Abstract
To determine the effects of penicillin and erythromycin on cytokine production induced by heat-killed Streptococcus pneumoniae (HKSP), we studied the effects of those drugs on cytokine production induced by S. pneumoniae in human whole blood in vitro and ex vivo. In whole blood in vitro, erythromycin, but not penicillin, caused a dose-dependent decrease in HKSP-induced production of tumor necrosis factor alpha (TNF) and interleukin 6 (IL-6), while the production of IL-10, IL-12, and gamma interferon was inhibited only at the highest erythromycin concentration tested (10−3 M). The production of TNF and IL-6 in whole blood obtained from healthy subjects after a 30-min infusion of erythromycin (1,000 mg) was lower after ex vivo stimulation with HKSP than that in blood drawn before the infusion. Inhibition of TNF contributed to erythromycin-induced inhibition of IL-6 synthesis. Inhibition of TNF and IL-6 production by erythromycin may have a negative impact on host defense mechanisms during pneumococcal pneumonia.
Bacterial pneumonia has an estimated incidence in the United States of four million cases per year, one-fifth of which require hospitalization. Streptococcus pneumoniae is the most commonly identified pathogen in community-acquired pneumonia, with a reported incidence of 27 to 46% (4, 23, 25). At present, penicillin is considered the first-choice antibiotic therapy for pneumococcal pneumonia in most parts of the world. Macrolide antibiotics, such as erythromycin, are given in cases of penicillin allergy. In addition, macrolides are used with increasing frequency for the treatment of pneumococcal pneumonia due to the emerging resistance of pneumococci to penicillin (9, 16).
Cytokines are small proteins involved in the orchestration of inflammatory processes. They interact in a network that consists of proinflammatory cytokines (e.g., tumor necrosis factor alpha [TNF], interleukin 6 (IL-6), gamma interferon [IFN-γ], and IL-12) and anti-inflammatory cytokines (e.g., IL-10). In patients with pneumonia, cytokines are produced within the lung at the site of the infection, where they are important for host defense (5, 8). Indeed, endogenous TNF, IL-6, and IL-12 are essential for limitation of bacterial growth in lungs in mouse models of pneumococcal and Klebsiella pneumonia, while IL-10 hampers antimicrobial defenses in such models (11, 12, 33–35).
Macrolide antibiotics have been found to influence the endotoxin-induced production of cytokines (13, 15, 20, 22, 29). However, the clinical relevance of this finding is uncertain, because macrolides are used mainly for the treatment of infections with gram-positive organisms. The effects of macrolides and β-lactam antibiotics on cytokine production induced by gram-positive organisms are unknown.
In the present study we sought to determine the effects of erythromycin and penicillin on cytokine production induced by heat-killed S. pneumoniae (HKSP) in human whole blood in vitro. In addition, we determined the capacity of whole blood obtained before and after an intravenous erythromycin infusion in healthy subjects to produce cytokines upon ex vivo stimulation with HKSP.
MATERIALS AND METHODS
Reagents.
Erythromycin and penicillin were purchased from Abbott (Amstelveen, The Netherlands) and Yamanouchi (Leiderdorp, The Netherlands), respectively. Anti-TNF F(ab′)2 fragment (MAK 195F) was kindly provided by Knoll, Ludwigshafen, Germany. MAK 195F is derived from a murine TNF-neutralizing monoclonal antibody (MAb), immunoglobulin G3 (IgG3), and neutralizes the biological activity of recombinant and naturally occurring human TNF (19). The concentration of MAK 195F used (10 μg/ml) represented a 1- to 2-log-unit excess neutralizing capacity over TNF concentrations detected after stimulation with pneumococci. Mouse IgG was purchased from Fluka Chemia, Buchs, Switzerland.
Whole-blood stimulation.
HKSP was obtained from a clinical isolate (serotype D9). The bacteria were cultured overnight in 1 liter of Todd-Hewitt broth (20 h) in 5% CO2 at 37°C, harvested by centrifugation, washed twice in pyrogen-free 0.9% NaCl, resuspended in 10 ml of 0.9% NaCl, and heat inactivated for 60 min at 80°C. A 500-μl sample on a blood agar plate did not show growth of bacteria.
Whole-blood stimulation was performed as described previously (7, 30, 31). Briefly, blood was collected aseptically from healthy subjects with a sterile collecting system consisting of a butterfly needle connected to a syringe (Becton Dickinson & Co., Rutherford, N.J.). Anticoagulation was obtained with endotoxin-free heparin (Heparine; Leo Pharmaceutical Products B. V., Weesp, The Netherlands) (final concentration, 10 U/ml of blood). Whole blood, diluted 1:1 in sterile RPMI 1640 (Gibco BRL, Life Technologies Inc., Paisley, Scotland), was stimulated for 4 to 24 h at 37°C with HKSP (amounts equivalent to a final concentration of 106 or 107 CFU/ml) in sterile polypropylene tubes (Becton Dickinson & Co.). For these experiments, polypropylene tubes were prefilled with 0.75 ml of RPMI 1640 with or without the appropriate concentrations of HKSP, erythromycin, penicillin, or anti-TNF, after which 0.75 ml of heparinized blood was added. Tubes were then gently mixed and placed in an incubator. After incubation, plasma was prepared by centrifugation and stored at −20°C until assays were performed.
Erythromycin infusion study.
In a separate series of experiments, six healthy subjects, aged 32 ± 2 years (mean ± standard error [SE]), received a 30-min intravenous infusion of erythromycin (1,000 mg in 250 ml of 0.9% NaCl). Blood was collected as described above directly before the infusion, immediately after the infusion, and at 1, 2, and 4 h after infusion was completed. Stimulation of whole blood was performed with HKSP (107 CFU/ml) for 16 h at 37°C as described above. All studies were approved by the institutional scientific and ethics committees.
Cell viability.
Cell viability was determined by trypan blue exclusion (24) and incorporation of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (21).
For trypan blue exclusion, aliquots of 0.75 ml of blood in 0.75 ml of RPMI 1640 with HKSP (107 CFU/ml) and/or erythromycin (10−5 to 10−3 M) were incubated for 16 h at 37°C. The supernatant was removed, and the cell pellet was resuspended in 2 ml of phosphate-buffered saline (PBS). The mononuclear cells were then isolated by standard Ficoll-Hypaque centrifugation and washed twice in PBS. The tubes containing the cell suspension were spun in polypropylene tubes (Becton Dickinson & Co.) at 1,000 × g for 10 min. Cells were stained with 0.04% trypan blue (Sigma, St. Louis, Mo.), and 100 viable or nonviable cells from incubations with different concentrations of erythromycin were counted with a standard microscope. MTT is a reagent that is metabolized to a dark blue end product by viable cells. For MTT incorporation, aliquots of blood from four volunteers, with and without HKSP and/or erythromycin, were incubated for 16 h at 37°C as described above, subjected to NH4Cl lysis to clear erythrocyte contamination (1.5 ml of culture plus 1.5 ml of NH4Cl lysis buffer), and centrifuged at 200 × g for 5 min. The pellet was resuspended in 3 ml of cold RPMI 1640, washed a second time, and resuspended in 0.5 ml of RPMI 1640. Duplicate aliquots of this cell suspension (200 μl) were placed in 96-well round-bottom plates, and 20 μl of MTT (5 mg/ml; Sigma) was added. The plates were incubated for 4 h at 37°C in a humidified atmosphere containing 5% CO2. After removal of 150 μl of the supernatant, 100 μl of 0.04 N HCl-isopropanol was added to solubilize the blue crystals and the absorbance was read at 550 nm.
Assays.
The following cytokines were tested with specific enzyme-linked immunosorbent assays (ELISAs) according to the instructions of the manufacturers (manufacturers’ names are in parentheses): TNF (Medgenix, Brussels, Belgium), IL-6 (Pharmingen, San Diego, Calif.), IL-10 (Pharmingen), and IFN-γ (Control Laboratory of The Netherlands Red Cross Blood Transfusion Service [CLB], Amsterdam, The Netherlands). Concentrations of IL-12 p40 and IL-12 p70 were determined by sandwich ELISAs. In short, 96-well Immuno Maxisorp plates (Nunc, Roskilde, Denmark) were coated overnight at 4°C with IL-12 p40-specific MAb C11.79 (2 μg/ml) or IL-12 p70-specific MAb 20C2 (1.25 μg/ml). The plates were washed with 0.2 M PBS–0.05% Tween 20, incubated with 2% milk in PBS for 1 h as a blocking step, and washed again. Samples and standards were diluted in high-performance ELISA buffer (CLB). Human recombinant IL-12 was used as the standard. Samples and standards were incubated together with biotinylated anti-human IL-12 p40 MAb C8.6 (final concentration, 0.5 μg/ml) for 1.5 h at room temperature. After five washes, bound IL-12 p40 or IL-12 p70 was detected with peroxidase-conjugated streptavidin (CLB) and ortho-phenylenediamine as the substrate. The color reaction was stopped after 10 min with 1 M H2SO4, and the absorbance was read at 490 and 650 nm. MAb 20C2 and human recombinant IL-12 were kindly provided by Maurice K. Gately, Hoffmann-La Roche Inc., Nutley, N.J., and the hybridomas producing the IL-12 p40-specific MAbs C11.79 and C8.6 were kindly provided by Giorgio Trinchieri, The Wistar Institute, Philadelphia, Pa.
Statistical analysis.
All values are means ± standard errors of the means. Two-sample comparisons were performed by using the Wilcoxon test for matched samples. A P value of <0.05 was considered to represent a statistically significant difference.
RESULTS
Time course of cytokine induction by HKSP.
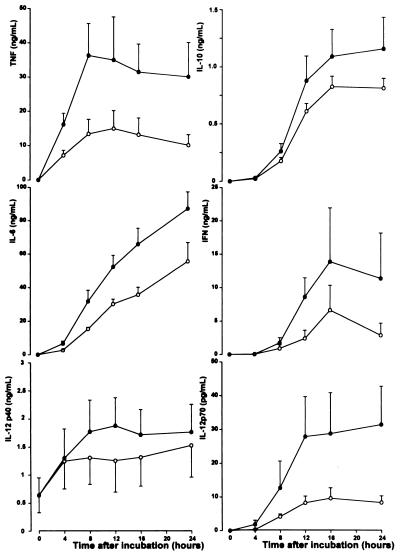
Incubation of whole blood without HKSP did not result in detectable cytokine production (data not shown). Incubation of whole blood with HKSP was associated with a dose- and time-dependent production of TNF, IL-6, IL-10, IFN-γ, IL-12 p40, and IL-12 p70. TNF was the first cytokine detectable, peaking after 8 h (36.3 ± 9.3 ng/ml), while the other cytokines reached peak concentrations at later time points (IL-12 p40 at 12 h [1.9 ± 0.5 ng/ml], IFN-γ at 16 h [13.8 ± 8.0 ng/ml], and IL-10, IL-6, and IL-12 p70 at 24 h [1.2 ± 0.3 ng/ml, 87.1 ± 10.1 ng/ml, and 31 ± 11 pg/ml, respectively]). Time curves for measured cytokines are shown in Fig. 1. Based on these experiments, a 16-h incubation with 107 CFU of HKSP/ml was chosen for further experiments.
FIG. 1.
Time-dependent release of TNF, IL-10, IL-6, IFN-γ, IL-12 p40, and IL-12 p70 in HKSP-stimulated blood. Whole blood diluted 1:1 in sterile RPMI 1640 was stimulated for 4 to 24 h at 37°C with HKSP (•, 107 CFU/ml; ○, 106 CFU/ml). Data are means ± standard errors of the means for six healthy donors.
Effects of penicillin and erythromycin on cytokine production induced by HKSP in vitro.
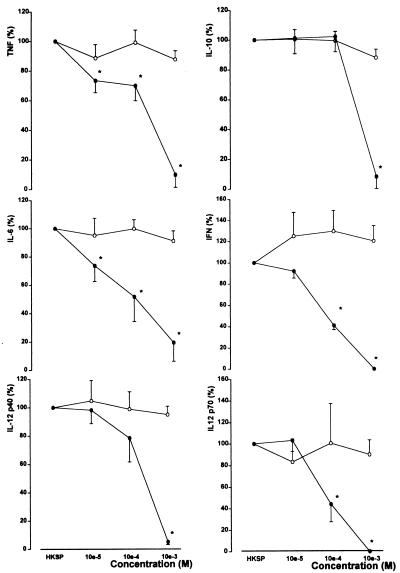
Penicillin (10−5 to 10−3 M) did not influence HKSP-induced cytokine production. By contrast, erythromycin (10−5 to 10−3 M) caused a dose-dependent inhibition of the production of all cytokines tested (Fig. 2). TNF and IL-6 production appeared most sensitive to erythromycin, with significant inhibition beginning after incubation with erythromycin at 10−5 M. IFN-γ production was inhibited by erythromycin at 10−4 M, while IL-10, IL-12 p40, and IL-12 p70 secretion was reduced only at the highest erythromycin concentration tested (10−3 M). The effects of erythromycin on cytokine production were not caused by a negative influence on the viability of leukocytes, as determined by trypan blue and MTT incorporation (data not shown).
FIG. 2.
Erythromycin, but not penicillin, influences the release of TNF, IL-10, IL-6, IFN-γ, IL-12 p40, and IL-12 p70 in HKSP-stimulated whole blood. Whole blood diluted 1:1 in sterile RPMI 1640 was stimulated for 16 h at 37°C with HKSP (107 CFU/ml) and erythromycin (•) or penicillin (○) (both at concentrations of 10−5 to 10−3 M). Values are expressed relative to production in the absence of penicillin and erythromycin (means ± SEs for six healthy donors). ∗, P < 0.05 versus value obtained after incubation without erythromycin. Concentrations after stimulation without penicillin (set at 100%) were 34.1 ± 5.2 ng/ml for TNF, 2.8 ± 0.5 ng/ml for IL-10, 75.7 ± 11.3 ng/ml for IL-6, 4.0 ± 1.6 ng/ml for IFN-γ, 2.1 ± 0.4 ng/ml for IL-12 p40, and 43 ± 21 pg/ml for IL-12 p70.
Effect of erythromycin infusion on ex vivo cytokine production.
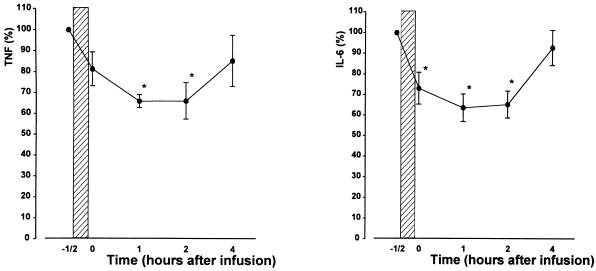
Inhibition of HKSP-induced cytokine production in vitro occurred at relatively high erythromycin concentrations. To evaluate the clinical relevance of our findings, we next infused six healthy subjects with 1,000 mg of erythromycin (the dose given to patients with severe infections) and determined the capacity of whole blood to produce cytokines after stimulation with HKSP ex vivo. In these experiments, erythromycin infusions influenced only HKSP-induced production of TNF and IL-6 (P < 0.05 [Fig. 3]), while IL-10, IFN-γ, IL-12 p40, and IL-12 p70 concentrations remained unchanged (data not shown). Erythromycin infusion did not affect leukocyte counts or differentials. Consequently, expression of cytokine levels corrected for the number of mononuclear cells yielded similar results (data not shown).
FIG. 3.
Erythromycin infusion inhibits the production of TNF and IL-6. Six healthy volunteers each received a 30-min intravenous infusion of 1,000 mg of erythromycin in 250 ml of 0.9% NaCl (hatched bar). Blood was collected directly before and directly after infusion and at 1, 2, and 4 h after the end of infusion. Whole blood diluted 1:1 in sterile RPMI 1640 was stimulated for 16 h at 37°C with HKSP (107 CFU/ml). Values are expressed relative to production before infusion of erythromycin (mean ± SEs for six healthy donors). Concentrations after stimulation and before infusion of erythromycin (set at 100%) were 18.4 ± 1.7 ng/ml for TNF and 165.8 ± 24.4 ng/ml for IL-6. ∗, P < 0.05 versus value obtained before infusion of erythromycin.
Erythromycin-induced inhibition of TNF production contributes to reduced IL-6 levels.
Since it has been reported that endogenously produced TNF in part mediates the production of IL-6 induced by endotoxin (27, 36), we investigated whether erythromycin-induced inhibition of TNF production was involved in the negative effect of erythromycin on the synthesis of other cytokines in HKSP-stimulated whole blood. To evaluate this possibility, we incubated whole blood with HKSP in the presence or absence of a neutralizing anti-TNF MAb (10 μg/ml). First, we demonstrated that polyclonal mouse IgG (final concentration, 10 μg/ml) did not influence HKSP-induced cytokine production (data not shown). Anti-TNF inhibited HKSP-induced production of IL-6, indicating that TNF is indeed partially responsible for HKSP-induced IL-6 production in whole blood (P < 0.05 [Table 1]). In the presence of anti-TNF, physiological concentrations of erythromycin failed to influence IL-6 concentrations in HKSP-stimulated whole blood (relative to IL-6 levels measured after incubation without erythromycin and in the presence of anti-TNF), suggesting that erythromycin exerts its effect on IL-6 production at least in part through the reduction of TNF concentrations.
TABLE 1.
Effect of anti-TNF MAb on erythromycin-induced inhibition of IL-6 production by HKSP-stimulated whole blood
| Erythromycin concn (M) | IL-6 production bya:
|
|||
|---|---|---|---|---|
| HKSP-stimulated blood
|
HKSP-stimulated blood + anti-TNF MAb
|
|||
| Concn (ng/ml) | % of control | Concn (ng/ml) | % of control | |
| 0 | 90.4 ± 9.5 | 100 | 63.1 ± 6.7c | 100 |
| 10−5 | 79.1 ± 6.4b | 88.6 ± 2.5 | 65.8 ± 5.3c | 107.3 ± 9.4 |
| 10−4 | 70.1 ± 4.7b | 79.6 ± 5.2 | 63.2 ± 5.9c | 101.0 ± 3.1 |
| 10−3 | 0.02 ± 0.01b | 0 | 0.02 ± 0.01b | 0 |
Whole blood diluted 1:1 in RPMI 1640 was incubated for 16 h with HKSP (amount equivalent to 107 CFU/ml) in the presence or absence of erythromycin (10−5 to 10−3 M) and/or anti-TNF MAb (10 μg/ml). IL-6 levels are means ± SEs for six volunteers.
P < 0.05 versus value obtained with HKSP in the absence of erythromycin.
P < 0.05 versus value obtained with HKSP in the absence of anti-TNF MAb.
DISCUSSION
In patients with unilateral pneumonia, much higher cytokine concentrations have been found in bronchoalveolar lavage fluid obtained from the infected lung than in lavage fluid from the uninvolved lung or in plasma (5, 8). This suggests that during clinical pneumonia cytokines are produced at the site of the infection. Mouse studies have indicated that locally produced cytokines are required for an effective host defense against bacterial pneumonia (11, 12, 33–35). Therefore, we considered it of interest to examine the effects of antimicrobial agents used for the treatment of pneumonia on cytokine production. In this study, we determined the capacities of erythromycin and penicillin to influence cytokine production. S. pneumoniae was used as a stimulus, since both antibiotics are commonly used to treat pneumococcal pneumonia. We specifically chose to use heat-killed bacteria rather than viable pneumococci, to allow us to study only direct effects on cytokine production and rule out indirect influences (i.e., consequences of an antimicrobial effect).
It was found that erythromycin, but not penicillin, inhibited the production of cytokines implicated in the pathogenesis of pneumonia. Importantly, in whole blood in vitro, erythromycin most potently attenuated the production of TNF and IL-6, and significant inhibition began at concentrations of 10−5 and 10−4 M, levels that are achieved in humans after intravenous administration (18). In contrast, the production of IFN-γ and in particular IL-10 and IL-12 was inhibited only at concentrations that exceeded the therapeutic range. Our in vitro findings were confirmed in vivo, since whole blood drawn from volunteers after intravenous infusions of erythromycin at a dose used in patients produced less TNF and IL-6 upon stimulation with HKSP than did blood obtained before the infusions. In view of the finding that during murine pneumococcal pneumonia a reduction in either TNF or IL-6 activity hampers bacterial clearance (34, 35), it is tempting to speculate that erythromycin-induced inhibition of TNF and IL-6 production is an undesired side effect of this antibiotic.
TNF is considered an endogenous mediator of IL-6 release induced by endotoxin in humans in vivo (27, 36). IL-6 release in endotoxemia is considered to be dependent on TNF production. We hypothesized that erythromycin-induced reduction of IL-6 release was in part the result of reduced TNF concentrations in the presence of erythromycin. Therefore, the effect of erythromycin on IL-6 release was investigated in the presence of a neutralizing anti-TNF MAb. Indeed, anti-TNF inhibited HKSP-induced IL-6 production in whole blood. Moreover, in the presence of anti-TNF, erythromycin at lower concentrations lost the ability to inhibit IL-6 release. Hence, these data suggest that erythromycin-induced inhibition of IL-6 release is predominantly the result of inhibition of TNF release by erythromycin.
In our study, blood mononuclear cells were likely the most important cytokine-producing cells (6). It can be argued that our results cannot be extrapolated to cytokine-producing cells within the lung. However, the design of our experiments, in which we considered it important to determine the effect of an in vivo infusion of a clinically relevant dose of erythromycin, did not allow for sampling of alveolar cells by repeated bronchoalveolar lavages for ethical reasons.
Previous studies focusing on the effects of erythromycin and other macrolides on inflammatory responses induced by endotoxin in vitro have yielded conflicting results. Macrolides have been reported to inhibit endotoxin-induced production of proinflammatory cytokines by monocytes in some but not all studies (2, 10, 14, 20). In one study, fosfomycin and clarithromycin were found to potentiate endotoxin-induced IL-10 production by human monocytes in vitro (20). To our knowledge, the effect of erythromycin on cytokine production induced by a pathophysiologically more relevant stimulus, e.g., S. pneumoniae, has not been examined. This seems important considering that cytokine production induced by gram-positive organisms may, in part, involve mechanisms different from those used by endotoxin (17). Studies on the mechanisms underlying the effect of erythromycin have produced inconsistent data with respect to the predominant mode of action of this drug. Macrolide antibiotics exhibit their antimicrobial activity by interfering with the protein production of microorganisms. They bind reversibly to the 50S ribosomal subunit of sensitive microorganisms, resulting in a dissociation of the tRNA from the ribosomes during translocation to the mRNA (26). In airway epithelial cells, erythromycin increases cyclic AMP (cAMP) levels (28). Elevation of cAMP levels has a marked effect on cytokine production induced by endotoxin, which includes inhibition of TNF and up regulation of IL-6 and IL-10 (3, 32). Hence, a possible increase in cellular cAMP levels by erythromycin would only partly explain our main findings.
Pneumonia is associated with local production of cytokines. We report here that erythromycin, but not penicillin, inhibits TNF and IL-6 production in whole blood stimulated with S. pneumoniae in vitro. This finding could be reproduced in blood obtained from healthy subjects infused with a clinically relevant dose of erythromycin. Inhibition of TNF and IL-6 production by erythromycin may negatively influence specific host defense mechanisms during pneumococcal pneumonia. Together with other reported anti-inflammatory effects of macrolides (1, 28), these data suggest that, during the treatment of pneumonia, the immunomodulatory actions of this group of antibiotics may be a disadvantage with respect to clearance of the infection.
ACKNOWLEDGMENTS
We thank J. Meeldijk of the Department of Medical Microbiology for his help in preparing heat-inactivated S. pneumoniae.
T. van der Poll is a fellow of the Royal Netherlands Academy of Arts and Sciences.
REFERENCES
- 1.Anderson R. Erythromycin and roxithromycin potentiate human neutrophil locomotion in vitro by inhibition of leukoattractant-activated superoxide generation and autooxidation. J Infect Dis. 1989;159:966–973. doi: 10.1093/infdis/159.5.966. [DOI] [PubMed] [Google Scholar]
- 2.Bailly S, Pocidalo J-J, Fay M, Gougerot-Pocidalo M-A. Differential modulation of cytokine production by macrolides: interleukin-6 production is increased by spiramycin and erythromycin. Antimicrob Agents Chemother. 1991;35:2016–2019. doi: 10.1128/aac.35.10.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailly S, Ferrua B, Fay M, Gougerot-Pocidalo M A. Differential regulation of IL-6, IL-1α, IL-1β and TNF-α production in LPS-stimulated monocytes: role of cyclic AMP. Cytokine. 1990;2:205–210. doi: 10.1016/1043-4666(90)90017-n. [DOI] [PubMed] [Google Scholar]
- 4.Bohte R, van Furth R, van den Broek P J. Aetiology of community-acquired pneumonia: a prospective study among adults requiring admission to hospital. Thorax. 1995;50:543–547. doi: 10.1136/thx.50.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boutten A, Dehoux M S, Seta N, Ostinelli J, Venembre P, Crestani B, Dombret M C, Durand G, Aubier M. Compartmentalized IL-8 and elastase release within the human lung in unilateral pneumonia. Am J Respir Crit Care Med. 1996;153:336–342. doi: 10.1164/ajrccm.153.1.8542140. [DOI] [PubMed] [Google Scholar]
- 6.Cassatella M A. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–23. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 7.DeForge L E, Kenney J S, Jones M L, Warren J S, Remick D G. Biphasic production of IL-8 in lipopolysaccharide (LPS)-stimulated human whole blood. Separation of LPS- and cytokine-stimulated components using anti-tumor necrosis factor and anti-IL-1 antibodies. J Immunol. 1992;148:2133–2141. [PubMed] [Google Scholar]
- 8.Dehoux M S, Boutten A, Ostinelli J, Seta N, Dombret M C, Crestani B, Deschenes M, Trouillet J L, Aubier M. Compartmentalized cytokine production within the human lung in unilateral pneumonia. Am J Respir Crit Care Med. 1994;150:710–716. doi: 10.1164/ajrccm.150.3.8087341. [DOI] [PubMed] [Google Scholar]
- 9.Friedland I R, McCracken G H. Management of infections caused by antibiotic-resistant Streptococcus pneumoniae. N Engl J Med. 1994;331:377–382. doi: 10.1056/NEJM199408113310607. [DOI] [PubMed] [Google Scholar]
- 10.Fujii T, Kadota J-I, Morikawa T, Matsubara Y, Kawakami K, Iida K, Shirai R, Taniguchi H, Kaseda M, Kawamoto S, Kohno S. Inhibitory effect of erythromycin on interleukin 8 production by 1α,25-dihydroxyvitamin D3-stimulated THP-1 cells. Antimicrob Agents Chemother. 1996;40:1548–1551. doi: 10.1128/aac.40.6.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberger M J, Strieter R M, Kunkel S L, Danforth J M, Goodman R E, Standiford T J. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J Immunol. 1995;155:722–729. [PubMed] [Google Scholar]
- 12.Greenberger M J, Kunkel S L, Strieter R M, Lukacs N W, Bramson J, Gauldie J, Graham F L, Hitt M, Danforth J M, Standiford T J. IL-12 gene therapy protects mice in lethal Klebsiella pneumonia. J Immunol. 1996;157:3006–3012. [PubMed] [Google Scholar]
- 13.Iiono Y, Toriyama M, Kudo K, Natori Y, Yuo A. Erythromycin inhibition of lipopolysaccharide stimulated tumor necrosis factor alpha production by human monocytes in vivo. Ann Otol Rhinol Laryngol. 1992;101:16–20. doi: 10.1177/0003489492101s1005. [DOI] [PubMed] [Google Scholar]
- 14.Keicho N, Sawada S, Kitamura K. Effects of an immunosuppressant, FK506, on interleukin alpha production by human macrophages and a macrophage-like cell line. Cell Immunol. 1991;132:285–294. doi: 10.1016/0008-8749(91)90028-a. [DOI] [PubMed] [Google Scholar]
- 15.Khair O A, Devalia J L, Abdelaziz M M. Effect of erythromycin on Haemophilus influenzae endotoxin induced release of IL-6, IL-8 and sICAM-1 by cultured human bronchial epithelial cells. Eur Respir J. 1995;8:1451–1457. [PubMed] [Google Scholar]
- 16.Klugman K P. Pneumococcal resistance to antibiotics. Clin Microbiol Rev. 1990;3:171–196. doi: 10.1128/cmr.3.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kusunoki T, Hailman E, Juan T S C, Lichenstein H S, Wright S D. Molecules from Staphylococcus aureus that bind CD14 and stimulate innate immune responses. J Exp Med. 1995;182:1673–1682. doi: 10.1084/jem.182.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambert H P, O’Grady F W. Macrolides. In: Lambert H P, O’Grady F W, editors. Antibiotic and chemotherapy. 6th ed. London, United Kingdom: Churchill Livingstone; 1992. pp. 168–179. [Google Scholar]
- 19.Möller A, Emling F, Blohm D, Schlick E, Schollmeier K. Monoclonal antibodies to human tumor necrosis factor α: in vitro and in vivo application. Cytokine. 1990;2:162–169. doi: 10.1016/1043-4666(90)90011-h. [DOI] [PubMed] [Google Scholar]
- 20.Morikawa K, Watabe H, Araake M, Morikawa S. Modulatory effect of antibiotics on cytokine production by human monocytes in vitro. Antimicrob Agents Chemother. 1996;40:1366–1370. doi: 10.1128/aac.40.6.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mossmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 22.Oishi K, Sonoda F, Kobayashi S, Iwagaki A, Nagatake T, Matsushima K, Matsumoto K. Role of interleukin-8 (IL-8) and an inhibitory effect of erythromycin on IL-8 release in the airways of patients with chronic airway diseases. Infect Immun. 1994;62:4145–4152. doi: 10.1128/iai.62.10.4145-4152.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortqvist A, Hedlund J, Grillner L. Aetiology, outcome and prospective factors in community acquired pneumonia requiring hospitalization. Eur Respir J. 1990;3:1105–1113. [PubMed] [Google Scholar]
- 24.Philips H J. Dye exclusion tests for cell viability. In: Kruse P P Jr, Patterson M K, editors. Tissue culture methods and applications. New York, N.Y: Academic Press; 1973. pp. 406–408. [Google Scholar]
- 25.Research Committee of the British Thoracic Society and the Public Health Laboratory Service. Community acquired pneumonia in adults in British hospitals in 1982–1983: a survey of aetiology, mortality, prognostic factors and outcome. Q J Med. 1987;62:195–220. [PubMed] [Google Scholar]
- 26.Sherris J C, Plorde J J. Antimicrobics and chemotherapy of bacterial and viral infections. In: Sherris J C, editor. Medical microbiology. An introduction to infectious diseases. 2nd ed. New York, N.Y: Elsevier Science Publishing Co., Inc.; 1990. pp. 213–214. [Google Scholar]
- 27.Suffredini A F, Reda D, Banks S M, Tropea M, Agosti J M, Miller R. Effects of recombinant dimeric TNF receptor on human inflammatory responses following intravenous endotoxin administration. J Immunol. 1995;155:5038–5045. [PubMed] [Google Scholar]
- 28.Takeyama K, Tamaoki Y, Chiotani A, Tagaya E, Konno K. Effect of macrolide antibiotics on ciliary motility in rabbit airway epithelium in vitro. J Pharm Pharmacol. 1993;45:756–758. doi: 10.1111/j.2042-7158.1993.tb07104.x. [DOI] [PubMed] [Google Scholar]
- 29.Takizawa H, Desaki M, Ohtoshi E. Erythromycin suppresses interleukin 6 expression by human bronchial epithelial cells: a potential mechanism of its anti-inflammatory action. Biochem Biophys Res Commun. 1995;210:781–786. doi: 10.1006/bbrc.1995.1727. [DOI] [PubMed] [Google Scholar]
- 30.Van der Poll T, Coyle S M, Barbosa K, Braxton C C, Lowry S F. Epinephrine inhibits tumor necrosis factor α and potentiates interleukin 10 release during human endotoxemia. J Clin Invest. 1996;97:713–719. doi: 10.1172/JCI118469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Poll T, Lowry S F. Lipopolysaccharide-induced interleukin 8 production by human whole blood is enhanced by epinephrine and inhibited by hydrocortisone. Infect Immun. 1997;65:2378–2381. doi: 10.1128/iai.65.6.2378-2381.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van der Poll T, Lowry S F. Epinephrine inhibits endotoxin-induced interleukin-1β production: roles of tumor necrosis factor-α and interleukin 10. Am J Physiol. 1997;273:R1885–R1890. doi: 10.1152/ajpregu.1997.273.6.R1885. [DOI] [PubMed] [Google Scholar]
- 33.Van der Poll T, Marchant A, Keogh C V, Goldman M, Lowry S F. Interleukin 10 impairs host defense in murine pneumococcal pneumonia. J Infect Dis. 1996;174:994–1000. doi: 10.1093/infdis/174.5.994. [DOI] [PubMed] [Google Scholar]
- 34.Van der Poll T, Keogh C V, Buurman W A, Lowry S F. Passive immunization against tumor necrosis factor α impairs host defense during pneumococcal pneumonia in mice. Am J Respir Crit Care Med. 1997;155:603–608. doi: 10.1164/ajrccm.155.2.9032201. [DOI] [PubMed] [Google Scholar]
- 35.Van der Poll T, Keogh C V, Guirao X, Buurman W A, Kopf M, Lowry S F. Interleukin 6 gene deficient mice are more susceptible to pneumococcal pneumonia. J Infect Dis. 1997;176:439–444. doi: 10.1086/514062. [DOI] [PubMed] [Google Scholar]
- 36.Van der Poll T, Coyle S M, Levi M, Jansen P M, Dentener M, Barbosa K, Buurman W A, Hack C E, ten Cate J W, Agosti J M, Lowry S F. Effects of recombinant dimeric TNF receptor on inflammatory responses to intravenous endotoxin in normal humans. Blood. 1997;89:3727–3736. [PubMed] [Google Scholar]