Abstract
Introduction
Young adults with HIV (YWH) experience worse clinical outcomes than adults and have high rates of substance use (SU) and mental illness that impact their engagement in care and adherence to antiretroviral therapy (ART). The intervention for Virologic Suppression in Youth (iVY) aims to address treatment engagement/adherence, mental health (MH) and SU in a tailored manner using a differentiated care approach that is youth friendly. Findings will provide information about the impact of iVY on HIV virological suppression, MH and SU among YWH who are disproportionately impacted by HIV and at elevated risk for poor health outcomes.
Methods and analysis
The iVY study will test the effect of a technology-based intervention with differing levels of resource requirements (ie, financial and personnel time) in a randomised clinical trial with an adaptive treatment strategy among 200 YWH (18–29 years old). The primary outcome is HIV virological suppression measured via dried blood spot. This piloted and protocolised intervention combines: (1) brief weekly sessions with a counsellor via a video-chat platform (video-counselling) to discuss MH, SU, HIV care engagement/adherence and other barriers to care; and (2) a mobile health app to address barriers such as ART forgetfulness, and social isolation. iVY has the potential to address important, distinct and changing barriers to HIV care engagement (eg, MH, SU) to increase virological suppression among YWH at elevated risk for poor health outcomes.
Ethics and dissemination
This study and its protocols have been approved by the University of California, San Francisco Institutional Review Board. Study staff will work with a Youth Advisory Panel to disseminate results to YWH, participants and the academic community.
Trial registration number
Keywords: MENTAL HEALTH, Telemedicine, Substance misuse, HIV & AIDS
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Intervention for Virologic Suppression in Youth (iVY) has the potential to impact HIV health outcomes and mental health (MH) and substance use (SU) among youth with HIV (YWH) who are disproportionately impacted by HIV and who experience more MH challenges than the general population.
iVY will enhance efficiency of care delivery because it provides a brief, out-of-facility, youth-friendly video-counselling and mobile health app to 18–29 years old that is customisable to the needs of YWH.
The study uses an adaptive treatment strategy to individualise the intervention to YWH based on their viral response to the intervention providing a differentiated care model which tailors care to individuals with the greatest need.
Given HIV-related stigma, the influence of the COVID-19 pandemic on MH and SU challenges, and other barriers to care, the use of video-counselling and provision of remote research and services will likely remain high.
The intervention will not be available to those who do not have a smartphone or who are Spanish speakers.
Introduction
Young adults with HIV (YWH) in the USA have the lowest level of virological suppression1–3 compared with older age groups. They experience significant health disparities in HIV clinical outcomes,4–8 including lower rates of antiretroviral therapy (ART) initiation,8 suboptimal ART adherence8–10 and retention in care,11 and higher virological failure rates.12 Lack of virological suppression is a major contributor to mortality, morbidity and secondary transmission events.13 14 Additionally, mental health (MH) and substance use (SU) impact every step of the HIV care continuum from diagnosis to virological suppression15–19 and exacerbate socioeconomic challenges of linkage and sustained access to healthcare.20–24 There is also an increased risk of SU disorders, psychiatric disorders and mortality with SU at a younger age.25–27 Overcoming these barriers is key to improving life expectancy, HIV-related disabilities and quality of life.28 29 Given the strong evidence for the influence of MH and SU on worsening HIV health outcomes, there is a clear need for increased access to and provision of MH and SU services. Despite the need to address these critical barriers to care among YWH, there is a severe shortage of MH professionals30–32 and evidence-based interventions for YWH.33 34
Addressing this gap calls for interventions with differentiated or individualised approaches to tailor care to individuals with the greatest need. Adaptive treatment strategies (ATSs) involve adapting a treatment to an individual’s changing needs using predefined decision rules,32–36 making them patient centric by design and potentially reducing cost by only giving the appropriate therapy rather than a one size fits all approach.
Study objective
We describe the protocol for a study to assess the efficacy of a technology-based intervention on HIV viral suppression (primary outcome). Intervention for Virologic Suppression in Youth (iVY) aims to address MH and SU in a tailored manner using a differentiated care approach that is youth-friendly.35 iVY combines two components to address SU and MH among YWH: (1) brief weekly sessions with a counsellor via a video-chat platform (video-counselling) to discuss MH, SU, HIV care engagement and other barriers to care37 38; and (2) a mobile health app called WYZ designed and developed using a Human Centered Design approach with YWH to address barriers.39 40 The primary goal is to address important, distinct and changing barriers to HIV care engagement (eg, MH, SU, forgetting, social isolation)41–44 among YWH.
Methods and analysis
Study overview and design
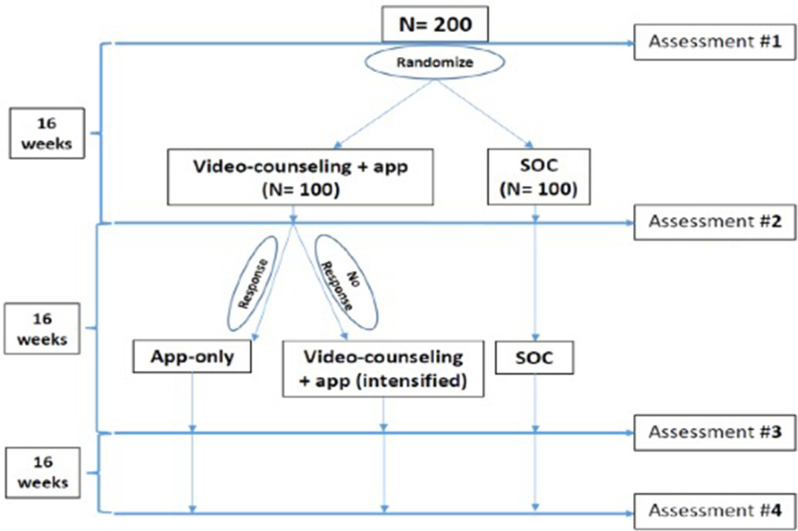
iVY is testing the effect of a technology-based mobile health app and video-counselling intervention in a randomised clinical trial (RCT) with an ATS45–49 among YWH (18–29 years old). Individuals who are not durably virologically suppressed are randomised (figure 1) to video-counselling+app or standard of care (SOC). At 16 weeks, HIV virological suppression between the intervention and control arms will be compared using data from home-collected HemaSpot test kits. Through this study, we are (1) testing the efficacy of video-counselling+app versus SOC on virological suppression and (2) assessing the impact of video-counselling+app versus SOC on MH and SU. We are evaluating HIV virological suppression, MH and SU differences between the intervention versus control arms at 16 weeks. We are also (3) exploring an ATS to individualise the intervention by assigning the virological ‘non-responders’ in the intervention arm to intensified video-counselling+app for 16 more weeks, and the virological ‘responders’ (responder=virologically suppressed; non-responder=virologically unsuppressed) in the intervention arm to continue only app use for 16 more weeks. Participants complete an assessment survey and home-collected viral load (VL) testing at baseline, 16, 32 and 48 weeks.
Figure 1.
Overview of study design. SOC, standard of care.
Study setting
We are working with AIDS Healthcare Foundation (AHF) to implement the study and recruit YWH receiving HIV primary care. In 2019, AHF served 1292 YWH in California, 927 (72%) of whom are in five AHF Healthcare Centers: Downtown Los Angeles, San Diego, Westside, Hollywood and Oakland. Among these 927 patients, 44% had a VL <200 copies/mL. Additionally, we are opening recruitment for YWH at other non-AHF CA clinics. All study activities are conducted remotely with previous methods successfully used by our team.50 51
Participant population
The study includes 200 YWH who are between the ages of 18–29, live and receive care in CA, have a lack of durable viral suppression (any HIV VL (≥20 copies/mL in the past 12 months), have access to a smartphone and speak English. We have chosen to include young adults aged 18–29 as they are in a distinct developmental phase with unique needs and challenges compared with individuals younger than 18 or older than 29 years or younger than 18. Those with a history of haemophilia or who are unable to conduct finger pricks at home for the VL test are excluded because participants are required to do at-home VL testing. Individuals with MH or SU challenges are included, unless their symptoms are too severe for them to safely participate in the study.
General study procedures
Recruitment
AHF is contacting patients from their database of YWH ages 18–29 who have a lack of durable viral suppression (any HIV VL (≥20 copies/mL in the past 12 months) to offer study information. Interested patients are handed off to the study team for further information, screening and enrolment; or they may be asked for permission to share their contact information with the study team to be contacted later. Non-AHF participants are recruited via social media and flyers posted at healthcare clinics serving YWH.
Screening, consent and enrolment
The study team screen interested individuals over the telephone to determine eligibility and enrol them in the study. Interested individuals are given adequate time to read the consent form; the study team member is available to answer any questions via phone. The consent form, to be signed electronically, includes information about potential risks and benefits, that participation is completely voluntary and will not affect SOC that participants receive, and that participants may withdraw from the study at any time. Any individual displaying MH or SU challenges that may impede the understanding and completion of consent is excluded and referred to appropriate services.
Randomisation
We are using a random number generator in REDCap (Research Electronic Data Capture) to randomise 1:1 allocation to intervention or SOC arms.52 Participants are assigned to groups via block randomisation with block size permuted to promote group balance on covariates. Given the lack of an appropriate time-matched and attention-matched control group and need to establish generalisable efficacy, we are comparing the video-counselling+app arm to a SOC arm. The SOC arm includes the current care delivery model: regularly scheduled visits with a healthcare provider and lab testing every 3–6 months or more/less frequently, depending on the individual’s HIV health outcomes (eg, viral suppression). The study team asks participants to complete the first study assessment. After completion of the survey, the participants will get randomised, and the study team will mail the participants’ baseline VL home-collected test. The VL sample is collected via a HemaSpot HF device, which uses advanced dried blood spot (DBS) technology. HemaSpot-HF is an improved collection over the traditional DBS cards because it protects the sample from contamination, allows for safe transport and easy storage and shipment, and provides a rapid sampling mechanism that does not require drying.
Patient retention and incentives
Retention is supported through the collection of detailed contact information, short message service (SMS) messaging and incentives. We have monthly check-ins with participants during which we ask about app use, provide support for logistical challenges with the app and update any contact information. We also work with a Youth Advisory Panel (YAP) on maintaining and enhancing participant retention throughout the study. Incentives include $20 for completing the baseline survey, $50 for the 16-week survey, $40 for the 32-week survey and $30 for the 48-week survey; $40 for the baseline, 16, 32 and 48-week home-collected VLs, plus a $10 for VL kits that are returned on time. Incentives are distributed through Venmo or CashApp via a study account that uses a study-specific mobile phone number and email and is accessible only by the study staff. Participants are asked to provide their CashApp or Venmo username, which is verified. The default is set to ‘private’ so that payment information cannot be viewed by others.
Risks to participants
Risks to participants are monitored regularly by trained study staff and documented at each intervention session and study assessment. At each assessment, we review participant responses to examine acute need for referral to medical, MH or SU services.
Adverse events and auditing
In this study, we do not anticipate moderate, severe, life-threatening, disabling or fatal adverse events. Potential adverse events include loss of confidentiality or emotional distress.
Patient and public involvement
To inform study implementation and dissemination, we have identified 10 YWH from AHF Healthcare Centers in CA to form the YAP. YAP meetings are held two times a year virtually and last for 2 hours. YAP members review study materials and provide input/feedback about the intervention, research questions, recruitment and retention strategies, next steps and dissemination of findings. In addition to keeping the YAP updated about study progress, we discuss any implementation challenges, engagement, attrition and other issues to obtain the YAP’s feedback to improve study implementation.
Intervention procedures
The intervention arm (video-counselling+app) receives 12 brief weekly counselling sessions (given over 16 weeks) with a masters level MH professional (eg, social worker), along with access to the WYZ app to use based on their needs. After 16 weeks, responders in the video-counselling+app arm continue to use the app only. Non-responders in the intervention arm continue with intensified video-counselling+app for 16 more weeks. The additional 16 weeks aims to reinforce counselling points by targeting participant-specific barriers to virological suppression based on the needs of non-responders.
Video-counseling
The goal of video counselling is to provide information, motivation and behavioural skills for dealing with MH, SU and HIV care engagement challenges to address mild–moderate MH symptoms or low–moderate levels of SU; and assist with linking/relinking to more extensive MH, SU and/or HIV treatment, as needed. The intervention tailors the counselling based on baseline factors as follows: (1) HIV care acuity: due to unsuppressed VL of all participants, each individual receives two core HIV sessions and sessions will be tailored based on barriers to achieving virological suppression; (2) MH acuity: based on elevated PHQ-953 54 (10+), GAD-7 (10+)55 or PCL-5; (33+)56 score or (3) SU acuity: based on elevated AUDIT (8+),57 DAST (3+),58 or monthly or more use of drugs (besides marijuana) or daily use of tobacco or marijuana as measured by ASSIST.59 60 Individuals with ‘high acuity’ receive two core sessions related to HIV care (2A/B), MH (2A/B) and/or SU (3A/B), each. ‘A’ sessions assess barriers and build motivation, while ‘B’ sessions provide information and deliver health education. A and B core sessions map onto the Information, Motivation, Behavior (IMB) model,61 which was used to develop the intervention.
For the remaining sessions, we use an integrated behavioural health and HIV care-focused approach to further the conversations in the core sessions. Participants choose from a list of menu sessions identified in the first session which allows the counsellor to spend more time on HIV care, MH or SU based on the participants’ needs. In this manner, the intervention is tailored to the unique and changing needs of participants and can address other specific topics related to their experiences of racism, transphobia, stigma, discrimination, gender identity, racial/ethnic identity, classism, and current events.
SMS messages are used to enhance the intervention and for participant retention by: (1) assessment of completion of health goals (ie, behavioural skills) set during weekly sessions (eg, schedule appointment with provider); (2) provision of MH/SU or community resources based on participant’s needs (eg, housing); (3) follow-up to ensure participant’s linkage to MH, SU or HIV care; (4) reminder of video-counselling session and (5) monthly check-in to update contact information.
Fidelity to intervention
A session fidelity checklist is completed for each session to ascertain whether the focus area and barriers were identified, education/information was provided, motivation was enhanced and problem-solving was initiated. The study team assesses intervention fidelity during weekly meetings with the counsellors to review each session’s length, technical issues, topics covered, goals established, and narrative progress notes.
Counsellor training
Prior to conducting sessions, counsellors participate in >25 hours of interactive training to learn the protocol and intervention manual intimately and role-play pre-prepared vignettes. Counsellors must attend the entire training and demonstrate proficiency of knowledge of the intervention manual.
WYZ app
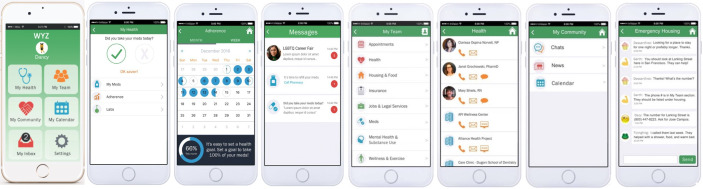
WYZ was designed and developed using a human-centred design approach with a YAP, formative research with YWH, and is grounded in the IMB model.39 40 WYZ contains three main features: My Health, My Community and My Team. Each of these features are described in more detail in table 1 and figure 2. Tailoring is achieved based on the individual’s needs at a given time (eg, My Community may be used more if social isolation is a barrier). For the My Community feature, all original posts must be approved by research staff before posting. On a weekly basis, research staff review new responses to all posts to ensure that there are not any critical issues to be addressed. Any issues are brought to the study team’s attention and referred to a clinical psychologist on the team if needed. Monthly check-ins help improve engagement through checking contact information and answering any questions.
Table 1.
WYZ features
| Feature | Function | Barriers addressed |
| My Health | Set customised ART adherence reminders (B), refill/injection reminders (B), graph adherence over time (M), keep track of VL and CD4+ test results (I/M) | Too busy, forgot, changed my routine, ran out of pills, unstructured lifestyle |
| My Team | Increase access to community organisations and resources using the participant’s geo-location (I) | Lack of access to MH and SU services or other community services, limited mobility |
| My Community | Allow users to interact with other YWH through moderated forums (M), stay up-to-date on health news (I), provide a calendar of community events (I) | Low health literacy, social isolation, stigma, lack of community support |
ART, antiretroviral therapy; MH, mental health; SU, substance use; VL, viral load; YWH, young adults with HIV.
Figure 2.
Mobile app screenshots.
Data collection procedures
Quantitative study assessments with home-collected HIV VL testing are done at baseline, 16, 32 and 48 weeks after enrolment and randomisation. Quantitative assessments collect information about sociodemographic characteristics, SU, technology use, MH, HIV-related outcomes and experiences with the intervention for those in the intervention arm. All measures are described in table 2. REDCap is used for all data collection such as surveys, intervention implementation and clinical data.52 REDCap is a secure web application for building and managing online surveys and databases, and data are stored and managed on a University of California San Francisco (UCSF) server.
Table 2.
Outcome and descriptive variables
| Variables | Items | Interpretation | |
| Outcome | |||
| 1o | HIV viral load (VL) | Virological suppression using HemaSpot device | Suppressed VL: <400 copies/mL Unsuppressed VL: ≥400 copies/mL |
| 2o | Mental health (MH) | Depressive symptoms: PHQ-964 (10 items; score=0–27) | Higher score indicates more depressive symptoms |
| Anxiety: GAD55 (7 items; score=0–21) | Higher score indicates more anxiety | ||
| Trauma: PCL-565 (score=0–80) | Higher score indicates more trauma | ||
| Substance use (SU) | DAST58 (10 items; score=0–10) | Higher score indicates more drug misuse | |
| AUDIT57 (10 items; scores=0–40) | Higher score indicates more alcohol misuse | ||
| ASSIST59 | Frequency of alcohol, smoking and SU | ||
| Other descriptive variables | |||
| Demographics | Age, sex/gender, race/ethnicity, sexual identity, education, income, work, school, living situation, city of residence, ever homeless or incarcerated | ||
| MH and SU | Type and frequency of MH and SU services ever received or being received; timeline follow-back method for more recent SU90 91; the Quick Inventory of Depressive Symptomatology (Self-Report) (QIDS-SR16)92 | ||
| Healthcare accessibility | Distance to get to HIV clinic, ease of getting appointments at clinic, ease of getting in touch with provider93 | ||
| Social isolation | PROMIS Item Bank94 (14 items, score=14–70) | Higher score indicates greater isolation | |
| Technology use | Use of technology to email providers, refill medications or making medical appointments, frequency of break in service or lost/stolen phone, reliability of service, access to Wi-Fi,95 mobile technology vulnerability scale (MTVS),96 System Usability Scale (SUS)97 for WYZ | MTVS range=0–17 with higher scores indicating more vulnerability,96 SUS range=0–100 and scores >68 considered above average98 | |
| Perceived engagement in HIV care | Index of Engagement in Care99 (10 items, score=0–10) | Higher score indicates higher antiretroviral therapy adherence, clinic attendance, VL suppression | |
| HIV knowledge | HIV treatment knowledge scale100 (15 items, score=0–15) | Higher score indicates more knowledge | |
| Engagement with provider | Healthcare provider engagement101 (13 items, score=0–52) | Higher score indicates poorer engagement | |
| Subsistence needs | Unmet subsistence needs42 102 (5 items, score=0–5) | Higher score indicates more subsistence needs | |
| Resilience | Brief resilience scale103 104 (6 items; score=1–5) | High score indicates more resilience | |
| App paradata | Number of minutes in app; change in app use over time; number of push notifications opened from those sent through the application; use of My Health (adherence tracking), My Team (identification of community services), My Community (chat with peers, use calendar) | ||
Qualitative exit interviews
At 16 and 32 weeks, we will conduct semi-structured individual exit qualitative interviews with a sample YWH from the intervention arm, stratified by response or non-response (ie, suppressed or unsuppressed HIV VL) and levels of engagement (attended ≥80% of counselling session vs <80% attendance). The interviews explore (1) barriers/facilitators to intervention participation, (2) barriers/facilitators to intervention response/non-response, (3) need for future modifications, and (4) preferences for longer term support.
Security and confidentiality
All data are stored on a secure, HIPAA compliant, password-protected server. Participants’ contact information and other identifying data are stored separately from other study data, the key linking names to study ID is stored separately and destroyed at the end of data collection, and all other study documents only have participant codes. Only the research team have access to participant identities. All data are de-identified prior to analysis and individuals will not be identified in any reports or publications of the research.
We use Zoom for video-counselling, which is the UCSF-preferred HIPAA-compliant video chat platform. We have developed study protocols related to video-counselling privacy and security. For SMS messages, study staff demonstrate how to set up privacy settings on smartphones, such as keeping SMS message previews from showing up on locked screens and adding a security code to lock the smartphone. For the WYZ app, we have partnered with the UCSF School of Medicine Technology (SOM Tech) team to maximise security. SOM Tech has built this app within best practices for developing HIPAA-compliant apps and has worked closely with the UCSF Enterprise Security team to review the app architecture in an iterative manner to ensure the highest level of security. All qualitative interviews are conducted remotely and audio-recorded via HIPAA-compliant web conference platform (eg, Zoom) with audio-recordings stored on a HIPAA-compliant server with access available only to select study staff. All audio-recorded files are deleted on completion of the study.
Data monitoring
A data safety monitoring board consisting of three external reviewers meet annually to review the research protocol and materials, evaluate the progress, and report on safety and concerns.
Study outcomes
The primary outcome is HIV VL using the HemaSpot-HF device.62 We mail participants home test kits (including the HemaSpot-HF device, gauze, bandages, lancets, alcohol wipes and pre-addressed stamped envelopes) at baseline, 16, 32 and 48 weeks. After a finger prick, they place two drops of blood on the HemaSpot-HF device. The device dries in 1 min; participants then close the lid and mail the device to the laboratory. The laboratory uses the Abbott RealTime HIV-1 DBS assay with a lower limit of 400 copies/mL.63 To minimise missing data, we request missing VL data (±1 month around the four VL time points) from participants’ clinical electronic medical records. The secondary outcomes are MH (PHQ-9,64 GAD-755 and PCL-565); and SU (AUDIT,57 DAST58 and ASSIST59).
Quantitative data analysis
Frequency tables for all variables and measures of central tendency and variability for continuous variables will be used to characterise the sample. In addition to describing important sample characteristics, these descriptive analyses will summarise the app paradata listed in table 2, which will provide important information on overall app engagement and specific features used most. If the study arms differ significantly at baseline on covariates, we will use methods based on the Rubin causal model (eg, propensity scores, double-robust estimation) to obtain the effect estimates under the counterfactual assumption of balanced groups.66–70 We will address missing data with multiple imputation.71
To assess efficacy of video-counselling+app versus SOC on virological suppression in YWH, we will compare HIV virological suppression of those randomised to the intervention versus control arms at 16 weeks. We hypothesise that at 16 weeks the odds of our primary outcome, virological suppression, will be higher for video-counselling+app intervention participants than for SOC participants. To test this comparison, we will fit a logistic regression model. To assess the impact of video-counselling+app versus SOC on the secondary outcomes, we will evaluate the MH and SU differences between the intervention versus control arms at 16 weeks. We will employ general linear modelling methods to test whether mean levels of MH and SU at 16 weeks are lower in the video-counselling+app group versus the SOC group. Demographic and prespecified covariates based on theory and literature will be included and moderated mediation will be explored using causal inference-based methods.
We will also explore differences between those who were (1) virological ‘non-responders’ in the intervention arm who received intensified video-counselling+app for 16 more weeks and (2) virological ‘responders’ in the intervention arm who continued only app use for 16 more weeks. Frequency tables for all variables and measures of central tendency and variability for continuous variables stratified by virological response status will characterise responders and non-responders on measures at 16 weeks including MH and SU. An exploratory multivariable logistic regression analysis will be performed on the subgroup of participants exposed to the intervention to predict which participants will be responders versus non-responders. Additionally, we will use logistic regression to explore whether responders versus non-responders at 16 weeks exhibit higher odds of virological suppression at 32 weeks.
Qualitative analysis
Data will be analysed using thematic and content analysis frameworks.72 Data analysis will draw on an inductive approach73 and by deductively applying codes developed from the interview guide. This dual approach will allow for themes to emerge from the data using inductive coding while also using an a priori template of code from which to frame the analysis.74
Power analysis
Power analyses were generated using NCSS PASS 202175 to compute the minimum detectable effect sizes for the proposed primary analyses. The study is beginning with 200 participants assigned to the video-counselling+app (N=100) intervention group and the SOC control group (N=100). Assuming 20% attrition based on our pilot studies40 76 and prior research among YWH,77 data from 160 participants will be available to test hypotheses. Assuming total N=160, α=0.05, power=0.80 and 44% in the control group virologically suppressed based on data supplied by AHF, we computed the minimum detectable OR, proportion difference (pdiff) and standardised proportion difference (h) for the proposed primary comparison, which yielded OR=2.46, pdiff=0.22 and h=0.44. For continuous MH and SU outcomes, we used the same inputs as above and computed the minimum detectable standardised mean difference d across the video-counselling+app and SOC groups, yielding d=0.45. Our proposed primary analyses can detect effects that are between small and medium.78 In addition, a 22% increase in virological suppression or an OR of 2.46 is an effect size equal to or smaller than other studies among YWH or older adults living with HIV79 and is clinically meaningful.
Ethics and dissemination
We received approval from the University of California, San Francisco Institutional Review Board to conduct this study and written consent from all participants. Reliance agreements were signed by RTI international and AHF. The study team will work with the YAP to disseminate results to YWH, participants and the academic community. A manuscript with the results of the primary study will be published in a peer-reviewed journal along with separate manuscripts for the secondary aims.
Discussion
Our intervention protocol uses a tailored approached to impact engagement in HIV care by focusing on MH, and SU among YWH using a tailored approach, focusing on various barriers to care to different degrees, based on their changing needs.41–44 Despite YWH experiencing more MH challenges than the general population substantially fewer interventions have been developed and evaluated in YWH compared with older adults.33 Most intervention studies for YWH have been conducted with small sample sizes, limited follow-up times, and not examined impact of the intervention to sustain or improve MH.33 80 While there are interventions for HIV prevention and SU among youth,81–84 they are limited by requiring lengthy in-person sessions with a trained counsellor, involving the youth’s families, focusing specifically on SU (vs a holistic approach), and were developed for younger adolescents. iVY is innovative because it provides a ‘self-service’ model that is customisable to the needs of YWH. Additionally, a recent review suggests the need for multi-component interventions that go beyond health facilities to address social barriers to engagement in HIV care.34 Thus, our study is significant and will enhance efficiency of care delivery because it provides a brief, out-of-facility, youth-friendly video-counselling and mobile health app to 18–29 years old with HIV, which focuses on HIV care, MH and SU, and connects participants to community resources to continue receiving MH and SU services, as needed.
We acknowledge that there will be more marginalised groups who may not have smartphone access (~4%) or may not have been diagnosed with HIV, and our study results may not be generalisable to them. Given this high level of smartphone ownership,85 we are confident that the vast majority of our target population will have a smartphone. Additionally, a Spanish version of the app is not currently available. Given the need to fully develop the intervention in a culturally relevant manner for non-English speakers, we aim to translate our intervention in future iterations of this project.
In summary, the remarkable biomedical advances in HIV prevention and treatment have resulted in discussions toward ending the HIV epidemic. However, this will not happen without addressing MH and SU barriers experienced by YWH.86 iVY will examine an innovative intervention to achieve the goal of ending the HIV epidemic. This study will provide valuable data about the characteristics of virological responders and non-responders to the intervention, individualisation of the intervention based on these variables, and linkage to MH and SU treatment services among those in need. If efficacious, in future research we will investigate the intervention’s sustainability for implementation across the USA. Given the influence of the COVID-19 pandemic on MH and SU challenges,87 the use of video-counselling and provision of remote research and services will remain high.88 89 iVY has the potential to impact HIV health outcomes and MH and SU among YWH who are disproportionately impacted by HIV and at elevated risk for poor health outcomes.
Supplementary Material
Acknowledgments
We would like to thank Gjermayne Wilson, Karla Quiteno and Sherrelle Banks for their contributions to the study and for help in recruiting participants. We would also like to thank the study participants and the Youth Advisory Panel for their time and effort.
Footnotes
PS and MCDS contributed equally.
Contributors: MCDS was the lead author of the manuscript with support and input from the study principal investigator, PS. CLM, CB and VG are developing and implementing the counselling intervention. KM, LS and DW are study coordinators. AS-C is overseeing work at AIDS Healthcare Foundation. BC is providing expertise on adaptive treatment strategies. TBN is providing mentorship on statistical methods and MOJ is providing mentorship and technical expertise on intervention development and testing. All coauthors reviewed and approved the manuscript and are involved in study implementation.
Funding: This work was supported by the National Institute of Mental Health (grant number R01MH131415).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1. Centers for disease control and prevention . Selected national HIV prevention and care outcomes 2017, Available: https://www.cdc.gov/hiv/pdf/library/slidesets/cdc-hiv-prevention-and-care-outcomes.pdf
- 2. Harris NS, Johnson AS, Huang Y-L, et al. Vital signs: status of human immunodeficiency virus testing, viral suppression, and HIV preexposure prophylaxis - United States, 2013-2018. MMWR Morb Mortal Wkly Rep 2019;68:1117–23. 10.15585/mmwr.mm6848e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kapogiannis BG, Koenig LJ, Xu J, et al. The HIV continuum of care for adolescents and young adults attending 13 urban US HIV care centers of the NICHD-ATN-CDC-HRSA SMILE collaborative. J Acquir Immune Defic Syndr 2020;84:92–100. 10.1097/QAI.0000000000002308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Doshi RK, Milberg J, Jumento T, et al. For many served by the ryan white HIV/AIDS program, disparities in viral suppression decreased, 2010-14. Health Aff (Millwood) 2017;36:116–23. 10.1377/hlthaff.2016.0655 [DOI] [PubMed] [Google Scholar]
- 5. Doshi RP, Aseltine RH, Sabina AB, et al. Racial and ethnic disparities in preventable hospitalizations for chronic disease: prevalence and risk factors. J Racial Ethn Health Disparities 2017;4:1100–6. 10.1007/s40615-016-0315-z [DOI] [PubMed] [Google Scholar]
- 6. Colasanti J, Kelly J, Pennisi E, et al. Continuous retention and viral suppression provide further insights into the HIV care continuum compared to the cross-sectional HIV care cascade. Clin Infect Dis 2016;62:648–54. 10.1093/cid/civ941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Castel AD, Kalmin MM, Hart RLD, et al. Disparities in achieving and sustaining viral suppression among a large cohort of HIV-infected persons in care - Washington, DC. AIDS Care 2016;28:1355–64. 10.1080/09540121.2016.1189496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zanoni BC, Mayer KH. The adolescent and young adult HIV cascade of care in the United States: exaggerated health disparities. AIDS Patient Care STDS 2014;28:128–35. 10.1089/apc.2013.0345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gross IM, Hosek S, Richards MH, et al. Predictors and profiles of antiretroviral therapy adherence among African American adolescents and young adult males living with HIV. AIDS Patient Care STDS 2016;30:324–38. 10.1089/apc.2015.0351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim S-H, Gerver SM, Fidler S, et al. Adherence to antiretroviral therapy in adolescents living with HIV: systematic review and meta-analysis. AIDS 2014;28:1945–56. 10.1097/QAD.0000000000000316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. California Department of Public Health . The continuum of HIV care -California 2018, Available: https://www.cdph.ca.gov/Programs/CID/DOA/CDPH%20Document%20Library/2018_HIV_CareContinuumFactSheet_AllLiving_ADA.pdf [Accessed 23 Apr 2021].
- 12. Wood SM, Lowenthal E, Lee S, et al. Longitudinal viral suppression among a cohort of adolescents and young adults with behaviorally acquired human immunodeficiency virus. AIDS Patient Care STDS 2017;31:377–83. 10.1089/apc.2017.0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crepaz N, Tang T, Marks G, et al. Durable viral suppression and transmission risk potential among persons with diagnosed HIV infection: United States, 2012-2013. Clin Infect Dis 2016;63:976–83. 10.1093/cid/ciw418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marks G, Gardner LI, Rose CE, et al. Time above 1500 copies. AIDS 2015;29:947–54. 10.1097/QAD.0000000000000640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gonzalez JS, Batchelder AW, Psaros C, et al. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr 2011;58:181–7. 10.1097/QAI.0b013e31822d490a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gamarel KE, Brown L, Kahler CW, et al. Prevalence and correlates of substance use among youth living with HIV in clinical settings. Drug Alcohol Depend 2016;169:11–8. 10.1016/j.drugalcdep.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krumme AA, Kaigamba F, Binagwaho A, et al. Depression, adherence and attrition from care in HIV-infected adults receiving antiretroviral therapy. J Epidemiol Community Health 2015;69:284–9. 10.1136/jech-2014-204494 [DOI] [PubMed] [Google Scholar]
- 18. Uthman OA, Magidson JF, Safren SA, et al. Depression and adherence to antiretroviral therapy in Low-, middle- and high-income countries: a systematic review and meta-analysis. Curr HIV/AIDS Rep 2014;11:291–307. 10.1007/s11904-014-0220-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown LK, Whiteley L, Harper GW, et al. Psychological symptoms among 2032 youth living with HIV: a multisite study. AIDS Patient Care STDS 2015;29:212–9. 10.1089/apc.2014.0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buckingham E, Schrage E, Cournos F. Why the treatment of mental disorders is an important component of HIV prevention among people who inject drugs. Adv Prev Med 2013;2013:690386. 10.1155/2013/690386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aidala AA, Wilson MG, Shubert V, et al. Housing status, medical care, and health outcomes among people living with HIV/AIDS: a systematic review. Am J Public Health 2016;106:e1–23. 10.2105/AJPH.2015.302905a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Feller DJ, Agins BD. Understanding determinants of racial and ethnic disparities in viral load suppression. J Int Assoc Provid AIDS Care 2017;16:23–9. 10.1177/2325957416667488 [DOI] [PubMed] [Google Scholar]
- 23. Centers for disease control and prevention . HIV surveillance report 2020. Available: https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2020-updated-vol-33.pdf
- 24. Centers for disease control and prevention (CDC) . Diagnoses of HIV infection in the United States and dependent areas 2020, Available: https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2018-updated-vol-31.pdf
- 25. Erskine HE, Moffitt TE, Copeland WE, et al. A heavy burden on young minds: the global burden of mental and substance use disorders in children and youth. Psychol Med 2015;45:1551–63. 10.1017/S0033291714002888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fishman M, Wenzel K, Scodes J, et al. Young adults have worse outcomes than older adults: secondary analysis of a medication trial for opioid use disorder. Journal of Adolescent Health 2020;67:778–85. 10.1016/j.jadohealth.2020.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Butelman ER, Chen CY, Brown KG, et al. Age of onset of heaviest use of Cannabis or alcohol in persons with severe opioid or cocaine use disorders. Drug Alcohol Depend 2021;226:108834. 10.1016/j.drugalcdep.2021.108834 [DOI] [PubMed] [Google Scholar]
- 28. Institute for Health Metrics and Evaluation (IHME) . GBD compare data visualization 2017
- 29. Remien RH, Stirratt MJ, Nguyen N, et al. Mental health and HIV/AIDS: the need for an integrated response. AIDS 2019;33:1411–20. 10.1097/QAD.0000000000002227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Satiani A, Niedermier J, Satiani B, et al. Projected workforce of psychiatrists in the United States: a population analysis. Psychiatr Serv 2018;69:710–3. 10.1176/appi.ps.201700344 [DOI] [PubMed] [Google Scholar]
- 31. Review of physician and advanced practitioner recruiting incentives 2017, Available: https://www.merritthawkins.com/uploadedFiles/MerrittHawkins/Pdf/2017_Physician_Incentive_Review_Merritt_Hawkins.pdf
- 32. USAFacts . Over one-third of Americans live in areas lacking mental health professionals 2021, Available: https://usafacts.org/articles/over-one-third-of-americans-live-in-areas-lacking-mental-health-professionals/
- 33. Vreeman RC, McCoy BM, Lee S. Mental health challenges among adolescents living with HIV. Journal of the International AIDS Society 2017;20. 10.7448/IAS.20.4.21497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Casale M, Carlqvist A, Cluver L. Recent interventions to improve retention in HIV care and adherence to antiretroviral treatment among adolescents and youth: a systematic review. AIDS Patient Care STDS 2019;33:237–52. 10.1089/apc.2018.0320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saberi P, Ming K, Dawson-Rose C. What does it mean to be youth-friendly? Results from qualitative interviews with health care providers and clinic staff serving youth and young adults living with HIV. Adolesc Health Med Ther 2018;9:65–75. 10.2147/AHMT.S158759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fisher JD, Fisher WA, Misovich SJ, et al. Changing AIDS risk behavior: effects of an intervention emphasizing AIDS risk reduction information, motivation, and behavioral skills in a college student population. Health Psychol 1996;15:114–23. 10.1037//0278-6133.15.2.114 [DOI] [PubMed] [Google Scholar]
- 37. McCuistian C, Wootton AR, Legnitto DA, et al. Development of a Video-counseling intervention to address HIV care, mental health, and substance use challenges for young adults. BMJ Open [Google Scholar]
- 38. Saberi P, McCuistian C, Agnew E, et al. Video-counseling intervention to address HIV care engagement, mental health, and substance use challenges: a pilot randomized clinical trial for youth and young adults living with HIV. Telemed Rep 2021;2:14–25. 10.1089/tmr.2020.0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saberi P, Lisha NE, Erguera XA, et al. WYZ: results of a pilot study of a mobile health application for engagement in care and antiretroviral therapy adherence among youth and young adults living with HIV. JMIR Form Res 2021;5:e26861. 10.2196/26861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Erguera XA, Johnson MO, Neilands TB, et al. WYZ: a pilot study protocol for designing and developing a mobile health application for engagement in HIV care and medication adherence in youth and young adults living with HIV. BMJ Open 2019;9:e030473. 10.1136/bmjopen-2019-030473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shubber Z, Mills EJ, Nachega JB, et al. Patient-reported barriers to adherence to antiretroviral therapy: a systematic review and meta-analysis. PLoS Med 2016;13:e1002183. 10.1371/journal.pmed.1002183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reeder C, Neilands TB, Palar K, et al. Food insecurity and unmet needs among youth and young adults living with HIV in the San Francisco Bay area. J Adolesc Health 2019;65:262–6. 10.1016/j.jadohealth.2019.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. MacDonell K, Naar-King S, Huszti H, et al. Barriers to medication adherence in behaviorally and Perinatally infected youth living with HIV. AIDS Behav 2013;17:86–93. 10.1007/s10461-012-0364-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Campbell CK, Dubé K, Sauceda JA, et al. Antiretroviral therapy experience, satisfaction, and preferences among a diverse sample of young adults living with HIV. AIDS Care 2022;34:1212–8. 10.1080/09540121.2021.2001783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lavori PW, Dawson R, Rush AJ. Flexible treatment strategies in chronic disease: clinical and research implications. Biol Psychiatry 2000;48:605–14. 10.1016/s0006-3223(00)00946-x [DOI] [PubMed] [Google Scholar]
- 46. Collins LM, Murphy SA, Bierman KL. A conceptual framework for adaptive preventive interventions. Prev Sci 2004;5:185–96. 10.1023/b:prev.0000037641.26017.00 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Murphy SA, Collins LM, Rush AJ. Customizing treatment to the patient: adaptive treatment strategies. Drug Alcohol Depend 2007;88 Suppl 2:S1–3. 10.1016/j.drugalcdep.2007.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lavori PW, Dawson R. Adaptive treatment strategies in chronic disease. Annu Rev Med 2008;59:443–53. 10.1146/annurev.med.59.062606.122232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chakraborty B, Moodie EEM. Statistical methods for dynamic treatment regimes. In: Statistical methods for dynamic treatment regimes: reinforcement learning, causal inference, and personalized medicine. New York, NY: Springer, 2013. 10.1007/978-1-4614-7428-9 [DOI] [Google Scholar]
- 50. Saberi P, Ming K, Legnitto D, et al. Feasibility and acceptability of novel methods to estimate antiretroviral adherence: a longitudinal study. PLoS One 2019;14. 10.1371/journal.pone.0210791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Saberi P, Ming K, Legnitto D, et al. Novel methods to estimate antiretroviral adherence: protocol for a longitudinal study. Patient Prefer Adherence 2018;12:1033–42. 10.2147/PPA.S166380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Harris PA, Taylor R, Minor BL, et al. The Redcap consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chenneville T, Gabbidon K, Drake H, et al. Comparison of the utility of the PHQ and CES-D for depression screening among youth with HIV in an integrated care setting. J Affect Disord 2019;250:140–4. 10.1016/j.jad.2019.03.023 [DOI] [PubMed] [Google Scholar]
- 54. Spitzer RL, Kroenke K, Williams JBW. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. J Am Med Assoc 1999;282:1737–44. 10.1001/jama.282.18.1737 [DOI] [PubMed] [Google Scholar]
- 55. Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–7. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 56. Lang AJ, Wilkins K, Roy-Byrne PP, et al. Abbreviated PTSD checklist (PCL) as a guide to clinical response. Gen Hosp Psychiatry 2012;34:332–8. 10.1016/j.genhosppsych.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Johnson JA, Lee A, Vinson D, et al. Use of AUDIT-based measures to identify unhealthy alcohol use and alcohol dependence in primary care: a validation study. Alcohol Clin Exp Res 2013;37 Suppl 1:E253–9. 10.1111/j.1530-0277.2012.01898.x [DOI] [PubMed] [Google Scholar]
- 58. Skinner HA. The drug abuse screening test. Addict Behav 1982;7:363–71. 10.1016/0306-4603(82)90005-3 [DOI] [PubMed] [Google Scholar]
- 59. Humeniuk R, Ali R, Babor TF, et al. Validation of the alcohol, smoking and substance involvement screening test (ASSIST). Addiction 2008;103:1039–47. 10.1111/j.1360-0443.2007.02114.x [DOI] [PubMed] [Google Scholar]
- 60. Humeniuk R, Henry-Edwards S, Ali R, et al. Manual for use in primary care. In: The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST). 2010. [Google Scholar]
- 61. Fisher JD, Fisher WA, Amico KR, et al. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychol 2006;25:462–73. 10.1037/0278-6133.25.4.462 [DOI] [PubMed] [Google Scholar]
- 62. Hall JM, Fowler CF, Barrett F, et al. HbA1C determination from Hemaspot™ blood collection devices: comparison of home prepared dried blood spots with standard venous blood analysis . Diabet Med 2020;37:1463–70. 10.1111/dme.14110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tang N, Pahalawatta V, Frank A, et al. HIV-1 viral load measurement in venous blood and Fingerprick blood using Abbott Realtime HIV-1 DBS assay. J Clin Virol 2017;92:56–61. 10.1016/j.jcv.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 64. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders Patient Health Questionnaire JAMA 1999. 10.1001/jama.282.18.1737 [DOI] [PubMed] [Google Scholar]
- 65. Weathers FW, Litz BT, Keane TM, et al. The PTSD checklist for DSM-5 (PCL-5) – standard. 2013. Available: http://www.ptsd.va.gov/
- 66. Luellen JK, Shadish WR, Clark MH. Propensity scores - an introduction and experimental test. Eval Rev 2005;29:530–58. 10.1177/0193841X05275596 [DOI] [PubMed] [Google Scholar]
- 67. Rubin DB. On principles for modeling propensity scores in medical research. Pharmacoepidemiol Drug Saf 2004;13:855–7. 10.1002/pds.968 [DOI] [PubMed] [Google Scholar]
- 68. Weitzen S, Lapane KL, Toledano AY, et al. Principles for modeling propensity scores in medical research: a systematic literature review. Pharmacoepidemiol Drug Saf 2004;13:841–53. 10.1002/pds.969 [DOI] [PubMed] [Google Scholar]
- 69. Seaman S, Copas A. Doubly robust generalized estimating equations for longitudinal data. Stat Med 2009;28:937–55. 10.1002/sim.3520 [DOI] [PubMed] [Google Scholar]
- 70. Shadish WR, Luellen JK, Clark MH. Propensity scores and quasi-experiments: A testimony to the practical side of Lee Sechrest. In: Bootzin RR, McKnight PE, eds. Strengthening research methodology: Psychological measurement and evaluation. Washington, DC: American Psychological Association, 2006: 143–57. 10.1037/11384-000 [DOI] [Google Scholar]
- 71. Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychological Methods 2002;7:147–77. 10.1037/1082-989X.7.2.147 [DOI] [PubMed] [Google Scholar]
- 72. Vaismoradi M, Turunen H, Bondas T. Content analysis and thematic analysis: implications for conducting a qualitative descriptive study. Nurs Health Sci 2013;15:398–405. 10.1111/nhs.12048 [DOI] [PubMed] [Google Scholar]
- 73. Ryan AB. Researching and writing your thesis: a guide for postgraduate students. In: Methodology: analysing qualitative data and writing up your findings. Maynooth Adult and Community Education: Mace, 2006: 92–108. [Google Scholar]
- 74. Fereday J, Muir E. Demonstarting rigor using thematic analysis: a hybrid approach of Inductive and deductive coding and theme development. Int J Qual Methods 2016. [Google Scholar]
- 75. NCSS Stastical Software . NCSS pass 2020; 2020.
- 76. Wootton AR, Legnitto DA, Gruber VA, et al. Telehealth and texting intervention to improve HIV care engagement, mental health and substance use outcomes in youth living with HIV: a pilot feasibility and acceptability study protocol. BMJ Open 2019;9:e028522. 10.1136/bmjopen-2018-028522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Agwu AL, Lee L, Fleishman JA, et al. Aging and loss to follow-up among youth living with human immunodeficiency virus in the HIV research network. J Adolesc Health 2015;56:345–51. 10.1016/j.jadohealth.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Crosby RA, Rothenberg R. In STI interventions, size matters. Sex Transm Infect 2004;80:82–5. 10.1136/sti.2003.007625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Puccio JA, Belzer M, Olson J, et al. The use of cell phone reminder calls for assisting HIV-infected adolescents and young adults to adhere to highly active antiretroviral therapy: a pilot study. AIDS Patient Care STDS 2006;20:438–44. 10.1089/apc.2006.20.438 [DOI] [PubMed] [Google Scholar]
- 80. Judd A, Sohn AH, Collins IJ. Interventions to improve treatment, retention and survival outcomes for adolescents with perinatal HIV-1 transitioning to adult care: moving on up. Curr Opin HIV AIDS 2016;11:477–86. 10.1097/COH.0000000000000302 [DOI] [PubMed] [Google Scholar]
- 81. Lauby J, Zhu L, Milnamow M, et al. Get real: evaluation of a community-level HIV prevention intervention for young MSM who engage in episodic substance use. AIDS Educ Prev 2017;29:191–204. 10.1521/aeap.2017.29.3.191 [DOI] [PubMed] [Google Scholar]
- 82. Letourneau EJ, McCart MR, Sheidow AJ, et al. First evaluation of a contingency management intervention addressing adolescent substance use and sexual risk behaviors: risk reduction therapy for adolescents. J Subst Abuse Treat 2017;72:56–65. 10.1016/j.jsat.2016.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Estrada Y, Rosen A, Huang S, et al. Efficacy of a brief intervention to reduce substance use and human immunodeficiency virus infection risk among latino youth. J Adolesc Health 2015. 10.1016/j.jadohealth.2015.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bhana A, McKay MM, Mellins C, et al. Family-based HIV prevention and intervention services for youth living in poverty-affected contexts: the CHAMP model of collaborative, evidence-informed programme development. J Int AIDS Soc 2010;13:S8. 10.1186/1758-2652-13-S2-S8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pew Research . Demographics of mobile device ownership and adoption in the United States. 2021. Available: https://www.pewresearch.org/internet/fact-sheet/mobile/
- 86. Remien RH, Patel V, Chibanda D, et al. Integrating mental health into HIV prevention and care: a call to action. J Int AIDS Soc 2021;24 Suppl 2:e25748. 10.1002/jia2.25748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Czeisler MÉ, Lane RI, Wiley JF, et al. Follow-up survey of US adult reports of mental health, substance use, and suicidal Ideation during the COVID-19 pandemic, September 2020. JAMA Netw Open 2021;4:e2037665. 10.1001/jamanetworkopen.2020.37665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fiorillo A, Gorwood P. The consequences of the COVID-19 pandemic on mental health and implications for clinical practice. Eur Psychiatr 2020;63. 10.1192/j.eurpsy.2020.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mdege ND, Chindove S, Ali S. The effectiveness and cost implications of task-shifting in the delivery of antiretroviral therapy to HIV-infected patients: a systematic review. Health Policy Plan 2013;28:223–36. 10.1093/heapol/czs058 [DOI] [PubMed] [Google Scholar]
- 90. Sobell LC, Sobell M. Timeline Followback Method (Drugs, Cigarettes, and Marijuana). Toronto, Ontario: Addictions Research Foundation, 1996. [Google Scholar]
- 91. Instrument . Timeline Followback method assessment, Available: https://cde.drugabuse.gov/instrument/d89c8e23-16e5-625a-e040-bb89ad43465d [Accessed 31 May 2021].
- 92. Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003;54:573–83. 10.1016/s0006-3223(02)01866-8 [DOI] [PubMed] [Google Scholar]
- 93. Agency for Healthcare research and quality . Consumer assessment of healthcare providers and systems (CAHPS), Available: https://www.ahrq.gov/cahps/index.html
- 94. Cella D, Riley W, Stone A, et al. The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. Journal of Clinical Epidemiology 2010;63:1179–94. 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Saberi P, Johnson MO. Correlation of Internet use for health care engagement purposes and HIV clinical outcomes among HIV-positive individuals using online social media. J Health Commun 2015;20:1026–32. 10.1080/10810730.2015.1018617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lisha NE, Neilands TB, Erguera XA, et al. Development of the mobile technology vulnerability scale among youth and young adults living with HIV. Int J Environ Res Public Health 2021;18:4170. 10.3390/ijerph18084170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lewis JR. The system usability scale: past, present, and future. International Journal of Human–Computer Interaction 2018;34:577–90. 10.1080/10447318.2018.1455307 [DOI] [Google Scholar]
- 98. Wootton AR, McCuistian C, Legnitto Packard DA, et al. Overcoming technological challenges: lessons learned from a telehealth counseling study. Telemed J E Health 2020;26:1278–83. 10.1089/tmj.2019.0191 [DOI] [PubMed] [Google Scholar]
- 99. Johnson MO, Neilands TB, Koester KA, et al. Detecting disengagement from HIV care before it is too late: development and preliminary validation of a novel index of engagement in HIV care. J Acquir Immune Defic Syndr 2019;81:145–52. 10.1097/QAI.0000000000002000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Balfour L, Kowal J, Tasca GA, et al. Development and psychometric validation of the HIV treatment knowledge scale. AIDS Care 2007;19:1141–8. 10.1080/09540120701352241 [DOI] [PubMed] [Google Scholar]
- 101. Chen W-T, Wantland D, Reid P, et al. Engagement with health care providers affects Self- efficacy, self-esteem, medication adherence and quality of life in people living with HIV. J AIDS Clin Res 2013;4:256. 10.4172/2155-6113.1000256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Riley ED, Moore K, Sorensen JL, et al. Basic subsistence needs and overall health among human immunodeficiency virus-infected homeless and unstably housed women. Am J Epidemiol 2011;174:515–22. 10.1093/aje/kwr209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Connor KM, Davidson JRT. Development of a new resilience scale: the Connor-Davidson resilience scale (CD-RISC). Depress Anxiety 2003;18:76–82. 10.1002/da.10113 [DOI] [PubMed] [Google Scholar]
- 104. Smith BW, Dalen J, Wiggins K, et al. The brief resilience scale: assessing the ability to bounce back. Int J Behav Med 2008;15:194–200. 10.1080/10705500802222972 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.