Abstract
Background/Aims
Endoscopic resection of all colorectal adenomatous lesions with a low complication rate, simplicity, and negative residuals is challenging. Hence, we developed a new method called “non-injection resection using bipolar soft coagulation mode (NIRBS)” method, adapted for colorectal lesions. In addition, we evaluated the effectiveness of this method.
Methods
We performed NIRBS throughout a 12-month period for all colorectal lesions which snare resection was acceptable without cancerous lesions infiltrating deeper than the submucosal layer.
Results
A total of 746 resected lesions were included in the study, with a 4.5 mm mean size (range, 1–35 mm). The major pathological breakdowns were as follows: 64.3% (480/746) were adenomas, and 5.0% (37/746) were intraepithelial adenocarcinomas (Tis lesions). No residuals were observed in any of the 37 Tis lesions (mean size, 15.3 mm). Adverse events included bleeding (0.4%) but no perforation.
Conclusions
NIRBS allowed the resection of multiple lesions with simplicity because of the non-injection and without perforating due to the minimal burn effect of the bipolar snare set in the soft coagulation mode. Therefore, NIRBS can be used to resect adenomatous lesions easily, including Tis lesions, from small to large lesions without leaving residuals.
Keywords: Bipolar snare, Colorectal lesion, Endoscopic mucosal resection, Non-injection, Soft coagulation mode
Graphical abstract
INTRODUCTION
Endoscopic resection of colorectal adenomatous lesions reduces morbidity and mortality rates in colorectal cancer patients.1-5 Therefore, resection of all adenomatous lesions is recommended, regardless of their size. Various resection methods use a snare, including endoscopic mucosal resection (EMR), hot snare polypectomy (HSP), and cold snare polypectomy (CSP). HSP, which is commonly used, has an inevitable delayed bleeding due to the burn effect of thermal coagulation, causing ulcers to gradually spread after the procedure and damaging the vessels in the submucosal layer.6-10 CSP, which uses a snare without thermal coagulation, has become more widespread owing to its high level of safety, usefulness, and short procedure time.7,10-12 However, one disadvantage of CSP is that the muscularis mucosae cannot always be cut because there is no burn effect, resulting in a high rate of incomplete resections (~8%).13-16 Alternatively, conventional EMR has the advantage of complete resection, even for outsized lesions of approximately 2 cm in diameter and cancerous lesions. Nevertheless, conventional EMR procedures have a high rate of delayed bleeding (7%–12%).17-21
Although flat or semipedunculated lesions ≤10 mm in diameter are considered candidates for CSPs14,22-29; careless CSPs may result in cancer recurrence, as 0.5% of 1 to 5 mm and 3.3% of 6 to 9 mm adenomatous lesions are cancerous.30 Therefore, it is necessary to distinguish cancerous from noncancerous lesions via magnification endoscopy to prevent cancer residuals, although this judgment is difficult for unskilled endoscopists. While conventional EMR can be performed on all lesions to prevent such residuals, it is difficult to resect all adenomatous lesions using this method because of the high frequency of delayed bleeding and the complexity of the procedure. Therefore, it is important to establish a new resection method that has the benefits of both conventional EMR and CSP. Consequently, we developed a new method in which the lesion was grasped, including the surrounding normal mucosa without injection into the submucosal layer, sufficiently squeezed, and resected with short-time energization by the bipolar snare set in the soft coagulation mode, which has a minimal burn effect. Hence, the technique was named “non-injection resection using bipolar soft coagulation mode” (NIRBS) and is adapted for colorectal lesions. Therefore, we evaluated the effectiveness of this method.
METHODS
Patients
Colorectal lesions detected via colonoscopy during a 12-month period from January to December 2016 at an institution (Daito Central Hospital, Osaka, Japan) were targeted, regardless of patient age and sex. The procedure was performed using a temporary suspension of antithrombotic drugs.
Target lesions
All colorectal lesions, excluding cancerous lesions infiltrating deeper than the submucosal layer and those in which the attachment portion between the lesion and the normal mucosa was more than 20 mm, were targeted. Therefore, the absolute values of the lesion’s size and shape were irrelevant.
Definition and procedure
NIRBS was defined as meetings the conditions (1) to (5). (1) The XEMEX Bipolar Snare S (width, 26 mm; length, 62 mm; Zeon Medical Inc., Tokyo, Japan) was used. (2) As the electrosurgery generator unit, VIO 300D (ERBE Elektromedizin GmbH Co., Ltd.) was used. The energization mode was set to soft coagulation mode at 30 W, and effect 5. If VIO 3 from the above company was used as the electrosurgery generator unit, the energization mode was set as the soft coagulation mode and effect 3. (3) The injection into the submucosal layer was not performed. (4) The lesion was grasped extensively, including the surrounding normal mucosa, and sufficiently squeezed by the snare. (5) Each lesion was resected according to its respective energization within 2 s in ≤5 mm, within 5 s in 6 to 10 mm, and within 10 s in >10 mm, while continuing to squeeze. Lesions >5 mm must be squeezed using a special technique (Supplementary Video 1) before energization, and this technique must be continued from energization to resection.
Figure 1 and Supplementary Video 2 illustrate the NIRBS procedure. All procedures were performed by an experienced endoscopist (>10,000 colonoscopy cases, >1,000 colorectal EMR, and >200 colorectal endoscopic submucosal dissections). The procedure was performed using one of three endoscopes (CF-Q240AI, CF-Q260AI, and CF-HQ290I; Olympus Medical Systems Co., Ltd.). After resection, if the operator noticed that the subject had an extensive mucosal resection or that the resected portion was at risk of delayed bleeding, prophylactic hemostasis was performed using a long clip (Olympus Medical Systems Co., Ltd.) or a disposable ligation device (DLD) (HX-400U-30; Olympus Medical Systems Co. Ltd.).
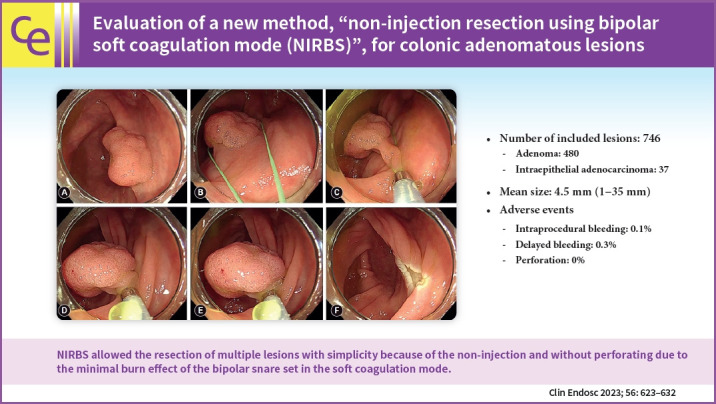
Fig. 1.
Non-injection resection using bipolar soft coagulation mode procedure (NIRBS) Procedure 1. (A) Cecum, 20 mm, sessile type (Is). (B, C) The lesion is grasped by the snare, including the surrounding normal mucosa, while sucking air. (D) Grasped. (E) Squeezed and energized. (F) Resected.
Terminology: operation definitions
“Negative” residuals or recurrences—if the resected lesion was pathologically cut-end negative or if the residual lesion was not detected via colonoscopy after six months of the resection until present, even when the cut-end was unknown.
“Unknown” residuals or recurrences—if the resected lesion was pathologically cut-end unknown and no colonoscopy was performed within six months of the resection until present.
“Prophylactic hemostasis”—clip-suturing or DLD-ligation before or after the resection.
1) Adverse events
This study only evaluated major adverse events such as bleeding during the procedure, delayed bleeding, and perforation. It did not evaluate minor symptoms, such as abdominal bloating, mild abdominal pain, nausea, dizziness, or a mild SpO2 decline due to sedation.
“Bleeding during the procedure”—profuse bleeding that occurred from the resected portion immediately after resection.
“Delayed bleeding”: a large amount of fresh bleeding or bloody stools that followed several hours after resection.
Statistical analysis
The relationship between lesion shape and pathology and between size and pathology was assessed using Spearman’s rank correlation test. SPSS Statistics Desktop for Japan, ver. 26 (IBM Japan, Ltd.), was used for the statistical analyses. The results were considered statistically significant when the p-values were <0.05.
Ethical statements
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board and Ethics Committee of Kansai Medical University (Osaka, Japan) and Daito Central Hospital (approval number: 2021379). In addition, this study was registered with the University Hospital Medical Information Network Clinical Trials Registry (number: UMIN000047558).
RESULTS
Table 1 presents the characteristics of the 746 lesions that were resected from 375 patients. The breakdown of lesion types was as follows: tubular adenoma with mild atypia, 30.7% (229/746); tubular adenoma with moderate atypia, 26.0% (194/746); tubular adenoma with severe atypia, 7.2% (54/746); serrated adenoma with mild atypia, 0.1% (1/746); serrated adenoma with moderate atypia, 0.3% (2/746); intraepithelial adenocarcinoma (Tis lesion), 5.0% (37/746 [well differentiated tubular adenocarcinoma in adenoma 36 lesions and moderately differentiated tubular adenocarcinoma in adenoma one lesion]); hyperplastic polyp, 21.4% (160/746); inflammatory polyp, 2.7% (20/746); pathological normal mucosa, 1.9% (14/746); leiomyoma, 0.3% (2/746); mucosal prolapse syndrome, 0.1% (1/746); and the lesion unable to be pathologically evaluated (undefined lesion), 4.3% (32/746).
Table 1.
The lesions’ characteristics (746 lesions of 375 patients)
| Characteristic | Value |
|---|---|
| Age (yr) | 68.7±10.7 |
| Sex, male | 477 (63.9) |
| Lesions’ location | |
| Rectum | 61 (8.2) |
| Left side colon | 283 (37.9) |
| Transverse colon | 176 (23.6) |
| Right side colon | 223 (29.9) |
| Unknown | 3 (0.4) |
| Pathological diagnosis | |
| Adenomatous lesion | 517 (69.3) |
| Tubular adenoma with mild atypia | 229 (30.7) |
| Tubular adenoma with moderate atypia | 194 (26.0) |
| Tubular adenoma with severe atypia | 54 (7.2) |
| Serrated adenoma with mild atypia | 1 (0.1) |
| Serrated adenoma with moderate atypia | 2 (0.3) |
| Intraepithelial adenocarcinoma (Tis lesion)a) | 37 (5.0) |
| BNAL | 197 (26.4) |
| Hyperplastic polyp | 160 (21.4) |
| Inflammatory polyp | 20 (2.7) |
| Pathological normal mucosa | 14 (1.9) |
| Leiomyoma | 2 (0.3) |
| Mucosal prolapse syndrome | 1 (0.1) |
| Undefined lesion | 32 (4.3) |
Values are presented as mean±standard deviation or number (%).
BNAL, benign and not adenomatous lesion; Undefined lesion, the lesion unable to be pathologically evaluated.
Intraepithelial adenocarcinoma (Tis lesion), well differentiated tubular adenocarcinoma in adenoma 36 lesions and moderately differentiated tubular adenocarcinoma in adenoma one lesion.
An overview of NIRBSs is presented in Table 2, where the overall mean±standard deviation (SD) size of the resected lesions was 4.5±3.6 mm. There were no residuals or recurrences in any of the 37 Tis lesions (mean size, 15.3 mm). In sessile-type (Ⅰs) lesions (n=539), the mean±SD size was 3.6±1.5 mm, and the rate of Tis lesions was 1.9% (10/539). In semipedunculated-type (Ⅰsp) lesions (n=47), the mean±SD size was 9.9±5.7 mm, and the rate of Tis lesions was 34.0% (16/47). In pedunculated-type (Ⅰp) lesions (n=16), the mean±SD size was 18.3±9.5 mm, and the rate of Tis lesions was 62.5% (10/16). There was a significant positive relationship between the proportion of cancer lesions and stalk length (p<0.001; 1.9% in Is, 34.0% in Isp, and 62.5% in Ip lesions). In lesions ≤5 mm in size (n=627), the mean±SD size was 3.4±0.8 mm, and the rate of Tis lesions was 0.3% (2/627). In lesions sized 6 to 10 mm (n=86), the mean±SD size was 7.5±1.5 mm, and the rate of Tis lesions was 15.1% (13/86). In lesions >10 mm in size (n=33), the mean±SD size was 17.7±6.8 mm, and the rate of Tis lesions was 66.7% (22/33). There was a significant positive relationship between the proportion of cancer lesions and lesion size (p<0.001; 0.3% in ≤5-mm, 15.1% in 6–10-mm, and 66.7% in >10-mm lesions). For prophylactic hemostasis, 0.5 clips and 0.03 DLDs per lesion were used.
Table 2.
Overview of non-injection resection using bipolar soft coagulation mode (NIRBS) procedure
| Total (n=746) | Lesions’ shape |
Lesions’ size (mm) |
Lesions’ pathology |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ⅱa (n=130) | Ⅰs (n=539) | Ⅰsp (n=47) | Ⅰp (n=16) | Unclassified (n=14) | ≤5 (n=627) | 6–10 (n=86) | >10 (n=33) | BNAL (n=197) | Adenoma (n=480) | Tis lesion (n=37) | Undefined (n=32) | ||
| Lesions’ size (mm) | 4.5±3.6 | 4.5±2.0 | 3.6±1.5 | 9.9±5.7 | 18.3±9.5 | 3.6±1.5 | 3.4±0.8 | 7.5±1.5 | 17.7±6.8 | 3.9±1.7 | 4.0±2.0 | 15.3±8.2 | 2.8±0.9 |
| Lesions’ pathology | |||||||||||||
| BNAL | 197 (26.4) | 52 (40.0) | 136 (25.2) | 6 (12.8) | 0 (0) | 3 (21.4) | 170 (27.1) | 27 (31.4) | 0 (0) | ||||
| Adenoma | 480 (64.3) | 73 (56.2) | 366 (67.9) | 25 (53.2) | 6 (37.5) | 10 (71.4) | 423 (67.5) | 46 (53.5) | 11 (33.3) | ||||
| Tis lesion | 37 (5.0) | 1 (0.8) | 10 (1.9) | 16 (34.0) | 10 (62.5) | 0 (0) | 2 (0.3) | 13 (15.1) | 22 (66.7) | ||||
| Undefined | 32 (4.3) | 4 (3.1) | 27 (5.0) | 0 (0) | 0 (0) | 1 (7.1) | 32 (5.1) | 0 (0) | 0 (0) | ||||
| p | <0.001a) | <0.001b) | |||||||||||
| Residuals or recurrences of all 517 adenomatous lesions | |||||||||||||
| Negative | 365 (76.0) | 37 (100) | |||||||||||
| Unknown | 115 (24.0) | 0 (0) | |||||||||||
| Positive | 0 (0) | 0 (0) | |||||||||||
| Prophylactic hemostasis | |||||||||||||
| Clip-number (mean) | 406 (0.5) | 126 (1.0) | 126 (0.2) | 114 (2.4) | 29 (1.8) | 11 (0.8) | 109 (0.2) | 190 (2.2) | 107 (3.2) | 94 (0.5) | 203 (0.4) | 109 (2.9) | 0 (0) |
| DLD-number (mean) | 23 (0.03) | 1 (0.007) | 2 (0.004) | 10 (0.2) | 10 (0.6) | 0 (0) | 1 (0.002) | 5 (0.06) | 17 (0.5) | 1 (0.005) | 5 (0.01) | 17 (0.5) | 0 (0) |
| Adverse event | 3 (0.4) | 1 (0.8) | 0 (0) | 1 (2.1) | 1 (6.3) | 0 (0) | 1 (0.2) | 0 (0) | 2 (6.0) | 1 (0.5) | 0 (0) | 2 (5.4) | 0 (0) |
Values are presented as mean±standard deviation or number (%), unless otherwise indicated.
Ⅱa, flat and elevated type; Is, sessile type; Isp, semipedunculated type; Ip, pedunculated type; BNAL, benign and not adenomatous lesion; Tis lesion, intraepithelial adenocarcinoma; undefined, the lesion unable to be pathologically evaluated; DLD, disposable ligation device.
p<0.001, Relationship between lesions’ shape and pathology were assessed using Spearman’s rank correlation test;
p<0.001, Relationship between lesions’ size and pathology were assessed using Spearman’s rank correlation test.
Table 3 presents the adverse events identified in this study. The rate of adverse events was 0.4% (3/746), of which 0.1% (1/746) experienced bleeding during the procedure and 0.3% (2/746) experienced delayed bleeding.
Table 3.
Adverse events
| Age (yr) | Sex | Lesions’ location | Lesions’ shape | Lesions’ size (mm) | Resection-times | Lesions’ pathology | Prophylactic hemostasis | Treatment | Prognosis | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Bleeding during the procedure | 70 | Male | Rectum | Ⅰp | 35 | 1 | Well differentiated tubular adenocarcinoma in adenoma | Not undertaken | Clip-suturing | Recover |
| 2 | Delayed bleeding | 39 | Male | Sigmoid colon | Ⅰsp | 20 | 1 | Well differentiated tubular adenocarcinoma in adenoma | Clip-suturing | Clip-suturing and DLD-ligation | Recover |
| 3 | Delayed bleeding | 78 | Female | Ascending colon | Ⅱa | 3 | 1 | Hyperplastic polyp | Not undertaken | Clip-suturing | Recover |
Ip, pedunculated type; Isp, semipedunculated type; Ⅱa, flat and elevated type; DLD, disposable ligation device.
DISCUSSION
Bipolar instruments used in NIRBS generally cause less tissue damage than monopolar instruments used in traditional HSP and EMR.31,32 Shinmura et al.33 reported a comparative study of the safety of endoscopic resection between monopolar and bipolar snares for 24 target lesions in the porcine rectum. This report showed that two perforations were found on the histology report after resection of target lesions using a monopolar snare. However, no perforation occurred during endoscopic resection when using a bipolar snare, and thermal denaturation during the resection procedure did not reach the muscularis propria layer, regardless of the size of the target lesion. Thus, fewer adverse events may occur with endoscopic resection procedures using the bipolar snare. However, Saraya et al.34 found that the bleeding and perforation rates during colonic lesion resection using the bipolar snare set in the forced coagulation mode were not significantly different from those with the monopolar snare. Notably, the crucial difference between the methods of previous reports using bipolar snare and NIRBS is that the latter is set in the soft coagulation mode, thus causing less tissue damage than the forced coagulation mode (Fig. 2). Owing to the bipolar snare set in the soft coagulation mode, NIRBS can achieve a complete “EMR” without submucosal injection, not “polypectomy”, such as CSP or HSP. Carbonization and burning are minimal in soft coagulation mode because the voltage is controlled and does not generate electrical sparks.35,36 Accordingly, NIRBS may also allow large lesions to be resected without submucosal injection and perforation, with less damage to the tissue and muscle layers. Moreover, using NIRBS, the vascular damage in the submucosal layer is reduced as the heat spread is small due to the minimal burn effect; as a result, delayed bleeding may be reduced compared with conventional EMR with the application of a monopolar snare. The reason no residuals in Tis lesions were observed in NIRBS is attributed to the minimal burn effect. The bipolar snare, which is flexible, can slide into the loosest submucosal layer existing between the mucosal and muscle layers, although the mucosa is widely grasped and squeezed in some cases. The tissue layers were then consistently separated. The bipolar snare could resect the tissue completely with a minimal burn effect, which would be residual if only the CSP method was used. Another advantage of the non-injection and minimal burn effect is that the resected portion can be easily sutured with the clip owing to the absence of mucosal swelling and minimal tissue solidification (Fig. 3).
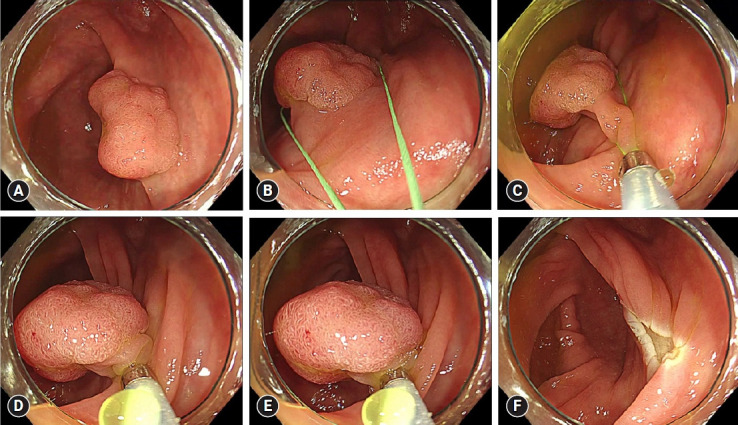
Fig. 2.
Comparison between non-injection resection using bipolar soft coagulation mode (NIRBS) and the conventional bipolar forced coagulation mode. (A) NIRBS. (B) Conventional bipolar forced coagulation mode (forced, 15 W; effect 2). The thermographs of A and B were taken during energization by attaching the snare to the surface of the saline. The graphs (A) and (B) show the relationship between the electric power and voltage (the red number is the electric voltage in the mode). A comparison of the two thermographs shows that the NIRBS mode has less heat spread to the surface of saline than the conventional bipolar forced coagulation mode. A comparison of the two graphs shows that the electric voltage in the NIRBS mode is approximately 1/4 that in the conventional bipolar forced coagulation mode.
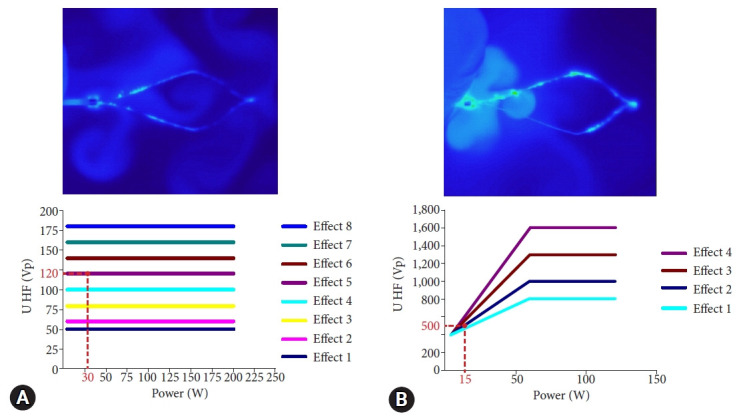
Fig. 3.
Non-injection resection using bipolar soft coagulation mode procedure 2. (A) Sigmoid colon, 20 mm, semipedunculated type (Isp). (B, C) Snaring. (D) Grasped and squeezed. (E) Resected portion. (F) The resected portion was completely sutured with the clip because there were no mucosal swelling and minimal tissue solidification.
To resect all colorectal adenomatous lesions, it may be necessary to satisfy the following conditions: (1) quick and easy to perform, (2) few complications, and (3) no residuals for large lesions. In the conventional EMR method, the lesion is widely resected using a monopolar snare following submucosal injection.28 In some cases, the resected portion is closed by clipping after the resection.28 Conventional EMR is unsuitable for multiple resections because peristaltic movement of the colon makes scope fixation and effective submucosal injection difficult.37 An increased procedure time is often associated with increased patient pain and the operator’s fatigue.38 Alternatively, CSP is recognized as an easier technique with fewer complications.7,10-12 However, CSP should not be performed for lesions >10 mm in size that are suspected to be cancerous.14,22-29 Therefore, CSP and conventional EMR are unsuitable for resecting all adenomatous colorectal lesions. Conversely, NIRBS may satisfy the above (1)–(3) conditions because the present study showed that NIRBS was resectable from small to large lesions of 1 to 35 mm, including Tis lesions, without residuals; moreover, it has a low complication rate (0.4%) and is easy to perform.
According to our findings, the proportion of cancer lesions increased exponentially as the lesion size and stalk length increased. Considering these, NIRBS may be preferred even if the lesion size is <5 mm because of the inability to exclude cancer for <5 mm lesions by less-experienced endoscopists.
We observed adverse events of NIRBS in three cases (0.4%), including one case with bleeding during the procedure and two cases with delayed bleeding, yet no perforation occurred. The first adverse event (spurting bleeding after resection) was reported in a patient with a large Ip lesion (35 mm). Although successful clipping hemostasis was immediately performed in this case, resections for a large Ip lesion should be carefully performed because thick arteries run in the stalk of the Ip lesions. When arterial spurting bleeding occurs, hemostasis becomes difficult. Generally, in cases with large Ip lesions, it is necessary to perform DLD-ligation before resection because visibility is poor suddenly and hemostasis is difficult when arterial spurting bleeding occurs. However, it is often difficult to pass through the largest part of the lesion and squeeze it to the stalk before resection because DLDs are very soft. Therefore, for complicated DLD-ligation before resection of Ip lesions, the stalk should be ligated by the bipolar snare, squeezed to the extent that it cannot be torn off without energization, and then energized and resected after confirmation of the ischemic lesion condition. This procedure prevented gushing bleeding immediately after the resection. Subsequently, the resected portion was immediately sutured or ligated using a clip or DLD. Notably, perforation was not observed in this study. NIRBS can hardly cause perforation. However, there is a possibility of perforation if the muscle layer is affected by snaring. Therefore, when the mucosa is extensively grasped, the area must be squeezed using a specific technique (Supplementary Video 1) to prevent involvement of the muscle layer.
Our study demonstrated that the NIRBS method is simple, has few complications, and has no residuals for Tis lesions. We found that numerous different lesions, including small and large lesions, can be easily resected using NIRBS. Therefore, the application of NIRBS may reduce the incidence of colorectal cancer and its associated mortality. The limitations of this study are its retrospective style and single facility-based nature, and it is unclear whether the bleeding rate is really low for large lesions because large lesions >10 mm were only observed in 33 cases. Additionally, the procedure time was not correctly measured in this study; therefore, it is unclear whether it was actually shorter than that of conventional EMR. Furthermore, it is unclear whether the same results could be reproduced, as one expert performed all procedures. If this novel treatment (NIRBS) is standardized and evaluated in a multicenter prospective randomized controlled trial, the outcome efficacy in patients with colorectal adenomatous lesions will be improved.
Footnotes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: MT; Data curation: MT, KK; Formal analysis: MT, KT, KK; Investigation: MT; Methodology: MT; Project administration: MT; Resources: MT; Validation: MS, HN, TO, MN; Writing–original draft: MT; Writing–review & editing: all authors.
Supplementary Material
In the case of lesions >5 mm, a specific technique is used. To avoid involvement of the muscle layer, the snare is quickly squeezed and loosened, and then resected in a short time (https://doi.org/10.5946/ce.2022.200.v1).
Basic NIRBS procedure (ascending colon, 16 mm; sessile type). No injection into the submucosal layer was performed. Instead, the lesion is grasped extensively, including the surrounding normal mucosa, and is sufficiently squeezed and then resected by energization within 2 s at ≤5 mm, within 5 seconds at 6–10 mm, and within 10 seconds at > 10 mm while continuing to squeeze (https://doi.org/10.5946/ce.2022.200.v2).
Supplementary materials related to this article can be found online at https://doi.org/10.5946/ce.2022.200.
REFERENCES
- 1.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood: Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–1371. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 3.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy: the National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 4.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–1477. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 5.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Horiuchi A, Nakayama Y, Kajiyama M, et al. Removal of small colorectal polyps in anticoagulated patients: a prospective randomized comparison of cold snare and conventional polypectomy. Gastrointest Endosc. 2014;79:417–423. doi: 10.1016/j.gie.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 7.Shinozaki S, Hayashi Y, Lefor AK, et al. What is the best therapeutic strategy for colonoscopy of colorectal neoplasia?: future perspectives from the East. Dig Endosc. 2016;28:289–295. doi: 10.1111/den.12566. [DOI] [PubMed] [Google Scholar]
- 8.Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296–1308. doi: 10.1111/j.1572-0241.2002.05812.x. [DOI] [PubMed] [Google Scholar]
- 9.Heldwein W, Dollhopf M, Rösch T, et al. The Munich Polypectomy Study (MUPS): prospective analysis of complications and risk factors in 4000 colonic snare polypectomies. Endoscopy. 2005;37:1116–1122. doi: 10.1055/s-2005-870512. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2015;27:417–434. doi: 10.1111/den.12456. [DOI] [PubMed] [Google Scholar]
- 11.Ichise Y, Horiuchi A, Nakayama Y, et al. Prospective randomized comparison of cold snare polypectomy and conventional polypectomy for small colorectal polyps. Digestion. 2011;84:78–81. doi: 10.1159/000323959. [DOI] [PubMed] [Google Scholar]
- 12.Uraoka T, Ramberan H, Matsuda T, et al. Cold polypectomy techniques for diminutive polyps in the colorectum. Dig Endosc. 2014;26 Suppl 2:98–103. doi: 10.1111/den.12252. [DOI] [PubMed] [Google Scholar]
- 13.Tutticci N, Burgess NG, Pellise M, et al. Characterization and significance of protrusions in the mucosal defect after cold snare polypectomy. Gastrointest Endosc. 2015;82:523–528. doi: 10.1016/j.gie.2015.01.051. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q, Gao P, Han B, et al. Polypectomy for complete endoscopic resection of small colorectal polyps. Gastrointest Endosc. 2018;87:733–740. doi: 10.1016/j.gie.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Kim JS, Lee BI, Choi H, et al. Cold snare polypectomy versus cold forceps polypectomy for diminutive and small colorectal polyps: a randomized controlled trial. Gastrointest Endosc. 2015;81:741–747. doi: 10.1016/j.gie.2014.11.048. [DOI] [PubMed] [Google Scholar]
- 16.Lee CK, Shim JJ, Jang JY. Cold snare polypectomy vs. cold forceps polypectomy using double-biopsy technique for removal of diminutive colorectal polyps: a prospective randomized study. Am J Gastroenterol. 2013;108:1593–1600. doi: 10.1038/ajg.2013.302. [DOI] [PubMed] [Google Scholar]
- 17.Lee CK, Lee SH, Park JY, et al. Prophylactic argon plasma coagulation ablation does not decrease delayed postpolypectomy bleeding. Gastrointest Endosc. 2009;70:353–361. doi: 10.1016/j.gie.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto M, Fukunaga S, Saito Y, et al. Risk factors for delayed bleeding after endoscopic resection for large colorectal tumors. Jpn J Clin Oncol. 2012;42:1028–1034. doi: 10.1093/jjco/hys131. [DOI] [PubMed] [Google Scholar]
- 19.Fujihara S, Mori H, Kobara H, et al. The efficacy and safety of prophylactic closure for a large mucosal defect after colorectal endoscopic submucosal dissection. Oncol Rep. 2013;30:85–90. doi: 10.3892/or.2013.2466. [DOI] [PubMed] [Google Scholar]
- 20.Burgess NG, Metz AJ, Williams SJ, et al. Risk factors for intraprocedural and clinically significant delayed bleeding after wide-field endoscopic mucosal resection of large colonic lesions. Clin Gastroenterol Hepatol. 2014;12:651–661. doi: 10.1016/j.cgh.2013.09.049. [DOI] [PubMed] [Google Scholar]
- 21.Turan AS, Moons LM, Schreuder RM, et al. Clip placement to prevent delayed bleeding after colonic endoscopic mucosal resection (CLIPPER): study protocol for a randomized controlled trial. Trials. 2021;22:63. doi: 10.1186/s13063-020-04996-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muniraj T, Sahakian A, Ciarleglio MM, et al. Cold snare polypectomy for large sessile colonic polyps: a single-center experience. Gastroenterol Res Pract. 2015;2015:175959. doi: 10.1155/2015/175959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirose R, Yoshida N, Murakami T, et al. Histopathological analysis of cold snare polypectomy and its indication for colorectal polyps 10-14 mm in diameter. Dig Endosc. 2017;29:594–601. doi: 10.1111/den.12825. [DOI] [PubMed] [Google Scholar]
- 24.Kawamura T, Takeuchi Y, Asai S, et al. A comparison of the resection rate for cold and hot snare polypectomy for 4-9 mm colorectal polyps: a multicentre randomised controlled trial (CRESCENT study) Gut. 2018;67:1950–1957. doi: 10.1136/gutjnl-2017-314215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinozaki S, Kobayashi Y, Hayashi Y, et al. Efficacy and safety of cold versus hot snare polypectomy for resecting small colorectal polyps: systematic review and meta-analysis. Dig Endosc. 2018;30:592–599. doi: 10.1111/den.13173. [DOI] [PubMed] [Google Scholar]
- 26.Qu J, Jian H, Li L, et al. Effectiveness and safety of cold versus hot snare polypectomy: a meta-analysis. J Gastroenterol Hepatol. 2019;34:49–58. doi: 10.1111/jgh.14464. [DOI] [PubMed] [Google Scholar]
- 27.Kawamura T, Takeuchi Y, Yokota I, et al. Indications for cold polypectomy stratified by the colorectal polyp size: a systematic review and meta-analysis. J Anus Rectum Colon. 2020;4:67–78. doi: 10.23922/jarc.2019-039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferlitsch M, Moss A, Hassan C, et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017;49:270–297. doi: 10.1055/s-0043-102569. [DOI] [PubMed] [Google Scholar]
- 29.Shaukat A, Kaltenbach T, Dominitz JA, et al. Endoscopic recognition and management strategies for malignant colorectal polyps: recommendations of the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2020;159:1916–1934. doi: 10.1053/j.gastro.2020.08.050. [DOI] [PubMed] [Google Scholar]
- 30.Sakamoto T, Matsuda T, Nakajima T, et al. Clinicopathological features of colorectal polyps: evaluation of the ‘predict, resect and discard’ strategies. Colorectal Dis. 2013;15:e295–e300. doi: 10.1111/codi.12210. [DOI] [PubMed] [Google Scholar]
- 31.Williams CB, de Peyer RC. Bipolar snare polypectomy: a safer technique for electrocoagulation of large polyp stalks. Endoscopy. 1979;11:47–50. doi: 10.1055/s-0028-1098324. [DOI] [PubMed] [Google Scholar]
- 32.Tucker RD, Platz CE, Sievert CE, et al. In vivo evaluation of monopolar versus bipolar electrosurgical polypectomy snares. Am J Gastroenterol. 1990;85:1386–1390. [PubMed] [Google Scholar]
- 33.Shinmura K, Ikematsu H, Kojima M, et al. Safety of endoscopic procedures with monopolar versus bipolar instruments in an ex vivo porcine model. BMC Gastroenterol. 2020;20:27. doi: 10.1186/s12876-020-1176-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saraya T, Ikematsu H, Fu KI, et al. Evaluation of complications related to therapeutic colonoscopy using the bipolar snare. Surg Endosc. 2012;26:533–540. doi: 10.1007/s00464-011-1914-8. [DOI] [PubMed] [Google Scholar]
- 35.Sakuragi T, Okazaki Y, Mitsuoka M, et al. Dramatic hemostasis of the transected pulmonary artery model using SOFT COAG electrosurgical output. Interact Cardiovasc Thorac Surg. 2008;7:764–766. doi: 10.1510/icvts.2008.177923. [DOI] [PubMed] [Google Scholar]
- 36.Sakuragi T, Ohma H, Ohteki H. Efficacy of SOFT COAG for intraoperative bleeding in thoracic surgery. Interact Cardiovasc Thorac Surg. 2009;9:767–768. doi: 10.1510/icvts.2009.212696. [DOI] [PubMed] [Google Scholar]
- 37.Dumoulin FL, Hildenbrand R. Endoscopic resection techniques for colorectal neoplasia: current developments. World J Gastroenterol. 2019;25:300–307. doi: 10.3748/wjg.v25.i3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Law KE, Lowndes BR, Kelley SR, et al. Surgeon workload in colorectal surgery: perceived drivers of procedural difficulty. J Surg Res. 2020;245:57–63. doi: 10.1016/j.jss.2019.06.084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In the case of lesions >5 mm, a specific technique is used. To avoid involvement of the muscle layer, the snare is quickly squeezed and loosened, and then resected in a short time (https://doi.org/10.5946/ce.2022.200.v1).
Basic NIRBS procedure (ascending colon, 16 mm; sessile type). No injection into the submucosal layer was performed. Instead, the lesion is grasped extensively, including the surrounding normal mucosa, and is sufficiently squeezed and then resected by energization within 2 s at ≤5 mm, within 5 seconds at 6–10 mm, and within 10 seconds at > 10 mm while continuing to squeeze (https://doi.org/10.5946/ce.2022.200.v2).


