Abstract
Introduction
Patients with hip fractures are almost always operated with quite extensive surgery and are often frail with a high risk of complications, increased dependency, and death. Orthogeriatric interdisciplinary care has shown better results compared with orthopaedic care alone. The best way of delivering orthogeriatric care, however, is still largely unknown. It is believed that a high degree of integration and shared care is better than on-demand consultative services. We aimed to evaluate two different orthogeriatric models for patients with hip fracture.
Methods
A prospective hip fracture quality database was used to evaluate two coexisting models of care from 2019 to 2021 in our hospital. An ‘integrated care model’ (ICM) was compared with a ‘geriatric consult service’ (GCS).
Results
516 patients were available for analysis, 360 from ICM and 156 from GCS. Mean age was 84 years. There were 370 (72%) women. American Society of Anesthesiologists class and prefracture cognitive impairment was similar between the groups. There were more patients with femoral neck fractures in the ICM group, and more patients were living independently prefracture. A logistic regression adjusting for the variables above showed that more patients in the ICM group were given a nerve block preoperatively (OR 2.0 (95% CI 1.31 to 2.97); p<0.01), had their urinary catheter removed the first day after surgery (OR 1.9 (95% CI 1.27 to 2.89); p<0.01), were mobilised to standing or seated in a chair beside the bed the first day after surgery (OR 1.5 (95% CI 1.03 to 2.30); p=0.033) and more ICM patients were considered for treatment against osteoporosis (OR 8.58 (95% CI 4.03 to 18.28); p<0.001). There were no significant differences in time to surgery, length of stay, discharge destination or mortality.
Conclusion
The ICM group performed equally good or better on all quality indicators than the GCS.
Keywords: Hip Fractures, Geriatrics, Healthcare quality improvement, Performance measures
WHAT IS ALREADY KNOWN ON THIS TOPIC
Orthogeriatric care improves the hip fracture patient’s opportunity to attain prefracture level of mobility, independency and health, but there is no consensus on which model of orthogeriatric care that is best to achieve patients prefracture function.
WHAT THIS STUDY ADDS
The integrated orthogeriatric care model with shared responsibility and decision-making between the orthopaedic surgeon and the geriatrician significantly improved most of the quality indicators measured but did not affect mortality.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study indicates that the integrated orthogeriatric model of care is superior to the geriatric consult service. However, more research is needed to conclude and randomised controlled trials between different orthogeriatric models of care with outcomes as delirium, readmission rates and health-related quality of life should be performed.
Introduction
Hip fracture remains one of the most important threats to health in elderly patients. Despite a reduction of incidence reported from several countries and regions,1 the burden of hip fracture is expected to increase due to an ageing population.2 Almost all patients with hip fracture need surgery, but they also need excellent perioperative care and rehabilitation due to concurrent medical problems. A patient with a hip fracture has an increased risk of death3 4 and may also experience loss of mobility and loss of independence,5 cognitive decline6 and new falls and fractures.7 8 Orthogeriatric care with comprehensive geriatric assessment is a well-established care model, which aims to handle these issues, as well as the need for timely high-quality surgery.9–12 However, orthogeriatric care can be organised in different ways and there is limited data on which system may provide the best possible outcome for patients.11–14 Various models of care have been suggested to categorise the interventions that have been reported. The models include various degrees of integration between the specialties (table 1).11 12
Table 1.
Different models of orthogeriatric care
| Models of orthogeriatric care | ||
| Kammerlander et al12 2010 | Grigoryan et al11 2014 | |
| Model 1 | Orthopaedic ward with geriatric consultative service by request | Orthopaedic ward with routine geriatric consultation |
| Model 2 | Orthopaedic ward with daily geriatric consultative service | Geriatric ward with orthopaedic consultative service |
| Model 3 | Geriatric and rehabilitation ward with orthopaedic consultant service | Shared care |
| Model 4 | Orthopaedic ward and integrated care | |
In our hospital, an orthogeriatric unit was established in 2018. The orthogeriatric unit treat most of the hip fracture patients and practice an integrated care model (ICM) with shared decision-making and responsibility for the patients between the orthopaedic surgeon and the geriatrician. However, due to lack of capacity in the orthogeriatric unit, some hip fracture patients were treated in the orthopaedic trauma ward. These patients were routinely offered a geriatric consult service (GCS) performed by one of the geriatricians from the orthogeriatric unit. According to Kammerlander et al and Grigoryan et al, we classified our orthogeriatric unit as model 4 ‘orthopaedic ward and integrated care’12 and model 3 ‘shared care’11 respectively, while the patients treated in the orthopaedic trauma ward were offered model 2 ‘orthopaedic ward with daily geriatric consultative service’ and model 1 ‘orthopaedic ward with routine geriatric consultation’ (table 1).
Although a systematic approach with interdisciplinary care by trained health professionals has been recommended,12 there is limited evidence to decide which care model is the best. We, therefore, aimed to compare an ICM with a GCS in a routine practice using data from the local hip fracture quality register.
Patients and methods
Study design
The hip fracture patients above 65 years of age have been offered orthogeriatric care at Oslo University Hospital (Oslo, Norway) since October 2018. The orthogeriatric unit was established within one of the three orthopaedic wards. The orthogeriatric team consisted of a geriatrician, an orthopaedic surgeon, a ‘hip fracture’ nurse (orthopaedic ward nurses with training in orthogeriatric care) and a physiotherapist. In addition, an orthopaedic surgeon (FF and LBS) and a nurse (IH) oversaw maintenance of the system and training of personnel. The orthogeriatric unit practises an ICM with shared decision-making and responsibility between the orthopaedic surgeon and the geriatrician. The hip fracture patients treated in the orthopaedic trauma ward were offered a GCS performed by one of the geriatricians from the orthogeriatric unit, but the orthopaedic surgeon remained responsible for the patients.
The local hip fracture quality register was in place from 2014 after a new monodisciplinary care pathway for hip fractures was established.15 16 In addition to the geriatrician (approximately 0.5 (full-time equivalent (FTE)) position initially, with an increase up to 1.0 (FTE) position), the orthogeriatric unit was reinforced with 1.0 (FTE) hip fracture nurse and 0.5 (FTE) ‘quality register nurse’. Other personnel resources were not increased.
There has been no systematic selection of hip fracture patients between the orthogeriatric unit and the orthopaedic trauma ward. We, therefore, designed a study to investigate if there were any differences between these two orthogeriatric models of care in our hospital using well-known quality indicators17 that were prospectively and routinely collected in our local hip fracture register.
The two orthogeriatric models
The orthogeriatric care was based on the Norwegian Guidelines for Interdisciplinary Care of hip fractures18 and adapted to fit the local organisation. The local hip fracture protocol was universal, that is, valid for all hip fracture patients regardless of which ward they stayed in (table 2). In brief, the patients entered the hospital through the emergency unit having their radiographs taken to confirm the hip fracture diagnosis and a standard set of blood tests performed. If they had an extracapsular fracture the haemoglobin was measured every 6 hours preoperatively. At the ward, they got a nerve block, and an evening round by the geriatrician on call (only orthogeriatric unit). Prior to surgery, the medical and nutritional status was evaluated by the geriatrician and the nurses. The patients were prioritised for surgery within 24–48 hours. All patients got prophylactic antibiotics prior to surgery, dexamethasone to prolong pain relief, tranexamic acid to prevent blood loss and thromboprophylaxis after surgery (and before if they waited for surgery more than 12 hours). The urinary catheter should be removed the first postoperative day and they should be assessed for delirium and constipation daily. The nurses and the physiotherapists mobilised the patients from the first postoperative day and every day (also in the weekends) at least three times (two by the nurses, one by the physiotherapist). The first postoperative day a discharge plan was made, and the patients were assessed for secondary fracture prevention. Blood tests (including haemoglobin) were taken postoperatively and every second day until discharge. The transfusion limit was 100 g/L for patients with a symptomatic cardiac disease and 80 g/L for those without.
Table 2.
Summary of local hip fracture protocol
| Orthogeriatric unit/integrated care model | Orthopaedic trauma ward/geriatric consult service | |
| Emergency unit | ||
| Triage and radiographs of pelvis/hip | x | x |
| Blood tests | x | x |
| Ward preoperatively | ||
| Nerve block | x | x |
| Evening round by the geriatrician on call | x | |
| Interdisciplinary rounds by the orthogeriatric team during weekdays. (Weekends: separate consultations by geriatrician and orthopaedic surgeon) | x | |
| Offer of geriatric consultation | x | |
| Review of comorbidities | x | x |
| Nutritional screening | x | x |
| Delirium screening (4AT) | x | x |
| Haemoglobin every 6 hours (extracapsular fractures) | x | x |
| Thromboprophylaxis (low molecular heparin 5000 IU daily) if time to surgery >12 hours | x | x |
| Operating room | ||
| Surgery within 24–48 hours | x | x |
| Prophylactic antibiotics, dexamethasone, tranexamic acid | x | x |
| Surgery according to guidelines | x | x |
| Ward postoperatively | ||
| Thromboprophylaxis (low molecular heparin 5000 IU daily) | x | x |
| Removal of urinary catheter day 1 | x | x |
| Discharge plan day 1 | x | x |
| Mobilisation day 1 | x | x |
| Mobilisation three times/day | x | x |
| Delirium assessment daily | x | x |
| Constipation assessment daily | x | x |
| Medications review | x | x |
| Blood tests (including haemoglobin) every second day until discharge. (Transfusion limit 80 g/L without symptomatic cardiac disease and 100 g/L with) | x | x |
| Secondary fracture prevention assessment and treatment | x | x |
The ICM group followed the routine in the orthogeriatric unit: Every day the orthogeriatric team (geriatrician, orthopaedic surgeon, hip fracture nurse and physiotherapist) met in the morning for the interdisciplinary rounds to the patients. During weekends the patients were seen separately by the geriatrician and the orthopeadic surgeon. The patients in the GCS group followed the routine in the orthopaedic trauma ward with rounds by the orthopaedic surgeon and nurse. In addition, they were offered a visit by the geriatrician from the orthogeriatric unit, however, without the orthogeriatric team, and often after the orthopaedic surgeon had left the ward. The physiotherapists did not participate in rounds.
Participants
The study was conducted at Oslo University hospital (Oslo, Norway) between January 2019 and December 2021. Patients with a hip fracture (International Classification of Diseases, 10th Revision diagnoses codes: S72.0 femoral neck fracture, S72.1 pertrochanteric fracture (PTFF) and S72.2 subtrochanteric fracture (STFF)) and age >65 years admitted to the hospital and registered in the local hip fracture register were considered for inclusion in the study.
Variables
Patient data were registered prospectively during the stay and entered into the local quality register at patient discharge. Information on patients who deceased after discharge were automatically received from the Norwegian Population Register. Data registered included demographic data, data on prefracture residence, morbidity (including prefracture cognitive impairment and American Society of Anesthesiologists physical Status Classification System (ASA class)) and function. Quality indicators were decided based on measurable data considered important for patient outcome and included: preoperative nerve block, time of surgery, removal of urinary catheter, mobilisation, secondary fracture prevention, time and place of discharged from hospital and mortality.
Bias
The decision to place a patient in the orthogeriatric unit or in any of the other orthopaedic trauma wards was done by a nurse in an administrative function, and solely based on whether there was room for another patient in the orthogeriatric unit or not. Prior to the analyses, and to ensure that the patients in the GCS group really had received a geriatric consultation, a research coordinator (EBV) registered the number of patients who had received a geriatric consultation in the GCS group, as this was not a prospectively registered variable.
Data access and cleaning methods
EBV and LBS had access to the register. EBV, LBS and FF had access to the anonymous data included in this study. The register is routinely cleaned twice a year by the research coordinator (EBV) and missing data are retrospectively included when possible, using the electronic patient files, the electronic patient medicine curve, and the schedule for the operation theatre as the sources for validation.
Statistical analyses
Continuous data were presented with means and SD, and categorical data were presented as frequencies and proportions. Bivariate analyses were performed with Student’s t-test or χ2 test. A binary logistic regression was used for the analyses of the performance indicators and mortality to adjust for potential confounding variables. Age, sex (female/male) and ASA class (dichotomised to 1/2 and 3/4/5) were prespecified as covariates as they were believed to potentially influence the outcomes. In addition, known cognitive impairment prefracture, fracture type (femoral neck fracture vs trochanteric/STFF) and prefracture dependency (independent: living in own home without any help; dependent: everyone else), were selected for adjustment based on possible imbalance between the two groups. A Cox regression model was used to evaluate mortality between the groups. Level of significance was set at p<0.05. Statistical analysis was performed using SPSS for Windows V.28 (SPSSL) and DATAtab: Online Statistics Calculator (DATAtab e.U. Graz, Austria. URL https://datatab.net).
Patients and public involvement
Patients and public were not involved in this study.
Data availability
All data relevant to the findings of this study are included in the article. Additional lower-level data are available on reasonable request from the corresponding author.
Results
Participants
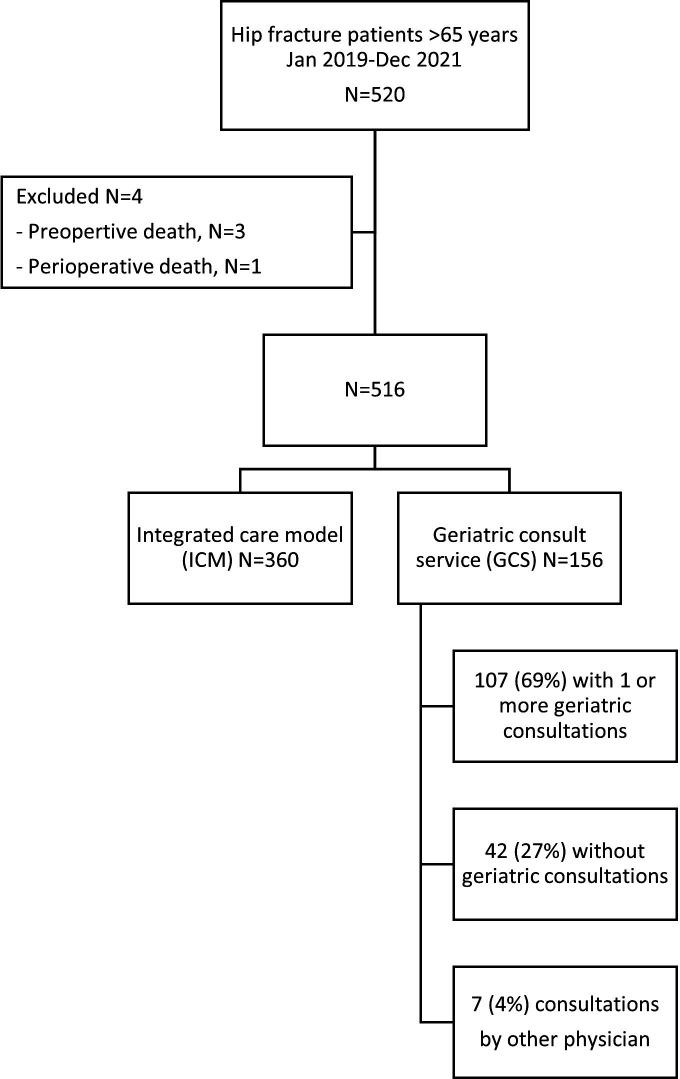
A total of 520 patients were identified and met the inclusion criteria in the period (hip fracture, >65 years). However, 4 patients died preoperatively or perioperatively in hospital and were excluded from the analyses, leaving 516 eligible patients: 360 patients in the ICM group and 156 in the GCS group (figure 1). In the GCS group, 107 (69%) received one or more geriatric consultation, 7 (4%) had a consultation by one or more other physician (cardiologist, haematologist or nephrologist) and 42 (27%) did not receive any consultation by a geriatrician or other physician. The median number of consults was 3 (range 1–10).
Figure 1.
Flow chart of hip fracture patients included/excluded in the study.
Baseline characteristics
Mean age in both groups was 84 years with a range from 66 to 100 in the ICM group and 66 to 104 in the GCS group. A total of 370 (72%) were females and a total of 136 (26%) patients were in ASA class 1 or 2 (table 3) with a mean ASA class on 2.8 vs 2.9 in the ICM vs GCS group, respectively. A total of 286 (55%) suffered from a femoral neck fracture (FNF), the rest had a PTFF or a STFF. Slightly more patients received a hemiarthroplasty in the ICM group (183 (51%) vs 61 (39%)). Prefracture cognitive impairment was documented in 177 (34%) of the patients and was fairly similar in both groups as was the prefracture mobility with 173 (48%) patients walking without aids in the ICM group vs 65 (42%) in the GCS group. More patients were living home without help in the ICM group prefracture, 174 (48%) vs 61 (39%) in the GCS group, indicating that the patients in the ICM group might have had a higher prefracture functional level than the patients in the GCS group. However, only ‘surgery with hemiarthroplasty’ and ‘living home without help prefracture’ were statistically significant different between the groups.
Table 3.
Baseline characteristics for the hip fracture patients in the ICM versus the GCS group
| ICM | GCS | Mean difference (95% CI) | P value | |
| No (N) | 360 (70%) | 156 (30%) | ||
| Age, mean (SD) | 84.1 (8.2) | 84.0 (8.6) | 0.1 (−0.15 to 0.17) | 0.92 |
| Sex (female) | 0.54 | |||
| Female | 263 (73%) | 107 (69%) | ||
| Male | 98 (27%) | 48 (31%) | ||
| ASA class, mean (SD) | 2.8 (0.63) | 2.9 (0.65) | −0.1 (−0.18 to 0.65) | 0.35 |
| ASA class 1 or 2 | 97 (29%) | 39 (26%) | 0.54 | |
| Fracture type | 0.15 | |||
| FNF | 209 (58%) | 77 (50%) | ||
| PTFF | 137 (38%) | 68 (44%) | ||
| STFF | 15 (4%) | 10 (6%) | ||
| Type of surgery | 0.04 | |||
| Hemiarthroplasty | 183 (51%) | 61 (39%) | ||
| Sliding hip screw | 134 (37%) | 71 (46%) | ||
| Intramedullary nail | 20 (6%) | 10 (6%) | ||
| Parallel screws | 18 (5%) | 7 (5%) | ||
| Other surgery/no surgery/unknown | 5 (1%) | 7 (5%) | ||
| Documented cognitive impairment prefracture | 121 (34%) | 56 (36%) | 0.61 | |
| Mobility pre-fracture | 0.32 | |||
| Cannot walk | 9 (3%) | 3 (2%) | ||
| Walk with aid | 162 (45%) | 79 (51%) | ||
| Walk without aid | 173 (48%) | 65 (42%) | ||
| Missing data | 16 (4%) | 9 (6%) | ||
| Residence prefracture | 0.01 | |||
| Home without help | 174 (48%) | 61 (39%) | ||
| Home with help | 101 (28%) | 41 (26%) | ||
| Nursing home | 62 (17%) | 36 (23%) | ||
| Other institution/hospital | 23 (6%) | 18 (12%) |
ASA, American Society of Anesthesiologists; FNF, femoral neck fracture; GCS, geriatric consult service; ICM, integrated care model; PTFF, pertrochanteric fracture; STFF, subtrochanteric fracture.
Quality indicators including mortality
The quality indicators measured are listed in table 4. Preoperative nerve block, removal of urinary catheter 1. postoperative day, mobilisation to standing or seated in a chair beside the bed 1 postoperative day and secondary fracture prevention (treatment with antiosteoporotic drugs) were all in favour of the ICM group. The measures were statistically significant different also after adjustment for potential confounding variables. Time to surgery was similar in the two groups with mean time of 20.5 hours (SD 15.5) in the ICM group and 20.1 hours (SD 12.8) in the GCS group (p=0.78), as was the number of patients who received surgery within 24 and 48 hours, 70% and 98%, respectively, in both groups. These results were not distorted by the adjustments.
Table 4.
Quality indicators, N (%)
| The whole population N* | Integrated care model | Geriatric consult service | Bivariate analysis p value |
Adjusted analysis† p value |
OR (95% CI)‡ | ||
| Preoperative nerve block | 368/503 (73%) | 272/352 (77%) | 96/151 (63%) | 0.001 | 0.001 | 2.0 (1.31 to 2.97) | |
| Surgery within 24 hours | 356/512 (70%) | 251/359 (70%) | 105/153 (70%) | 0.58 | 0.51 | 1.2 (0.76 to 1.73) | |
| Surgery within 48 hours | 501/512 (98%) | 350/359 (98%) | 151/153 (99%) | 0.79 | 0.76 | 1.2 (0.39 to 3.61) | |
| Removal of urinary catheter 1 postoperative day | 230/510 (45%) | 177/356 (50%) | 53/154 (34%) | 0.002 | 0.002 | 1.9 (1.27 to 2.89) | |
| Mobilised to standing or seated in a chair beside the bed 1 postoperative day | 318/506 (63%) | 235/358 (66%) | 83/148 (55%) | 0.01 | 0.033 | 1.5 (1.03 to 2.30) | |
| Treatment with antiosteoporosis drugs in hospital (patients already on medication not included in analysis) | 345/491 (70%) | 259/339 (76%) | 86/152 (57%) | <0.001 | <0.001 | 2.1 (1.39 to 3.10) | |
| Treatment for osteoporosis considered during stay | 476/507 (94%) | 350/359 (98%) | 126/148 (85%) | <0.001 | <0.001 | 8.58 (4.03 to 18.28) | |
| Mortality | HR (95% CI) | ||||||
| 30 days | 49 (9%) | 33 (9%) | 16 (10%) | 0.7 | 0.78 | 0.92 (0.50 to 1.68) | |
| 1 year | 140 (27%) | 90 (25%) | 50 (32%) | 0.1 | 0.22 | 0.80 (0.57 to 1.14) |
Colour codes17: light grey=structure indicator; dark grey=process indicator; black=outcome indicator.
*N varies in analyses of quality indicators because some variables are missing for some patients.
†The adjusted analyses were adjusted for: age, sex, ASA class, cognitive impairment prefracture, fracture type (femoral neck fracture versus pertrochanteric/subtrochanteric fracture) and prefracture dependency.
‡95% CI of OR.
ASA, American Society of Anesthesiologists.
Length of stay (LOS) was similar with a total mean of 5.55 days (5.72 in the ICM group and 5.39 in the GCS group), ranging from 1 to 19 days in the ICM group and from 2 to 20 days in the GCS group.
Few patients were discharged to home: 16 (4%) in the ICM group vs 7 (5%) in the GCS group. Most of the patients were discharged to a rehabilitation unit or short-term nursing home 253 (70%) in the ICM group vs 84 (54%) in the GCS group. A number of patients discharged for long-term nursing homes were almost the same as the number of patients living in long-term nursing homes prefracture: 62 (17%) and 36 (23%) prefracture vs 64 (18%) and 35 (22%) at discharge in the ICM and GCS group, respectively.
There was no significant difference in mortality between the groups, neither the 30 days nor the 1-year mortality, however, a tendency to lower mortality rates in the ICM group was seen (1-year mortality 25% in the ICM group vs 32% in the GCS group). We also investigated if COVID-19 may have influenced the mortality rates as our data covers mortality between 2019 and 2022: An increase in mortality from 2019 (before COVID-19) to 2021 was observed: 30 days mortality 7% vs 13% and 1-year mortality 23% vs 35%, respectively. However, adjusted for fracture type, age, gender, ASA, dependency and prefracture cognitive impairment the increase was not significant (30 days mortality: HR 0.64 95% CI (0.32 to 1.27), 1-year mortality: HR 0.67, 95% CI (0.45 to 1.01)).
Discussion
We found that the ICM with comanagement of the patients and shared decision-making and responsibility between the orthopaedic surgeon and the geriatrician, provided equally good or better results on all the quality indicators measured. Although it was a slight tendency towards decreased 1-year mortality in the ICM group, the difference was not significant. The quality indicators regarding postoperative care (removal of urinary catheter 1 postoperative day, mobilisation 1 postoperative day and treatment with antiosteoporotic drugs) seemed to have the largest measured effect in favour of the ICM in addition to preoperative nerve block. These quality indicators may to a larger extent be influenced by an active orthogeriatric comanagement team ensuring that the patients get optimal treatment, while time to surgery may be influenced by other factors that may not be different between the two types of care. These may include emergency room routines, willingness to give priority to patients with hip fractures and available operating theatres. In our study, two different models of orthogeriatric care were directly compared prospectively. Few such comparisons are known from the literature, and in recent systematic reviews and meta-analyses most of the studies included compared the different orthogeriatric care models with usual care.11–14 Only Middleton et al19 and Baroni et al20 have directly compared the ICM with a geriatric consultation service in their hospitals. Middleton and coworkers investigated the impact of a change from geriatric consultation service to an ICM in an orthogeriatric ward and found reduced LOS, shorter time to surgery and reduced 30-day mortality. All parameters were significant also after controlling for confounders.19 Baroni et al demonstrated shorter time to surgery and more antiosteoporotic drugs prescribed postfracture in the ICM group compared with the GCS group. However, no difference in complications or mortality were seen between the two orthogeriatric models.20 The results from these studies are comparable to what we found in our study, and it seems to be some evidence for recommending an ICM rather than a geriatric consultation service. However, the studies are few and observational. A few randomised studies comparing consultative GCA with usual care in the orthopaedic ward have reported better results with GCA.21 22 However, both these studies had a structured system with interdisciplinary consults 2–3 times per week. On the other hand, a meta-analysis including studies on mainly non-orthopaedic patients concluded that there is conflicting evidence for geriatric consultative services, whereas more integrated services demonstrated good results.23
Limitations
This study has several limitations. There were more trochanteric fractures and more surgeries with internal fixation in the GCS group. Patients with internal fixation of trochanteric fractures may be more difficult to mobilise postoperatively, and this could affect the performance indicators. We did, however, adjust for fracture type in the analyses. The quality indicators used may not be comprehensive enough to measure the quality of care. However, the quality indicators we did use were established in 2014 and intended to be manageable also in the setting of usual care in the orthopaedic ward. Some variables in the register, notably fall prevention and delirium during the stay, were too incomplete to be used for this report. Potentially relevant variables such as a medication review are not included in the register, neither are any patient-reported measures. Apart from mortality, which the study is clearly underpowered for, no indicators after discharge are registered. Measures such as readmissions, complications, new long-term care admissions, mobility, cognition and health related quality of life would have been of interest. Strengths of the study include fairly large groups, well balanced on age, sex, ASA class and prefracture cognitive state. It may also be a strength that data were collected during the same period from both groups, hence avoiding a bias from general improvement of care that may be an issue with before/after studies. We believe that the improvement seen in our unit of the performance indicators is due to the integrated orthogeriatric approach, but we cannot exclude that it simply due to extra resources spent on the patients with hip fractures during the period, and that similar results could have been obtained by adding more orthopaedic personnel, without the special training.
Conclusion
An ICM provides better acute care for the hip fracture patients measured by selected quality indicators. However, more research which embraces a wider spectrum is needed to clearly state that a model of comanagement between the orthopaedics and the geriatricians affects important outcome measures, such as mobility, postdischarge dependency and quality of life.
Footnotes
Contributors: LBS and FF directed and supervised the study design, data collection, analysis, interpretation of data and wrote the manuscript. EBV and IH collected the data, managed data storage and reviewed the manuscript. MVA, NO and MW contributed to the data interpretation and reviewed the manuscript. All authors contributed to the implementation of this programme and study and reviewed the analysis, results and manuscript. LBS: guarantor.
Funding: This study was funded by Oslo University Hospital, no award/grant number.
Competing interests: FF has received honorarium from UCB and Amgen and is president elect in Fragility Fracture Network. The others have no competing interests.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request. All data relevant to the findings of this study are included in the article. Additional lower-level data are available on reasonable request from the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The local hip fracture register and data collection were approved and has a preapproved waiver for quality audit. Hospital Data Protection Officer (PVO 2014/12309, PVO 2015/18831, and PVO 2020/11011) and Regional Ethics Committee South Eastern Norway (2014/1433).
References
- 1.Veronese N, Maggi S. Epidemiology and social costs of hip fracture. Injury 2018;49:1458–60. 10.1016/j.injury.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 2.Cooper C, Cole ZA, Holroyd CR, et al. Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos Int 2011;22:1277–88. 10.1007/s00198-011-1601-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abrahamsen B, van Staa T, Ariely R, et al. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int 2009;20:1633–50. 10.1007/s00198-009-0920-3 [DOI] [PubMed] [Google Scholar]
- 4.Haentjens P, Magaziner J, Colón-Emeric CS, et al. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med 2010;152:380–90. 10.7326/0003-4819-152-6-201003160-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dyer SM, Crotty M, Fairhall N, et al. A critical review of the long-term disability outcomes following hip fracture. BMC Geriatr 2016;16:158. 10.1186/s12877-016-0332-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uzoigwe CE, O’Leary L, Nduka J, et al. Factors associated with delirium and cognitive decline following hip fracture surgery. Bone Joint J 2020;102-B:1675–81. 10.1302/0301-620X.102B12.BJJ-2019-1537.R3 [DOI] [PubMed] [Google Scholar]
- 7.Berry SD, Samelson EJ, Hannan MT, et al. Second hip fracture in older men and women: the Framingham study. Arch Intern Med 2007;167:1971–6. 10.1001/archinte.167.18.1971 [DOI] [PubMed] [Google Scholar]
- 8.Lönnroos E, Kautiainen H, Karppi P, et al. Incidence of second hip fractures. A population-based study. Osteoporos Int 2007;18:1279–85. 10.1007/s00198-007-0375-3 [DOI] [PubMed] [Google Scholar]
- 9.Handoll HH, Cameron ID, Mak JC, et al. Multidisciplinary rehabilitation for older people with hip fractures. Cochrane Database Syst Rev 2021;11:CD007125. 10.1002/14651858.CD007125.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nordström P, Thorngren K-G, Hommel A, et al. Effects of geriatric team rehabilitation after hip fracture: meta-analysis of randomized controlled trials. J Am Med Dir Assoc 2018;19:840–5. 10.1016/j.jamda.2018.05.008 [DOI] [PubMed] [Google Scholar]
- 11.Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma 2014;28:e49–55. 10.1097/BOT.0b013e3182a5a045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kammerlander C, Roth T, Friedman SM, et al. Ortho-geriatric service--a literature review comparing different models. Osteoporos Int 2010;21:S637–46. 10.1007/s00198-010-1396-x [DOI] [PubMed] [Google Scholar]
- 13.Moyet J, Deschasse G, Marquant B, et al. Which is the optimal orthogeriatric care model to prevent mortality of elderly subjects post hip fractures? A systematic review and meta-analysis based on current clinical practice. Int Orthop 2019;43:1449–54. 10.1007/s00264-018-3928-5 [DOI] [PubMed] [Google Scholar]
- 14.Mordant G, Dupont J, Van Heghe A, et al. Effects of orthogeriatric care models on outcomes of hip fracture patients: a systematic review and meta-analysis. Calcif Tissue Int 2022;110:761–3. 10.1007/s00223-021-00943-z [DOI] [PubMed] [Google Scholar]
- 15.Ingstad F, Solberg LB, Nordsletten L, et al. Vitamin D status and complications, readmissions, and mortality after hip fracture. Osteoporos Int 2021;32:873–81. 10.1007/s00198-020-05739-9 [DOI] [PubMed] [Google Scholar]
- 16.Svenøy S, Watne LO, Hestnes I, et al. Results after introduction of a hip fracture care pathway: comparison with usual care. Acta Orthop 2020;91:139–45. 10.1080/17453674.2019.1710804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sermon A, Slock C, Coeckelberghs E, et al. Quality indicators in the treatment of geriatric hip fractures: literature review and expert consensus. Arch Osteoporos 2021;16:152. 10.1007/s11657-021-00995-6 [DOI] [PubMed] [Google Scholar]
- 18.Ranhoff AH, Saltvedt I, Frihagen F, et al. Interdisciplinary care of hip fractures.: orthogeriatric models, alternative models, interdisciplinary teamwork. Best Pract Res Clin Rheumatol 2019;33:205–26. 10.1016/j.berh.2019.03.015 [DOI] [PubMed] [Google Scholar]
- 19.Middleton M, Wan B, da Assunçao R. Improving hip fracture outcomes with integrated orthogeriatric care: a comparison between two accepted orthogeriatric models. Age Ageing 2017;46:465–70. 10.1093/ageing/afw232 [DOI] [PubMed] [Google Scholar]
- 20.Baroni M, Serra R, Boccardi V, et al. The orthogeriatric comanagement improves clinical outcomes of hip fracture in older adults. Osteoporos Int 2019;30:907–16. 10.1007/s00198-019-04858-2 [DOI] [PubMed] [Google Scholar]
- 21.Naglie G, Tansey C, Kirkland JL, et al. Interdisciplinary inpatient care for elderly people with hip fracture: a randomized controlled trial. CMAJ 2002;167:25–32. [PMC free article] [PubMed] [Google Scholar]
- 22.Kennie DC, Reid J, Richardson IR, et al. Effectiveness of geriatric rehabilitative care after fractures of the proximal femur in elderly women: a randomised clinical trial. BMJ 1988;297:1083–6. 10.1136/bmj.297.6656.1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pilotto A, Cella A, Pilotto A, et al. Three decades of comprehensive geriatric assessment: evidence coming from different healthcare settings and specific clinical conditions. J Am Med Dir Assoc 2017;18:192. 10.1016/j.jamda.2016.11.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the findings of this study are included in the article. Additional lower-level data are available on reasonable request from the corresponding author.
Data are available on reasonable request. All data relevant to the findings of this study are included in the article. Additional lower-level data are available on reasonable request from the corresponding author.