Abstract
Ischemic colitis is an inflammatory condition of the colon that results from insufficient blood supply commonly caused by enterocolitis, vessel occlusion, or shock. In contrast, pseudomembranous colitis is a clinical manifestation of Clostridioides difficile infection (CDI). Ischemic colitis caused by CDI has rarely been reported. Fecal microbiota transplantation (FMT) is an efficient treatment for refractory or fulminant CDI, and the indications for its use have recently expanded. However, performing FMT in patients with ischemic colitis is challenging because of the risk of perforation. Here, we have presented a case of ischemic colitis caused by CDI that was successfully treated with FMT via sigmoidoscopy.
Keywords: Clostridioides difficile infection, Fecal microbiota transplantation, Ischemic colitis, Pseudomembranous colitis
INTRODUCTION
Clostridioides difficile infection (CDI) is a common diarrheal disease that can be induced by prolonged antibiotic use. Clinical manifestations of CDI vary according to comorbidities and region of infection.1 Ischemic colitis is an inflammatory condition of the large bowel that commonly results from insufficient blood supply due to enterocolitis, vessel occlusion, or shock. It has a different diagnosis from that of pseudomembranous colitis (PMC), which is a clinical manifestation of CDI.2 Ischemic colitis caused by CDI has been rarely reported.3,4 However, C. difficile was not the causative organism in these cases. Fecal microbiota transplantation (FMT) is highly efficacious for recurrent or refractory CDI.5-7 However, performing FMT in patients with ischemic colitis is challenging because of the risk of perforation. Here, we have presented a case of ischemic colitis caused by CDI that was successfully treated with FMT.
CASE REPORT
A 53-year-old woman without comorbidities presented to the emergency room of our hospital complaining of headache. She was diagnosed with subarachnoid hemorrhage, for which she underwent coil embolization. During hospitalization, however, she developed pneumonia and was treated with mechanical ventilation. Methicillin-resistant Staphylococcus aureus was isolated from her blood culture, and third-generation cephalosporins and intravenous vancomycin were administered to treat the pneumonia. Diarrhea (5 times/day) and abdominal distension developed 2 weeks after the initiation of antibiotic treatment.
Sigmoidoscopy revealed yellowish plaques covering the sigmoid colon, suggesting the possibility of PMC (Fig. 1). Additionally, C. difficile toxins were detected and isolated from her stool samples. Therefore, oral metronidazole (500 mg × 3 times/day) was administered for 14 days. After resolution of CDI, septic arthritis developed as a complication, thus, continuous administration of broad-spectrum antibiotics was needed. The diarrhea reoccurred and C. difficile toxins were detected in the stool. Oral vancomycin (125 mg) was administered four times daily to treat the recurrent CDI.
Fig. 1.
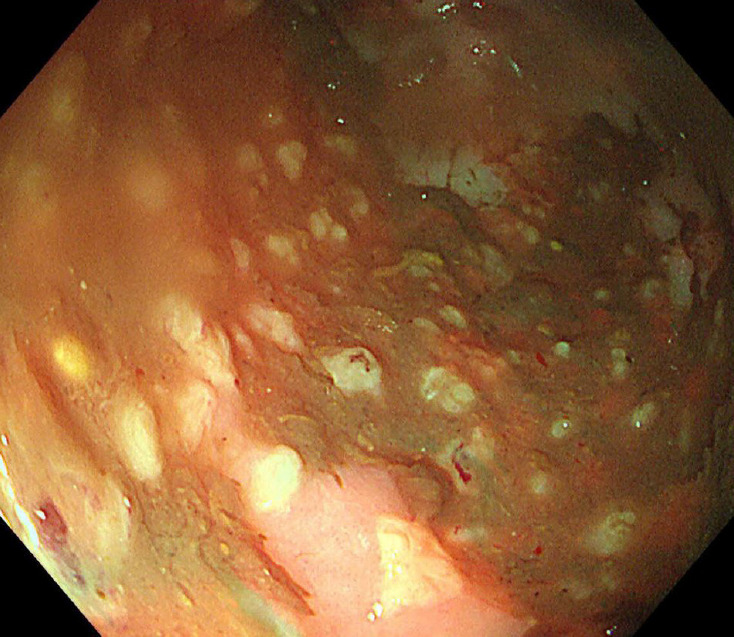
Sigmoidoscopic finding. Sigmoidoscopy showing multiple yellowish plaques in the sigmoid colon.
Seven days after vancomycin treatment, the patient developed hematochezia, prompting the increase in vancomycin dosage to 500 mg × 4 times/day, supplemented with intravenous metronidazole (500 mg × 3 times/day). Subsequently, the patient developed abdominal distension and ileus, prompting insertion of a nasogastric tube for decompression. Four days after the change in medication, the volume and frequency of bloody diarrhea increased to 8 times/day. Sigmoidoscopy revealed multiple various-sized bullae and hemorrhages from the rectum to the sigmoid colon. Yellowish pseudomembranes were also observed in the bullae (Fig. 2).
Fig. 2.
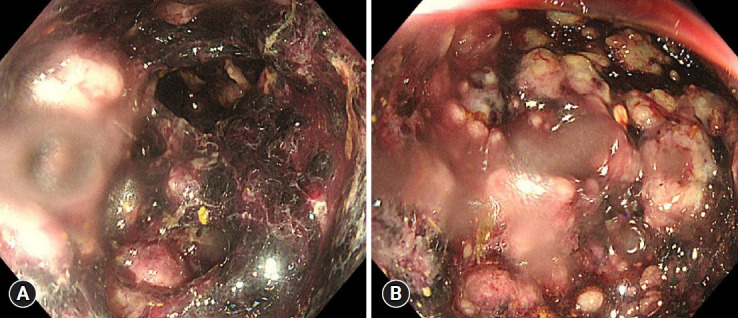
Endoscopic image showing ischemic colitis. (A) Sigmoidoscopy showing multiple various-sized bullae with hemorrhage from the rectum to the distal transverse colon. (B) Focal yellowish pseudomembranes can been seen among the bullae.
Owing to the complications that arose despite the interventions, we established that the ongoing medical treatment for the patient’s CDI was ineffective, leading us to perform FMT as an alternative treatment. After obtaining informed consent, FMT was performed on hospitalization day 70. Fecal matter for the FMT was obtained from the patient’s 28-year-old son. Hepatitis B surface antigen, hepatitis C antibody, syphilis reagin test, C. difficile toxins, and human immunodeficiency virus were not detected in the stool sample. The donor’s stool was collected 1 day before the FMT procedure and stored in a commercial freezer at –20 °C. A mixture containing 80 g of stool and 240 mL of normal saline (1:3) was placed in a commercial blender and ground for 4 minutes until no visible particles remained.
The patient’s vital signs on the day of the FMT were as follows: blood pressure, 116/70 mmHg; pulse rate, 90/min; and body temperature, 37.2 °C. The laboratory test results on the day of the FMT were as follows: hemoglobin, 10.9 g/dL; platelet count, 126 ×109/L; white blood cell count, 13,200/mm3; C-reactive protein, 45.8 mg/dL; albumin, 2.2 g/dL; and creatinine, 1.37 mg/dL.
FMT was performed using sigmoidoscopy with CO2 gas to minimize inflation. Multiple various-sized bullae with hemorrhage were observed covering the rectum, sigmoid colon, and descending colon. The colonoscope was inserted cautiously until the normal colonic mucosa was observable at a position that was thought to be the transverse colon. The fecal mixture was transferred to the recipient’s intestines through the working channel of the endoscope. The procedure was performed with the patient in the right lateral position to prevent rapid evacuation of the fecal suspension. The total operation time was approximately 15 minutes; however, the patient was kept in the right lateral position for an additional hour. Oral vancomycin and intravenous metronidazole were continuously administered after FMT.
One day after the procedure, the patient’s abdominal distension improved slightly, and the number of diarrhea episodes decreased to 3 times/day. Four days after FMT, the bloody diarrhea stopped, therefore, antibiotic therapy for CDI was discontinued. Sigmoidoscopy was performed without enema 5 days after the procedure and showed that all bullae had regressed to crusted blood (Fig. 3). The patient was discharged on hospitalization day 105, which was 35 days after FMT. This case is summarized in Table 1.
Fig. 3.
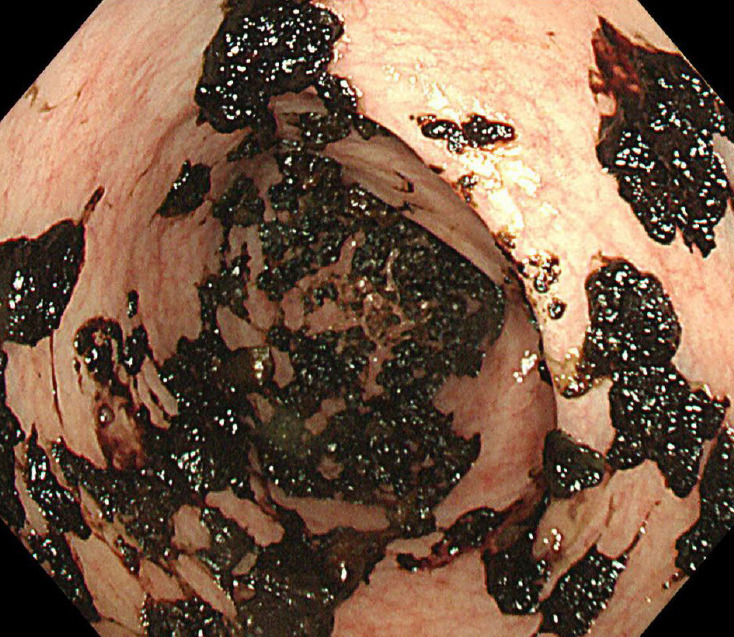
Resolution of ischemic colitis. Sigmoidoscopy showing regression of hemorrhagic bullae to crusted blood. Majority of the colon mucosa has recovered.
Table 1.
Case summary
| Hospital course | Event | Treatment |
|---|---|---|
| Hospital day #14 | CDI, first episode | Oral metronidazole |
| Hospital day #34 | Septic arthritis | Broad spectrum antibiotics |
| Hospital day #53 | CDI recurrence | Oral vancomycin (125 mg × 4) |
| Hospital day #60 | Symptom aggravation | Oral vancomycin (500 mg × 4) and intravenous metronidazole |
| Hospital day #64 | Sigmoidoscopy revealed ischemic colitis | |
| Hospital day #70 | Fecal microbiota transplantation | |
| Hospital day #74 | Diarrhea stop | |
| Hospital day #74 | Improvement of ischemic colitis | |
| Hospital day #105 | Discharge |
CDI, Clostridioides difficile infection.
DISCUSSION
In this case report, we have described the use of FMT for the treatment of ischemic colitis caused by CDI. Despite the risk of perforation, FMT was performed through sigmoidoscopy because the patient developed abdominal distension and ileus. The patient was cured after only one session of FMT. To the best of our knowledge, successfully using FMT to treat ischemic colitis caused by CDI is a novel achievement in this field.
The presence of multiple, yellowish plaques in the colon is a typical endoscopic finding of PMC.1 Additionally, multiple bullae with hemorrhage were also observed in the patient’s colon, suggesting that the PMC associated with CDI had progressed to ischemic colitis. However, it was difficult to determine whether the ischemic colitis was complicated by PMC because the yellowish plaques were found in small numbers. Most of the colon showed ischemic changes up to the descending colon. We suspected a relationship between CDI and ischemic colitis because C. difficile toxins were detected in the patient’s stool.
Although there is no grading system for PMC, we believe that ischemic colitis represents its most severe form. Manifestations of severe, complicated CDI include shock and ileus.1 This patient developed ileus and had a risk of perforation because the colon mucosa was friable, a condition consistent with severe CDI, which has a mortality rate as high as 50%.8 Recently, the indications for FMT have been expanded to include severe, complicated CDI that is refractory to medical treatment within 48 hours.5 FMT has favorable efficacy in the treatment of fulminant CDI.9
Colonoscopic infusion is the preferred route for FMT, but alternative routes include the upper gastrointestinal (GI) tract or enema.10 The upper GI tract route can be chosen for unconscious patients or those with poor medical conditions.11 However, this route is contraindicated in patients with ileus drainage because of the risk of serious adverse events such as vomiting or aspiration. FMT can also be performed using enema, which is less invasive than colonoscopic or sigmoidoscopic infusion. Nonetheless, we did not consider enema in this case because the patient was critically ill and confused. Fecal suspension should be retained for at least 30 minutes after infusion, but the patient did not seem to be able to retain it because of her condition. Therefore, in this case report, we successfully treated fulminant CDI using sigmoidoscopic FMT.12
CDI is the most common etiology of PMC, and its incidence is positively correlated with the increase in population age and comorbidities.13 Manifestations of CDI have worsened and the recurrence rate has also escalated.1 The incidence of ischemic colitis caused by CDI is anticipated to increase in the future. Multiple FMTs can be considered in cases of early failure or ineffectiveness of the first FMT.14 We considered that the patient would possibly need multiple applications of FMT, but fortunately, she recovered after only a single session of FMT. Our report suggests that FMT can be considered an effective treatment option for patients with ischemic colitis complicated by CDI.
Footnotes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (grant number: NRF-2021R1G1A1094049).
Author Contributions
Conceptualization: TGG; Data curation: HH; Formal analysis: SHK; Investigation: MKB; Methodology: TGG; Supervision; TGG; Validation: TGG; Writing–original draft: SHK; Writing–review & editing: all authors.
REFERENCES
- 1.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 2.Trotter JM, Hunt L, Peter MB. Ischaemic colitis. BMJ. 2016;355:i6600. doi: 10.1136/bmj.i6600. [DOI] [PubMed] [Google Scholar]
- 3.Moulis H, Vender RJ. Antibiotic-associated hemorrhagic colitis. J Clin Gastroenterol. 1994;18:227–231. doi: 10.1097/00004836-199404000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Kendrick JB, Risbano M, Groshong SD, et al. A rare presentation of ischemic pseudomembranous colitis due to Escherichia coli O157:H7. Clin Infect Dis. 2007;45:217–219. doi: 10.1086/518990. [DOI] [PubMed] [Google Scholar]
- 5.Moore T, Rodriguez A, Bakken JS. Fecal microbiota transplantation: a practical update for the infectious disease specialist. Clin Infect Dis. 2014;58:541–545. doi: 10.1093/cid/cit950. [DOI] [PubMed] [Google Scholar]
- 6.Kelly CR, Ihunnah C, Fischer M, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109:1065–1071. doi: 10.1038/ajg.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gweon TG, Na SY. Next generation fecal microbiota transplantation. Clin Endosc. 2021;54:152–156. doi: 10.5946/ce.2021.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaber MR, Olafsson S, Fung WL, et al. Clinical review of the management of fulminant clostridium difficile infection. Am J Gastroenterol. 2008;103:3195–3203. doi: 10.1111/j.1572-0241.2008.02198.x. [DOI] [PubMed] [Google Scholar]
- 9.Fischer M, Sipe BW, Rogers NA, et al. Faecal microbiota transplantation plus selected use of vancomycin for severe-complicated Clostridium difficile infection: description of a protocol with high success rate. Aliment Pharmacol Ther. 2015;42:470–476. doi: 10.1111/apt.13290. [DOI] [PubMed] [Google Scholar]
- 10.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53:994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 11.Gweon TG, Kim J, Lim CH, et al. Fecal microbiota transplantation using upper gastrointestinal tract for the treatment of refractory or severe complicated Clostridium difficile infection in elderly patients in poor medical condition: the first study in an Asian country. Gastroenterol Res Pract. 2016;2016:2687605. doi: 10.1155/2016/2687605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gweon TG, Lee KJ, Kang DH, et al. A case of toxic megacolon caused by Clostridium difficile infection and treated with fecal microbiota transplantation. Gut Liver. 2015;9:247–250. doi: 10.5009/gnl14152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin JSH, Monaghan TM, Wilcox MH. Clostridium difficile infection: epidemiology, diagnosis and understanding transmission. Nat Rev Gastroenterol Hepatol. 2016;13:206–216. doi: 10.1038/nrgastro.2016.25. [DOI] [PubMed] [Google Scholar]
- 14.Fischer M, Kao D, Mehta SR, et al. Predictors of early failure after fecal microbiota transplantation for the therapy of Clostridium difficile infection: a multicenter study. Am J Gastroenterol. 2016;111:1024–1031. doi: 10.1038/ajg.2016.180. [DOI] [PubMed] [Google Scholar]


