain why PVFs were more frequently detected ahead of PVMs, possibly laying down a permissive environment for PVM migration. There is some evidence that fibroblasts and macrophages form stable interactions in vitro via CSF1 and PDGF-B signaling (Zhou et al., 2018). It is possible that the proximity of PVFs and PVMs facilitates stable cellular communication circuits, and this signaling network may underlie the development and maintenance of these two cell types. PVFs may also function in producing molecular signals required for development and maintenance of the PVS and neurovasculature. Our lab has shown previously that in a mouse model of stroke, in addition to their fibrotic role, PVFs producing retinoic acid (RA) at the site of injury (Kelly et al., 2016). Developmental factors produced by meningeal fibroblasts, in particular RA, are required for cortical development (Siegenthaler et al., 2009) and cerebrovascular development (Bonney et al., 2016; Mishra et al., 2016). We found previously that adult brain PVFs express some of the components of the RA synthesis pathway (RALDH1/RALDH2) (Kelly et al., 2016), and we show here that meningeal-located PVFs express RALDH2. It is possible that PVFs are a local source for RA in the brain during postnatal development, and their heightened production of RA during injury reflects a re-activation of developmental pathways.
Our study provides an important framework for the cellular mechanisms driving PVF development in the mouse brain. A major outstanding question is: what are the molecular mechanisms controlling PVF development? We highlight integrins/ECM modification and RA signaling as potential functions for PVFs; it is possible that PVFs require these mechanisms for their own development as well. Us and others have previously shown that PVFs express both PDGF receptors α and β (Fernández-Klett et al., 2013; Kelly et al., 2016; Riew et al., 2020; Vanlandewijck et al., 2018). PDGFB ligand produced by the endothelium is required for recruitment of pericytes to the brain vasculature during embryonic development (Lindahl et al., 1997); it is possible that PVFs rely on a similar mechanism for their progression on the vasculature during postnatal development. A recent single-cell RNA-sequencing study shows that microglia and macrophages express PDGFB in the adult mouse brain (Marsh et al., 2022); these cell types may also act as a source of ligand attracting PVFs into PVSs. In addition, interstitial fluid flow within perivascular spaces or increased pulsatility of large vessels may play a role by providing the molecular cues or mechanical stimulus required for PVF migration and proliferation on vessels. Given their positioning within perivascular spaces, it is likely that PVFs receive developmental cues via cell-cell signaling with other proximal neurovascular cell types. Future studies aimed at understanding the specific signals required for PVF development, and how PVFs fit into the overall development of PVSs and the neurovascular unit are now possible using our model of the timing and cellular mechanisms of PVF development.
MATERIALS AND METHODS
Animals
Mice used for experiments were housed in specific pathogen-free facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care and procedures were performed in accordance with animal protocols approved by the Institutional Animal Care and Use Committee at The University of Colorado, Anschutz Medical Campus and Seattle Children's Research Institute. Mouse lines used in this study include: (1) Col1a1-GFP: Tg(Col1a1-EGFP)#Dab (MGI 4458034; Yata et al., 2003); (2) Col1a2-CreERT2:Tg(Col1a2-cre/ERT,-ALPP)7Cpd (The Jackson Laboratory, #029567, RRID: IMSR_JAX:029567); (3) Ai14(tdTomato)-flox: B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J (The Jackson Laboratory, #007914, RRID: IMSR_JAX:007914); (4) Brainbow-flox: Gt(ROSA)26Sortm1(CAG-Brainbow2.1)Cle (The Jackson Laboratory, #013731, RRID: IMSR_JAX:013731). For flat-mount and cleared tissue analyses, adult mice were crossed to generate embryonic (E12-E16) or postnatal litters collected at ages E14, E16, P0, P2, P3, P5, P6, P7, P8, P9, P10, P11 and P14. For lineage tracing experiments, P1 mice were injected into the milk sac with 50 µl of 1 mg/ml Ai14-flox or 1.5 mg/ml Brainbow-flox of tamoxifen (Sigma-Aldrich, T5648) in corn oil (Sigma-Aldrich, C8267) to achieve Cre-activated recombination. For two-photon imaging studies on P8 Col1a1-GFP pups, we followed our previous methods that are comprehensively described in Coelho-Santos et al. (2021) and Coelho-Santos et al. (2022). For EdU-based proliferation assays, we intraperitoneally injected 2.5 mg/ml EdU (Thermo Fisher Scientific, NC1495577) into pregnant mice for embryonic collections (150 µl) or postnatal mice (50 µl) 2 h before collection.
Tissue collection and immunohistochemistry
All procedures and reagents used for collecting and processing meningeal whole mounts and cleared brain slices for immunohistochemistry, EdU detection and imaging are described at length in our methods paper (Jones et al., 2022). The following primary antibodies were used in meningeal whole mount, sectioned and cleared tissue staining: rabbit anti-Desmin (Cell Signaling Technology, D93F5, 1:100), rat anti-CD31/PECAM (BD Pharmingen, 561814, 1:100), rabbit anti-S100A6 (Novus, NBP1-89388, 1:100), rabbit anti-RALDH2 (Sigma-Aldrich, HPA010022, 1:100) goat anti-CD206 (R&D Systems, AF2535, 1:100), rabbit anti-AQP4 (Alomone Labs, 249-323, 1:100) and Cy3-directly conjugated mouse anti-aSMA (Sigma-Aldrich, C6198, 1:100). Appropriate species-specific Alexa-Fluor secondary antibodies (Invitrogen) were used at a dilution of 1:500 in all cases: goat anti-rat 647 (A21247), goat anti-rabbit 594 (A11037) and donkey anti-goat 555 (A32816). In cleared tissue, vasculature was labeled by DyLight Tomato Lectin 649 (Vector Laboratories, DL-1178-1). EdU detection was performed using the Click-iT Plus EdU Alexa Fluor 647 Imaging Kit (Thermo Fisher Scientific, C10640). Images were obtained using a Zeiss 900 LSM confocal microscope and Zen Blue software (Zeiss) and processed in FIJI.
Image acquisition and quantification
Images were obtained using a Zeiss 900 LSM microscope and Zen Blue software. All processing and image quantification was performed in FIJI. For analysis of cell depth, percent coverage and cell density in Figs 1 and 4, maximum projections of 300-500 µm subvolumes were created from raw images, and individual vessels were selected for analysis by observing the labeled vessel channel only (lectin, far red) and annotating penetrating vessels in which the entire length of vessel could be seen from the meninges to the terminus. For cell depth analysis, the distance from the surface of the meninges to the soma of the furthest-migrated cell on the vessel was measured using the segmented line tool in FIJI. For percent coverage measurements, the depth measurement (PVF or PVM ‘coverage’ length) was divided by the total length of the vessel. For density measurements, the coverage length was divided by the number of cells found within the covered length of the vessel then multiplied by 100 µm to normalize measurements to a given length. Similar analyses were conducted for density measurements of meningeal PVFs (Fig. 3D); PVFs characterized by Col1a1-GFP labeling and elongated morphology aligned to the vessel was counted over a measured length of large-diameter (>8 µm) vessels, then multiplied by 100 µm to normalize measurements to a given length. For analyses of Brainbow-labeled PVF clones in Fig. 5E,F, the number of PVF per labeled clone was counted to obtain ‘# of PVFs per clone’ and the length of vessel covered by each clone was measured using the segmented line tool in FIJI. For clones in which multiple branches of the vessel were covered, individual length measurements were taken and added together. For measurements of migratory dynamics of PVFs in Fig. 6, detailed methods for in vivo image acquisition and calculation of PVF displacement from pial vessel branch points are described in Bonney et al. (2022). Characterization of pial and penetrating vessel types were determined by branching patterns and vessel morphology as previously described (Coelho-Santos et al., 2021). Leading PVFs were classified as the deepest two PVFs while the remaining superficial PVFs were defined as trailing on the corresponding penetrating vessel. Further calculations of total displacement over time were conducted by summing displacement values for individual PVFs across time points. For analysis of cell proliferation in meninges flat-mounts and cleared brains in Fig. 6, the number of EdU+ nuclei were counted and divided by the total number of GFP+ PVFs or meningeal cells counted across all images for each replicate, then multiplied by 100 to obtain a percentage.
Statistical analysis
All statistical analyses were performed in GraphPad Prism (v.8.2.0). Normality tests were performed before statistical analyses where necessary. Respective statistical tests and replicate numbers are reported in figure legends. Where shown, lines and error bars represent mean and standard deviation.
Supplementary Material
Acknowledgements
The authors would like to thank the members of the Siegenthaler Lab and members of H.E.J.’s thesis committee (Dr Santos Franco, Dr Katie Fantauzzo, Dr Linda Barlow, Dr Joe Brzezinski and Dr Fabrice Dabertrand) for fruitful discussions and feedback that helped shape this project. All schematics found in the figures were created with BioRender.com.
Footnotes
Author contributions
Conceptualization: H.E.J., J.A.S.; Methodology: H.E.J., J.A.S.; Formal analysis: H.E.J., S.K.B., K.A.A.; Investigation: H.E.J., V.C.-S., S.K.B., K.A.A.; Writing - original draft: H.E.J.; Writing - review & editing: H.E.J., V.C.-S., S.K.B., K.A.A., A.Y.S., J.A.S.; Visualization: H.E.J., V.C.-S., S.K.B.; Supervision: A.Y.S., J.A.S.; Funding acquisition: H.E.J., A.Y.S., J.A.S.
Funding
This work was supported by funding from the National Institute of Neurological Disorders and Stroke (F31NS125875-01 to H.E.J., R01 NS098273 to J.A.S., F32NS117649 to S.K.B.), American Heart Association (Postdoctoral Fellowship to V.C.-S.), and National Institute of General Medical Sciences (1T32GM141742-01 support for the Developing Scholars summer research program for K.A.A. and H.E.J.). Deposited in PMC for release after 12 months.
Data availability
Raw 2-photon imaging files have been deposited in Figshare.
Peer review history
The peer review history is available online at https://journals.biologists.com/dev/lookup/doi/10.1242/dev.201805.reviewer-comments.pdf
References
- Bonney, S., Harrison-Uy, S., Mishra, S., Macpherson, A. M., Choe, Y., Li, D., Jaminet, S.-C., Fruttiger, M., Pleasure, S. J. and Siegenthaler, J. A. (2016). Diverse functions of retinoic acid in brain vascular development. J. Neurosci. 36, 7786-7801. 10.1523/JNEUROSCI.3952-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney, S. K., Sullivan, L. T., Cherry, T. J., Daneman, R. and Shih, A. Y. (2022). Distinct features of brain perivascular fibroblasts and mural cells revealed by in vivo two-photon imaging. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 42, 966-978. 10.1177/0271678X211068528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho-Santos, V. and Shih, A. Y. (2020). Postnatal development of cerebrovascular structure and the neurogliovascular unit. Wiley Interdiscip. Rev. Dev. Biol. 9, e363. 10.1002/wdev.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho-Santos, V., Berthiaume, A.-A., Ornelas, S., Stuhlmann, H. and Shih, A. Y. (2021). Imaging the construction of capillary networks in the neonatal mouse brain. Proc. Natl. Acad. Sci. USA 118, e2100866118. 10.1073/pnas.2100866118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho-Santos, V., Tieu, T. and Shih, A. Y. (2022). Reinforced thinned-skull window for repeated imaging of the neonatal mouse brain. Neurophotonics 9, 031918. 10.1117/1.NPh.9.3.031918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desisto, J., O'rourke, R., Jones, H. E., Pawlikowski, B., Malek, A. D., Bonney, S., Guimiot, F., Jones, K. L. and Siegenthaler, J. A. (2020). Single-cell transcriptomic analyses of the developing meninges reveal meningeal fibroblast diversity and function. Dev. Cell 54, 43-59.e4. 10.1016/j.devcel.2020.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrier, C. E., Aran, D., Haenelt, E. A., Sheehy, R. N., Hoi, K. K., Pintarić, L., Chen, Y., Lizama, C. O., Cautivo, K. M., Weiner, G. A.et al. (2021). CNS fibroblasts form a fibrotic scar in response to immune cell infiltration. Nat. Neurosci. 24, 234-244. 10.1038/s41593-020-00770-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Klett, F., Potas, J. R., Hilpert, D., Blazej, K., Radke, J., Huck, J., Engel, O., Stenzel, W., Genové, G. and Priller, J. (2013). Early loss of pericytes and perivascular stromal cell-induced scar formation after stroke. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 33, 428-439. 10.1038/jcbfm.2012.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis, F., Ballerini, L. and Wardlaw, J. M. (2019). Perivascular spaces and their associations with risk factors, clinical disorders and neuroimaging features: a systematic review and meta-analysis. Int. J. Stroke Off. J. Int. Stroke Soc. 14, 359-371. 10.1177/1747493019830321 [DOI] [PubMed] [Google Scholar]
- Freitas-Andrade, M., Comin, C. H., Van Dyken, P., Ouellette, J., Raman-Nair, J., Blakeley, N., Liu, Q. Y., Leclerc, S., Pan, Y., Liu, Z.et al. (2023). Astroglial Hmgb1 regulates postnatal astrocyte morphogenesis and cerebrovascular maturation. Nat. Commun. 14, 4965. 10.1038/s41467-023-40682-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, F. J., Sun, N., Lee, H., Godlewski, B., Mathys, H., Galani, K., Zhou, B., Jiang, X., Ng, A. P., Mantero, J.et al. (2022). Single-cell dissection of the human brain vasculature. Nature 603, 893-899. 10.1038/s41586-022-04521-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannocks, M.-J., Pizzo, M. E., Huppert, J., Deshpande, T., Abbott, N. J., Thorne, R. G. and Sorokin, L. (2018). Molecular characterization of perivascular drainage pathways in the murine brain. J. Cereb. Blood Flow Metab. 38, 669-686. 10.1177/0271678X17749689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff, J. J., Wang, M., Liao, Y., Plogg, B. A., Peng, W., Gundersen, G. A., Benveniste, H., Vates, G. E., Deane, R., Goldman, S. A.et al. (2012). A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 4, 147ra111. 10.1126/scitranslmed.3003748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, H. E., Abrams, K. A. and Siegenthaler, J. A. (2022). Techniques for visualizing fibroblast-vessel interactions in the developing and adult CNS. Neurophotonics 9, 021911. 10.1117/1.NPh.9.2.021911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam, M., Janbon, H., Malkinson, G. and Brunet, I. (2022). Heterogeneity and developmental dynamics of LYVE-1 perivascular macrophages distribution in the mouse brain. J. Cereb. Blood Flow Metab. 42, 1797-1812. 10.1177/0271678X221101643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, K. K., Macpherson, A. M., Grewal, H., Strnad, F., Jones, J. W., Yu, J., Pierzchalski, K., Kane, M. A., Herson, P. S. and Siegenthaler, J. A. (2016). Col1a1+ perivascular cells in the brain are a source of retinoic acid following stroke. BMC Neurosci. 17, 49. 10.1186/s12868-016-0284-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl, P., Johansson, B. R., Levéen, P. and Betsholtz, C. (1997). Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277, 242-245. 10.1126/science.277.5323.242 [DOI] [PubMed] [Google Scholar]
- Marsh S. E., Walker A. J., Kamath T., Dissing-Olesen L., Hammond T. R., de Soysa T. Y., Young A. M. H., Murphy S., Abdulraouf A., Nadaf N.et al. (2022). Dissection of artifactual and confounding glial signatures by single-cell sequencing of mouse and human brain. Nat. Neurosci. 25, 306-316. 10.1038/s41593-022-01022-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Månberg, A., Skene, N., Sanders, F., Trusohamn, M., Remnestål, J., Szczepińska, A., Aksoylu, I. S., Lönnerberg, P., Ebarasi, L., Wouters, S.et al. (2021). Altered perivascular fibroblast activity precedes ALS disease onset. Nat. Med. 27, 640-646. 10.1038/s41591-021-01295-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda, T., Amann, L., Monaco, G., Sankowski, R., Staszewski, O., Krueger, M., Del Gaudio, F., He, L., Paterson, N., Nent, E.et al. (2022). Specification of CNS macrophage subsets occurs postnatally in defined niches. Nature 604, 740-748. 10.1038/s41586-022-04596-2 [DOI] [PubMed] [Google Scholar]
- Mestre, H., Hablitz, L. M., Xavier, A. L., Feng, W., Zou, W., Pu, T., Monai, H., Murlidharan, G., Castellanos Rivera, R. M., Simon, M. J.et al. (2018). Aquaporin-4-dependent glymphatic solute transport in the rodent brain. eLife 7, e40070. 10.7554/eLife.40070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra, S., Choe, Y., Pleasure, S. J. and Siegenthaler, J. A. (2016). Cerebrovascular defects in Foxc1 mutants correlate with aberrant WNT and VEGF-A pathways downstream of retinoic acid from the meninges. Dev. Biol. 420, 148-165. 10.1016/j.ydbio.2016.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk, A. S., Wang, W., Bèchet, N. B., Eltanahy, A. M., Cheng, A. X., Sigurdsson, B., Benraiss, A., Mäe, M. A., Kress, B. T., Kelley, D. H.et al. (2019). PDGF-B is required for development of the glymphatic system. Cell Rep. 26, 2955-2969.e3. 10.1016/j.celrep.2019.02.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes, I., Himmels, P. and Ruiz De Almodóvar, C. (2018). Neurovascular communication during CNS development. Dev. Cell 45, 10-32. 10.1016/j.devcel.2018.01.023 [DOI] [PubMed] [Google Scholar]
- Plog, B. A. and Nedergaard, M. (2018). The glymphatic system in CNS health and disease: past, present and future. Annu. Rev. Pathol. 13, 379-394. 10.1146/annurev-pathol-051217-111018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, L., Yang, D., Yi, W., Cao, H. and Xiao, G. (2021). Roles of leader and follower cells in collective cell migration. Mol. Biol. Cell 32, 1267-1272. 10.1091/mbc.E20-10-0681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, J., Gauert, A., Briones Montecinos, L., Fanlo, L., Alhashem, Z. M., Assar, R., Marti, E., Kabla, A., Härtel, S. and Linker, C. (2016). Leader cells define directionality of trunk, but not cranial, neural crest cell migration. Cell Rep. 15, 2076-2088. 10.1016/j.celrep.2016.04.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riew, T.-R., Jin, X., Kim, H. L., Kim, S. and Lee, M.-Y. (2020). Ultrastructural and molecular characterization of platelet-derived growth factor beta-positive leptomeningeal cells in the adult rat brain. Mol. Neurobiol. 57, 1484-1501. 10.1007/s12035-019-01793-5 [DOI] [PubMed] [Google Scholar]
- Siegenthaler, J. A., Ashique, A. M., Zarbalis, K., Patterson, K. P., Hecht, J. H., Kane, M. A., Folias, A. E., Choe, Y., May, S. R., Kume, T.et al. (2009). Retinoic acid from the meninges regulates cortical neuron generation. Cell 139, 597-609. 10.1016/j.cell.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, A. J., Yao, X., Dix, J. A., Jin, B.-J. and Verkman, A. S. (2017). Test of the “glymphatic” hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. eLife 6, e27679. 10.7554/eLife.27679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderblom, C., Luo, X., Blumenthal, E., Bray, E., Lyapichev, K., Ramos, J., Krishnan, V., Lai-Hsu, C., Park, K. K., Tsoulfas, P.et al. (2013). Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J. Neurosci. 33, 13882-13887. 10.1523/JNEUROSCI.2524-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlandewijck, M., He, L., Mäe, M. A., Andrae, J., Ando, K., Del Gaudio, F., Nahar, K., Lebouvier, T., Laviña, B., Gouveia, L.et al. (2018). A molecular atlas of cell types and zonation in the brain vasculature. Nature 554, 475-480. 10.1038/nature25739 [DOI] [PubMed] [Google Scholar]
- Wardlaw, J. M., Benveniste, H., Nedergaard, M., Zlokovic, B. V., Mestre, H., Lee, H., Doubal, F. N., Brown, R., Ramirez, J., Macintosh, B. J.et al. (2020). Perivascular spaces in the brain: anatomy, physiology and pathology. Nat. Rev. Neurol. 16, 137-153. 10.1038/s41582-020-0312-z [DOI] [PubMed] [Google Scholar]
- Yang, A. C., Vest, R. T., Kern, F., Lee, D. P., Agam, M., Maat, C. A., Losada, P. M., Chen, M. B., Schaum, N., Khoury, N.et al. (2022). A human brain vascular atlas reveals diverse mediators of Alzheimer's risk. Nature 603, 885-892. 10.1038/s41586-021-04369-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yata, Y., Scanga, A., Gillan, A., Yang, L., Reif, S., Breindl, M., Brenner, D. A. and Rippe, R. A. (2003). DNase I-hypersensitive sites enhance alpha1(I) collagen gene expression in hepatic stellate cells. Hepatology 37, 267-276. 10.1053/jhep.2003.50067 [DOI] [PubMed] [Google Scholar]
- Zhang, E. T., Inman, C. B. and Weller, R. O. (1990). Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J. Anat. 170, 111-123. [PMC free article] [PubMed] [Google Scholar]
- Zheng, B., Zhang, Z., Black, C. M., De Crombrugghe, B. and Denton, C. P. (2002). Ligand-dependent genetic recombination in fibroblasts. Am. J. Pathol. 160, 1609-1617. 10.1016/S0002-9440(10)61108-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, X., Franklin, R. A., Adler, M., Jacox, J. B., Bailis, W., Shyer, J. A., Flavell, R. A., Mayo, A., Alon, U. and Medzhitov, R. (2018). Circuit design features of a stable two-cell system. Cell 172, 744-757.e17. 10.1016/j.cell.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.