Abstract
Background
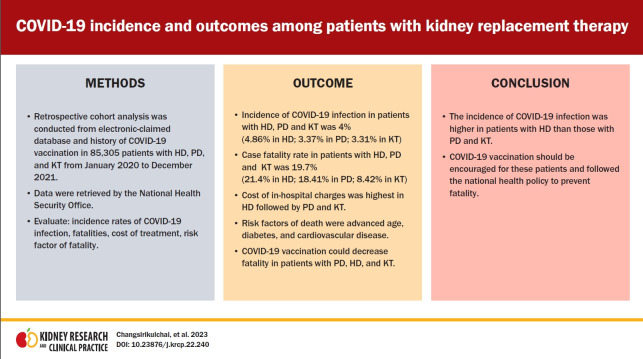
We aimed to investigate the incidence, fatality, and associated factors in patients with hemodialysis (HD), peritoneal dialysis (PD), and kidney transplantation (KT) hospitalized for coronavirus disease 2019 (COVID-19) infection and reimbursed from the National Health Security Office (NHSO).
Methods
The retrospective cohort analysis was conducted from an electronic-claimed database, and COVID-19 vaccination status was evaluated in patients with HD, PD, and KT from January 2020 to December 2021. There were 85,305 patients reimbursed for HD, PD, and KT by the NHSO. The rates of COVID-19 infection, COVID-19 vaccination, comorbidities, fatalities, and the cost of treatment were evaluated.
Results
COVID-19 infection was observed in 1,799 of 36,982 HD cases (4.9%), 1,531 of 45,453 PD cases (3.4%), and 95 of 2,870 KT cases (3.3%). Patients receiving COVID-19 vaccinations were most common in the KT group, followed by those with HD and PD (76.93% vs. 70.65% vs. 51.34%, respectively). KT patients had a lower fatality rate compared to those with PD and HD (8.42% vs. 18.41% vs. 21.40%, respectively). Advanced age, diabetes, cardiovascular diseases, and COVID-19 vaccination status were associated with fatality. The adjusted odds ratios of fatality after receiving one or two doses of vaccines were 0.7 (95% confidence interval [CI], 0.6–0.9) and 0.3 (95% CI, 0.2–0.4), respectively. The cost of treatment was highest in patients with HD, followed by PD and KT.
Conclusion
The incidence of COVID-19 infection was higher in patients with HD than in those with PD or KT. COVID-19 vaccination following the national health policy should be encouraged for these patients to prevent fatality.
Keywords: COVID-19, Dialysis, Kidney transplantation, Peritoneal dialysis, Renal dialysis, Renal replacement therapy
Graphical abstract
Introduction
Coronavirus disease 2019 (COVID-19) infection is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and was first recognized in December 2019 in Wuhan, China [1]. Patients with end-stage kidney disease (ESKD) have high mortality rates following COVID-19 infection; mortality rates in previous cohort studies were 20% to 30% [2–6]. The risk factors related to death from COVID-19 infections are increasing age, comorbid conditions, and frailty [7–11]. Patients with maintenance dialysis are susceptible to COVID-19 infection as they have difficulty preventing COVID-19 transmission [12]. Patients with hemodialysis (HD) have frequent visits to dialysis units to get treatment and have limited abilities to perform physical distancing measures in closed spaces [9,10,13]. The rates of COVID-19 infection in patients with peritoneal dialysis (PD) are lower than in those with HD [14–16]. PD is a home-based dialysis modality, so the risks of exposure to highly transmissible infections are less than those for HD [16,17]. Patients with kidney transplantation (KT) have risks of critical illness from COVID-19 infection due to their use of immunosuppressive medication [18,19].
In Thailand, the first case of COVID-19 infection was confirmed in mid-January 2020 [20]. The first wave of the COVID-19 pandemic from a domestic infection occurred in March 2020 [21]. The Thai government responded rapidly to mitigate the COVID-19 pandemic by using legislative, social, and health measures, in addition to full-scale lockdown measures [21]. The second wave of the pandemic was triggered in December 2020 by illegal migrants, which led to widespread infections. The national health strategies used public health measures to reduce COVID-19 transmission rather than locking down the entire country [22]. Public and private health resources were used to identify index cases and tracers, test all high-risk contacts, and isolate cases of COVID-19 infection. Early hospitalization and treatment would be provided to infected patients with severe comorbidities [21,23].
The kidney replacement therapy (KRT) of Thai patients with ESKD is funded by the national health budget through one of the three healthcare schemes (the civil servant medical health scheme, social security health scheme, and universal health coverage scheme [UHC]). The majority of people are reimbursed by the National Health Security Office (NHSO) under the UHC. The modalities of KRT in these patients include HD, PD, and KT. PD is the only home dialysis modality, while HD is performed for in-hospital dialysis or outpatient dialysis clinics. Protocols to prevent COVID-19 infection in dialysis units were implemented during the outbreak [24,25]. Patients with HD, PD, or KT were encouraged to receive COVID-19 vaccinations. COVID-19 infections were diagnosed by the positive reverse transcriptase-polymerase chain reaction (RT-PCR) test. The “PD First” policy was adopted as the main dialysis modality in patients with ESKD under the UHC in 2008. It was changed to a shared decision-making policy in February 2022. We are interested in studying the outcomes of the COVID-19 pandemic across different KRT modalities during the period of the PD First policy. The results may be different from previous studies. In addition, it could provide information to healthcare providers to assist in the prevention of COVID-19 outbreaks, which may occur from crowded patients with HD when the policy is changed in the future. This study aims to investigate the incidence rates, fatality, and factors associated with death among patients with PD, HD, and KT who were hospitalized due to COVID-19 infection. We also analyzed and compared the cost of treatment during hospitalization for these patients.
Methods
This study was approved in the exempt category by the Institutional Review Committee for Research in Human Subjects, Faculty of Medicine, Srinakharinwirot University (No. SWUEC-M-067/2565X). As the study subjects were de-identified, the need for written consent from the patients was waived.
Study population
The data of patients receiving KRT who were admitted due to COVID-19 from January 2020 to December 2021 were retrospectively reviewed. All patients with KRT who had COVID-19 infections were hospitalized because they were considered high-risk patients. The cost of hospitalization was claimed by the NHSO. The sources of databases for analysis were the files of inpatients (electronic-claimed data), files of patients with a history of COVID-19 vaccination, and files of KRT reimbursement that were retrieved by the NHSO. The NHSO granted only one of our investigators (TI) access to the database with this patient information. The personal data of each patient was redacted before data sharing. All analyses were performed on the cloud using the Rstudio server provided by the NHSO, where SQL was used to query data from the NHSO data storage server. No data were transferred to any of the researchers’ personal computers or notebooks. We selected patients at least 18 years of age receiving either HD, PD, or KT. The International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) codes U071, U072, U099, and U109 were used to identify patients with COVID-19 infections who tested COVID-19-positive by RT-PCR and were admitted to hospitals. They were de-identified by the NHSO data controller before analysis.
Data collection and variables
The following demographic and clinical characteristics of the patients were collected: age at hospital admission due to COVID-19 infection, sex, comorbidities, complications, history of COVID-19 vaccination, and KRT modalities. The type of KRT in each patient was determined from the modality of long-term chronic dialysis and KT at the first date of hospital admission due to COVID-19. Comorbidities were defined according to the ICD-10 codes for diabetes, cardiovascular disease, cerebrovascular disease, malignancy, hypertension, airway disease, liver disease, human immunodeficiency virus disease, and psychiatric problems. The complications from COVID-19 infection were classified as sepsis, pneumonia, respiratory failure, volume overload, and heart failure. The type and number of COVID-19 vaccinations prior to or after COVID-19 infection were evaluated. In addition, the time intervals between the date of the last vaccination and the date of admission were assessed.
Outcomes and definitions
The primary outcome was fatality rates during admission and associated factors. The secondary outcomes were the length of hospitalization and the cost of treatment. The length of hospitalization was calculated from the first date of admission to the date of discharge or death. The cost of treatment due to COVID-19 infection was calculated from the total charges from hospitals, which included the cost of personal protective equipment during hospitalization. The cost of dialysis or immunosuppressive drugs prescribed to KT patients was calculated from the file of the KRT reimbursement. The incidence rates and primary and secondary outcomes of COVID-19 infection were compared among PD, HD, and KT patients. Age, sex, comorbidities, the number of vaccinations, and types of COVID-19 vaccines were assessed as being associated with fatalities. These factors were analyzed separately in HD, PD, and all modalities of KRT.
Statistical analysis
Descriptive analysis is presented as numbers with percentages for categorical variables and median with interquartile range (IQR) for continuous variables. The case fatality rate was calculated from the number of in-hospital deaths divided by the total number of patients admitted after COVID-19 diagnosis and presented as percentages. The comorbidity conditions, complications during hospitalization, and cost of treatment were compared among PD, HD, and KT by chi-square test for categorical variables and t test or Wilcoxon rank-sum test for continuous variables. The factors associated with death in hospitalization were determined by using logistic regression models and presented as odd ratios (ORs) and 95% confidence intervals (95% CIs) with adjustments including variables that were significant in the univariate analysis. The variables for the final multivariate model were selected using the backward stepwise method based on Akaike Information Criteria [26]. The missing data were verified and managed by the NHSO since they were used to reimburse the cost of treatment. The final data set for analysis had no missing data. All statistical analyses were performed using the R program version 3.6.2 (R Core Team). A p-value of less than 0.05 was considered statistically significant.
Results
Patient characteristics and incidence of COVID-19 infection
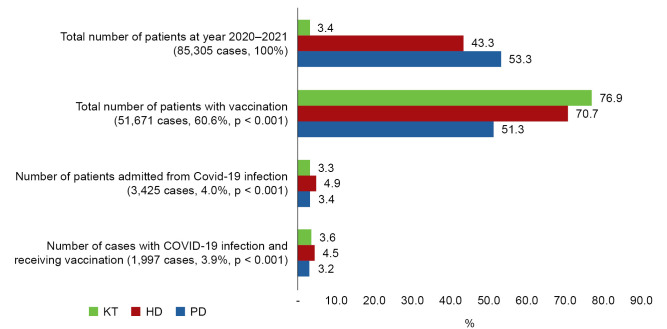
Fig. 1 shows the percentages of COVID-19 infections and vaccinations in KRT patients classified into PD, HD, and KT groups. There were 85,305 patients receiving KRT from January 2020 to December 2021. The number of patients with PD was 45,453 (53.3%), that of HD was 36,982 (43.3%), and that of KT was 2,870 (3.4%). A total of 3,425 patients (4.0%) with either PD, HD, or KT were admitted due to COVID-19 infection. All cases had only a single COVID-19 infection during the period of the study. The number of HD, PD, and KT patients infected with COVID-19 was 1,799 (4.9%), 1,531 (3.4%), and 95 (3.3%), respectively. The percentage of hospital admissions due to COVID-19 infection in patients with PD was significantly less than in those with HD. The total number of KRT patients receiving COVID-19 vaccinations was 51,671 (60.6%). The number of patients with KT and vaccinations was 2,208 out of 2,870 cases (76.9%), that of HD was 26,126 out of 36,982 cases (70.7%), and that of PD was 23,337 out of 45,453 cases (51.3%). The percentage of vaccinated patients was highest in the KT group, followed by the HD and PD groups. There were 1,997 out of 51,671 KRT patients (3.9%) who received vaccinations and became infected with COVID-19. The number of COVID-19 infections in vaccinated patients with PD was 744 out of 23,337 cases (3.2%), that of HD was 1,173 out of 26,126 cases (4.5%), and that of KT was 80 out of 2,208 cases (3.6%). The percentage of vaccinated patients with PD who became infected with COVID-19 was significantly lower than that of those with HD or KT.
Figure 1. Percentage of patients with different types of kidney replacement therapy receiving COVID-19 vaccination and becoming infected with COVID-19.
p < 0.001 compared between HD and PD.
COVID 19, coronavirus disease 2019; HD, hemodialysis; KT, kidney transplantation; PD, peritoneal dialysis.
Table 1 shows the characteristics of patients infected with COVID-19. The median age at the admission of these patients was 58 years (IQR, 48–67 years), which was lowest in patients with KT, followed by those with PD and HD. Patients with KT tended to have less severe comorbidity conditions than those with HD or PD. The complications during hospitalization were not different among PD, HD, and KT patients. Table 2 shows the fatality rates, length of admission, and cost of treatment in patients with COVID-19 infection during hospitalization. The median length of hospitalization across all modalities of KRT was 13 days (IQR, 7–17 days). The median cost of treatment in a hospital, not including the cost of dialysis (calculated in US dollars [USD]; 1 USD is equal to 35 baht), in patients with HD was 1,458.03 USD (IQR, 274.28–3,831.94 USD). This was higher than in those with PD or KT. The median costs of treatment in patients with PD and KT were 1,272.63 USD (IQR, 298.71–3,058.71 USD) and 761.20 USD (IQR, 285.71–4,431.34 USD), respectively. The case fatality rate in patients with HD was higher than in those with PD (21.4% vs. 18.41%), while it was lowest in patients with KT (8.4%). The median length of admission in patients who died during treatment was 11 days (IQR, 6–19 days). The median cost of treatment in patients who died during treatment was 2,528.57 USD (IQR, 1,046.40–5,194.29 USD), and this cost was the highest in those with KT.
Table 1.
Characteristics of comorbidities, complications, fatality rates, length of admission, and cost of treatment in patients with COVID-19 infection requiring hospitalization
| Characteristic | PD group | HD group | KT group | Total |
|---|---|---|---|---|
| No. of patients | 1,531 | 1,799 | 95 | 3,425 |
| Age at admission for COVID-19 infection (yr)a | 57 (47–66) | 60 (50–69) | 46 (33–58) | 58 (48–67) |
| Comorbidity | ||||
| Diabetesb | 988 (64.5) | 1,159 (64.4) | 24 (25.3) | 2,276 (63.3) |
| Cardiovascular diseasesb | 600 (39.2) | 824 (45.8) | 17 (17.9) | 1,497 (41.6) |
| Cerebrovascular diseases | 229 (15.0) | 260 (14.5) | 10 (10.5) | 517 (14.4) |
| Malignancyb | 48 (3.1) | 86 (4.8) | 1 (1.1) | 152 (4.2) |
| Hypertensionb | 1,494 (97.6) | 1,716 (95.4) | 72 (75.8) | 3,432 (95.4) |
| Airway diseasesb | 162 (10.6) | 186 (10.3) | 1 (1.1) | 366 (10.2) |
| Liver diseases | 227 (14.8) | 329 (18.3) | 14 (14.7) | 599 (16.7) |
| HIV infection | 9 (0.6) | 3 (0.2) | 0 (0) | 12 (0.4) |
| Psychiatric problem | 84 (5.5) | 128 (7.1) | 3 (3.2) | 219 (6.1) |
| Complications during hospitalization | ||||
| Sepsis | 243 (15.9) | 253 (14.1) | 11 (11.6) | 528 (14.7) |
| Pneumonia | 1,062 (69.4) | 1,299 (72.2) | 60 (63.2) | 2,530 (70.3) |
| Respiratory failure | 42 (2.7) | 55 (3.1) | 5 (5.3) | 106 (2.9) |
| Volume overload | 112 (7.3) | 113 (6.3) | 1 (1.1) | 235 (6.5) |
| Heart failure | 45 (2.9) | 67 (3.7) | 2 (2.1) | 131 (3.6) |
Data are expressed as number only, median (interquartile range), or number (%).
COVID 19, coronavirus disease 2019; HD, hemodialysis; HIV, human immunodeficiency virus; KT, kidney transplantation; PD, peritoneal dialysis.
p < 0.05 compared between PD and HD.
p < 0.05 compared among PD, HD, and KT.
Table 2.
The fatality rates, length of admission, and cost of treatment in patients with COVID-19 infection requiring hospitalization
| Outcome | PD group (n = 1,531) | HD group (n = 1,799) | KT group (n = 95) | Total (n = 3,425) |
|---|---|---|---|---|
| Length of admission (day) | 12 (8–17) | 13 (8–18) | 12 (6–16) | 13 (7–17) |
| Cost of treatment in all casesa,b (USD) | 1,272.63 (298.71–3,058.71) | 1,458.03 (274.28–3,831.94) | 761.20(285.71–4,431.34) | 1,314.57 (273.40–3,397.00) |
| No. of patients that died from COVID-19a,b | 282 (18.4) | 385 (21.4) | 8 (8.4) | 675 (19.7) |
| Days to death during hospitalization (day) | 11 (6–21) | 11 (5–18) | 14 (8–23) | 11 (6–19) |
| Cost of treatment in cases resulting in death (USD) | 2,605.60 (1,245.59–5,179.00) | 2,381.66 (906.57–5,064.04) | 5,292.09 (887.29–7,488.05) | 2,528.57 (1,046.40–5,194.29) |
Data are expressed as median (interquartile range) or number (%).
COVID 19, coronavirus disease 2019; HD, hemodialysis; KT, kidney transplantation; PD, peritoneal dialysis; USD, US dollars (1 USD equals 35 baht).
p < 0.05 compared between PD and HD.
p < 0.05 compared among PD, HD, and KT.
Characteristics of COVID-19 vaccination in patients with COVID-19 infection
Table 3 shows the characteristics of COVID-19 vaccination in patients with PD, HD, and KT who developed COVID-19 infections. Most of them did not receive COVID-19 vaccinations or received a single dose of vaccination before COVID-19 infection. The number of patients with PD, HD, and KT who did not receive vaccination prior to contacting COVID-19 was 1,966 (57.40%). The percentage of unvaccinated patients who were admitted due to COVID-19 infection was highest in the PD group (66.5%), followed by the KT (51.6%) and HD groups (50.0%). The number of patients with PD, HD, and KT who received at least one vaccination dose before COVID-19 infection was 1,459 (42.6%). The percentage of patients who received a COVID-19 vaccination before infection was highest in those with HD (50.0%), followed by those with KT and PD (48.4% and 33.5%, respectively). There were 538 patients (15.7%) who had COVID-19 infections and received COVID-19 vaccinations after they were discharged from the hospital. The total number of patients with PD, HD, and KT receiving vaccination before or after COVID-19 infection was 1,997 (58.3%). Most patients received live, attenuated, or viral vector vaccines. The median interval between the date of the last vaccination and the date of admission was 30 days (IQR, 15–58 days), which was the longest in patients with PD, followed by those with HD and KT.
Table 3.
Characteristics of COVID-19 vaccination among patients with COVID-19 infection
| Variable | PD group (n = 1,531) | HD group (n = 1,799) | KT group (n = 95) | Total group (n = 3,425) |
|---|---|---|---|---|
| Hospitalizing COVID-19 infections (%) | 44.7 | 52.5 | 2.8 | 100 |
| Cases without vaccination before infection* | 1,018 (66.5) | 899 (50.0) | 49 (51.6) | 1,966 (57.4) |
| Cases of vaccination before infection | 513 (33.5) | 900 (50.0) | 46 (48.4) | 1,459 (42.6) |
| Single dose | 326 (63.6) | 594 (66.0) | 31 (67.4) | 951 (65.2) |
| Two doses | 181 (35.3) | 298 (33.1) | 15 (32.6) | 494 (33.9) |
| At least three doses | 6 (1.2) | 8 (0.9) | 0 (0) | 14 (1.0) |
| Cases of vaccination after infectiona | 231 (15.1) | 273 (15.2) | 34 (35.8) | 538 (15.7) |
| Cases of vaccination before or after infection | 744 (48.6) | 1,173 (65.2) | 80 (84.2) | 1,997 (58.3) |
| Cases of only lived, attenuated vaccine administrationa | 131 (8.6) | 187 (10.4) | 13 (13.7) | 331 (9.7) |
| Cases of only viral vector vaccine administration | 289 (18.9) | 605 (33.6) | 28 (29.5) | 922 (26.9) |
| Cases of only mRNA vaccine administration | 29 (1.9) | 25 (1.4) | 2 (2.1) | 56 (1.6) |
| Cases of mixed-type vaccine administration with any mRNA vaccine | 1 (0.1) | 2 (0.1) | 0 (0) | 3 (0.1) |
| Cases of mixed-type vaccine administration without any mRNA vaccine | 63 (4.1) | 81 (4.5) | 3 (3.2) | 147 (4.3) |
| Cases of any mRNA vaccine administration | 30 (2.0) | 27 (1.5) | 2 (2.1) | 59 (1.7) |
| Interval between the last vaccination and admission date (day)a | 34.0 (16.0–61.0) | 28.5 (14.0–56.0) | 24.0 (15.0–38.3) | 30.0 (15.0–58.0) |
Data are expressed as percentage only, number (%), or median (interquartile range).
COVID-19, coronavirus disease 2019; HD, hemodialysis; KT, kidney transplantation; mRNA, messenger RNA; PD, peritoneal dialysis.
p < 0.05.
Characteristics of patients with fatal COVID-19 infections and associated factors
Table 4 shows the characteristics of KRT patients with COVID-19 infection who died during admission compared with those who survived at hospital discharge. Patients in the survival group had a significantly lower age at the time of COVID-19 infection; a higher chance of being vaccinated against COVID-19, especially with double doses of the vaccine; and a longer interval between the last date of vaccination and date of admission than those in the death group. The case fatality rate in patients who did not receive COVID-19 vaccinations was 23.1%; in contrast, it was 15.1% for those who received COVID-19 vaccinations. There were only 59 patients in the dead and surviving groups who received the messenger RNA (mRNA) vaccine. It was a small number, and there was not a significant difference between those who died and survived.
Table 4.
Characteristics of patients with fatal and non-fatal hospitalizing COVID-19 infections
| Characteristic | Dead group (n = 675) | Surviving group (n = 2,750) | Total (n = 3,425) |
|---|---|---|---|
| Agea (yr) | 63 (55–71) | 57 (47–66) | 58 (48–67) |
| Vaccination statusa | |||
| No. of patients with COVID-19 vaccination | 221 (32.7) | 1,238 (45.0) | 1,459 (42.6) |
| No. of patients without COVID-19 vaccination | 454 (67.3) | 1,512 (55.0) | 1,966 (57.4) |
| Doses of vaccinationa | |||
| No. of patients with a single dose | 178 (80.5) | 773 (62.4) | 951 (65.2) |
| No. of patients with two doses | 42 (19.0) | 452 (36.5) | 494 (33.9) |
| No. of patients with at least three doses | 1 (0.5) | 13 (1.1) | 14 (1.0) |
| Any mRNA vaccine | 5 (2.3) | 54 (4.4) | 59 (4.0) |
| Interval between the last vaccination and admission date (day)a | |||
| Single dose | 24.0 (13.2–43.8) | 29.0 (15.0–53.0) | 27.0 (15.0–52.0) |
| Two doses | 17.0 (7.0–48.0) | 40.0 (19.0–70.2) | 39.5 (17.2–69.0) |
| At least three doses | NA | NA | NA |
| All doses of vaccination | 24.0 (12.0–45.0) | 32.0 (16.0–59.8) | 30.0 (15.0–58.0) |
Data are expressed as median (interquartile range) or number (%).
COVID 19, coronavirus disease 2019; mRNA, messenger RNA; NA, not available.
p < 0.05 compared between dead and surviving groups.
The factors associated with death in KRT patients after COVID-19 infection are shown in Table 5. Increased age, diabetes, and cardiovascular diseases were demonstrated as significant factors associated with death. The modes of KRT were not factors associated with death after being adjusted for age, comorbidities, and the status of COVID-19 vaccination. Receiving COVID-19 vaccination or the interval between the date of the last vaccination and the date of admission for COVID-19 were protective factors against death after COVID-19 infection. The factors associated with fatality in the subgroup analysis of patients with HD and PD have shown similar results (Supplementary Table 1, available online). The adjusted OR of fatality in HD patients with double COVID-19 vaccinations was 0.32 (95% CI, 0.21–0.49). The adjusted ORs of fatality in PD patients with COVID-19 vaccination (one and double doses) were 0.46 (95% CI, 0.31–0.66) and 0.24 (95% CI, 0.12–0.42), respectively.
Table 5.
Factors associated with death in KRT patients with COVID-19 infection
| Factor | Crude OR (95% CI) | p-value | Adjusted ORb (95% CI) | p-value |
|---|---|---|---|---|
| Age | 1.03 (1.03–1.04) | <0.001 | 1.03 (1.02–1.04) | <0.001 |
| Malea | 1 | 1 | ||
| Female | 0.96 (0.81–1.13) | 0.60 | 0.85 (0.71–1.02) | 0.08 |
| Diabetes | 1.63 (1.35–1.96) | <0.001 | 1.26 (1.03–1.53) | 0.03 |
| Cardiovascular disease | 1.64 (1.38–1.94) | <0.001 | 1.44 (1.20–1.72) | <0.001 |
| Cerebrovascular disease | 1.33 (1.06–1.67) | 0.01 | 1.16 (0.92–1.47) | 0.20 |
| Malignancy | 0.97 (0.62–1.48) | 0.90 | 0.90 (0.56–1.39) | 0.60 |
| Hypertension | 1.45 (0.92–2.38) | 0.12 | 0.96 (0.60–1.62) | 0.90 |
| Airway disease | 0.91 (0.68–1.20) | 0.50 | 0.76 (0.56–1.02) | 0.07 |
| Liver disease | 1.01 (0.80–1.26) | >0.90 | 0.99 (0.78–1.25) | 0.90 |
| HIV | 1.36 (0.30–4.57) | 0.60 | 1.39 (0.28–5.42) | 0.70 |
| Psychiatric problem | 1.59 (1.16–2.16) | 0.003 | 1.15 (0.83–1.59) | 0.40 |
| Mode of KRT | ||||
| HDa | 1 | 1 | ||
| KT | 0.34 (0.15–0.66) | 0.004 | 0.51 (0.22–1.04) | 0.09 |
| PD | 0.83 (0.70–0.98) | 0.03 | 0.85 (0.71–1.02) | 0.09 |
| No. of vaccination doses | ||||
| No vaccinationa | 1 | 1 | ||
| Single dose | 0.77 (0.63–0.93) | 0.007 | 0.71 (0.58–0.87) | <0.001 |
| Double dose | 0.31 (0.22–0.43) | <0.001 | 0.30 (0.21–0.42) | <0.001 |
| At least three doses | 0.26 (0.01–1.29) | 0.20 | 0.28 (0.02–1.44) | 0.20 |
| Type of vaccine without mRNAa | 1 | 1 | ||
| With mRNA | 2.68 (1.18–7.73) | 0.04 | 1.50 (0.63–4.42) | 0.40 |
| Interval between the last vaccination and admission date (day) | 0.99 (0.98–0.99) | <0.001 | 0.99 (0.98–0.99) | <0.001 |
CI, confidence interval; COVID 19, coronavirus disease 2019; HD, hemodialysis; HIV, human immunodeficiency virus; KT, kidney transplantation; KRT, kidney replacement therapy; mRNA, messenger RNA; OR, odds ratio; PD, peritoneal dialysis.
Reference to calculate ORs.
Adjusted for age, sex, diabetes, cardiovascular disease, cerebrovascular disease, malignancy, hypertension, airway disease, liver disease, HIV, psychiatric problems, mode of KRT, number of vaccination doses, types of vaccine, and interval between the date of the last vaccination and the date of hospital admission due to COVID-19 infection.
Discussion
We report the incidence of COVID-19 infection, case fatality rates, and associated factors in patients with HD, PD, and KT during 2 years of the COVID-19 pandemic in Thailand. We found that the overall incidence of COVID-19 infection in patients with KRT (HD, PD, and KT) was 4% compared to the rate of 3.4% for the general population nationwide [22,27]. This incidence was probably underestimated due to the fact that some patients died at home. This happened despite the national policy response to the COVID-19 pandemic during the period of study, including contact tracing by surveillance and rapid response team engagement from village health volunteers to identify, isolate, and quarantine cases and admit all cases with positive RT-PCR results. The in-hospital case fatality rate in these patients was 19.7% versus 1% for nationwide patients [22]. The incidence and fatality rates of COVID-19 infections varied among countries and the times of outbreak [4,9,10,15,16,20,28,29]. There are reasons to explain these variations. The incidence and mortality of COVID-19 may be impacted by the enrollment of patients at different times during outbreaks, as this could be influenced by the severity of virulence and transmission diversity of the disease. The preparedness of national health policies, the adherence of people to preventive measures, and the availability of healthcare facilities, e.g., intensive care units or mechanical ventilation, could also affect outcomes [9,10,20,28,29].
In Thailand, the number of COVID-19 diagnoses was much higher in the second wave compared to the first wave. There were only 3,042 cumulative cases of COVID-19 infection with 57 deaths (1.5% case fatality rate) countrywide, and only eight patients with KRT had COVID-19 infections in the first wave [21]. In contrast, the total number of cases of COVID-19 infection in the second wave was more than two million cases by the end of December 2021, which led to increasing numbers of KRT patients infected with COVID-19 [22]. We suspect that the main cause of the increasing incidence of COVID-19 infection in the second wave was the highly contagious COVID-19 clades due to differences in the processes of lockdown between the first and second waves of the COVID-19 outbreaks [30]. The nationwide lockdown included curfews between 10.00 PM to 04.00 AM, canceling national holidays, and suspending international flights for tourists, which led to a huge negative impact on the economy during the first wave. Therefore, the policy was changed to lockdown specific areas with COVID-19 outbreaks, find active cases, speed up COVID-19 vaccinations for the elderly and patients with chronic disease, and practice early admission for treatment in patients with a high risk of mortality in the second wave of the COVID-19 pandemic. Patients with KRT who were infected with COVID-19 and confirmed by RT-PCR would be admitted to hospitals and receive treatment early as they were considered vulnerable people with high risks of mortality.
Patients with PD, HD, and KT had different incidence rates of COVID-19 infection in our study, which are similar to previous publications [9,10,28,29]. The proportions of KRT modalities in the 3,425 patients with COVID-19 infection were 52.5% from HD, 44.7% from PD, and 2.8% from KT. Patients with HD had a higher chance of COVID-19 infection than those with PD. The main reasons were that they had to travel two to three times per week and remained in clinics for at least 4 hours in each HD session to get dialyzed. It was not from a greater opportunity to screen for COVID-19 in patients with HD since the national health policy was that the test would be done in all index cases and cases with high-risk contacts. RT-PCR was the only method used to test for COVID-19 during the study period. Antigen test kits were not available at that time; therefore, there was no policy to test patients with HD regularly during the second wave of COVID-19. Our study showed that patients with KT had the lowest incidence of COVID-19 infection compared to those with dialysis. We proposed that this was due to KT patients tending to have younger ages and fewer comorbidities than patients with HD or PD [8,31].
Our study showed the results of short-term fatality and risk factors, which were similar to previous reports from other countries. Previous studies showed that the fatality rates from COVID-19 infection in patients with dialysis were 20% to 30% [8–10,14,16,28,32–34]. Advanced age, multiple comorbid conditions, diabetes, frailty, and the need for ventilation were risk factors related to death [4,8–10,28,32,33]. The overall fatality rate of COVID-19 infection in our patients with KRT was 19.71%. It was highest in patients with HD, followed by those with PD and KT. Advanced age, diabetes, and cardiovascular diseases were risk factors related to fatality. The KRT modalities were not related to fatality after adjusting for age, comorbidities, and the status of COVID-19 vaccination. COVID-19 vaccination, especially two doses of a vaccine, decreased fatality from COVID-19 infection in patients with PD, HD, and KT. Patients with HD tended to be vaccinated against COVID-19 more than those with PD or KT. This was probably due to the fact that they attended HD clinics two to three times per week, and they had more chances to access the COVID-19 vaccination than patients with PD or KT. Previous studies demonstrated a diminished antibody response and a rapid decline in concentration following COVID-19 vaccination in these patients [35–37]. A current research letter shows that patients with maintenance dialysis developed robust antibody responses after being vaccinated with a booster dose of an mRNA vaccine [38]. We strongly suggest that these patients should be prioritized and encouraged to receive COVID-19 vaccinations with boosters.
We would like to address the limitations of our study. First, we could not calculate the cost of HD and PD from the KRT reimbursement files because we found that there was no reimbursement for the cost of dialysis in 26.2% of PD and 47.2% of HD patients during COVID-19 infection. Although the cost of dialysis was not included in the analysis of expenses for patients with PD, it was still lower than for those with HD. The average costs of PD and HD reimbursed from the NHSO in patients under UHC were 472.57 and 514.29 USD/patient/month, respectively. Second, we could not identify the locations of HD units or whether patients received maintenance HD in-hospital or in outpatient dialysis clinics. This may have impacted the analysis of the risk of infection, as it is increased in crowded dialysis clinics with small areas. Third, we did not evaluate body mass index, frailty parameters, the treatment protocol for COVID-19 in individual cases, medication usage for the treatment of COVID-19 infection, or the use of immunosuppressive medications in patients with KT due to the lack of this information in the database. These may be associated with fatality. Fourth, we reported in-hospital fatality rates, which may be lower than the actual death rates because we could not follow the status of patients after discharge from hospitals. Some patients may have expired shortly after they were discharged from the hospital. Fourth, it is an observational cohort study that could not match patients to compare the effects of KRT modalities on COVID-19 infection. Fifth, we could not compare the risk factors of fatality in KRT patients versus the general population due to the inability to access the national database of COVID-19 infection, treatment, and vaccination. Although this study has several limitations, we believe that it has strengths and benefits to share. First, it is a study from a middle-income country that provides PD, HD, and KT for all under UHC. Second, the databases are from the NHSO, which has covered the majority of KRT patients under UHC. The median cost of in-hospital charges was highest in HD, followed by PD and KT. The cost of treatment during admission for each KRT modality reflects the financial burden of COVID-19 infection. PD had beneficial treatment options and resulted in a lower risk of COVID-19 infection during the pandemic than HD because it is the main home-based dialysis modality available in developing countries. This information should be emphasized during the shared decision-making process regarding KRT modalities. Third, KT should be done early for appropriate patients. Fourth, vulnerable patients should not hesitate to receive available COVID-19 vaccines while awaiting more effective and safer ones.
In conclusion, the COVID-19 pandemic has had an impact on KRT patients. The results from this study provide information on the effects of KRT modalities on COVID-19 incidence, risk factors for in-hospital fatality, and the cost of treatment. COVID-19 vaccinations could reduce fatality in patients with KRT, especially those with advanced age and comorbid conditions. These findings could be of benefit to the healthcare authorities to prepare measures to prevent outbreaks of COVID-19 infection that may occur in crowded patients undergoing HD after the policy changes from PD First to shared decision-making regarding dialysis modalities in the future. Policymakers and healthcare providers should promote KT and home treatment programs, such as PD and telemedicine, to mitigate the crowded dialysis centers. Home treatment programs are strategic treatments that can reduce the pressure on hospitals from the risk of spreading diseases with high severity and transmission and mitigate the financial burden in case of future pandemic infections.
Acknowledgments
We would like to acknowledge the National Health Security Office for providing the data for analysis. The interpretation and reporting of these data are solely the responsibility of the authors and do not represent the view of the National Health Security Office or the policy implications of the Thai government. Mr. Robert Cho is acknowledged for manuscript preparation.
Footnotes
Conflicts of interest
Siribha Changsirikulchai reports receiving a speaker honorarium from Baxter Healthcare and Fresenius Medical Care, as well as a fee paid by the George Institute for Global Health for serving on their clinical advisory board of Ellen Medical Devices. We would also like to report grants from the Health Systems Research Institute outside of the submitted work. Pornpen Sangthawan reports receiving a speaker honorarium from Baxter Healthcare. Thammasin Ingviya reports having research and workshop grant support from the National Institutes of Health (NIH) (grant No. D43TW009522) for research and training on epidemiology related to TB infection. There are no conflicts of interest related to this research.
Data sharing statement
The data presented in this study are available on request from the corresponding author.
Authors’ contributions
Conceptualization: SC, PS
Data curation: SC, PS, SR, TI
Formal analysis: SR, TI
Methodology: SC, PS, SR, TI, JJ
Writing–original draft: SC
Writing–review & editing: SC
All authors read and approved the final manuscript.
Supplementary Materials
Supplementary data are available at Kidney Research and Clinical Practice online (https://doi.org/10.23876/j.krcp.22.240).
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher M, Yunes M, Mokrzycki MH, Golestaneh L, Alahiri E, Coco M. Chronic hemodialysis patients hospitalized with COVID-19: short-term outcomes in the Bronx, New York. Kidney360. 2020;1:755–762. doi: 10.34067/KID.0003672020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goicoechea M, Sánchez Cámara LA, Macías N, et al. COVID-19: clinical course and outcomes of 36 hemodialysis patients in Spain. Kidney Int. 2020;98:27–34. doi: 10.1016/j.kint.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomacruz ID, So PN, Pasilan RM, Camenforte JK, Duavit MI. Clinical characteristics and short-term outcomes of chronic dialysis patients admitted for COVID-19 in Metro Manila, Philippines. Int J Nephrol Renovasc Dis. 2021;14:41–51. doi: 10.2147/IJNRD.S287455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valeri AM, Robbins-Juarez SY, Stevens JS, et al. Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol. 2020;31:1409–1415. doi: 10.1681/ASN.2020040470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiong F, Tang H, Liu L, et al. Clinical characteristics of and medical interventions for COVID-19 in hemodialysis patients in Wuhan, China. J Am Soc Nephrol. 2020;31:1387–1397. doi: 10.1681/ASN.2020030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gansevoort RT, Hilbrands LB. CKD is a key risk factor for COVID-19 mortality. Nat Rev Nephrol. 2020;16:705–706. doi: 10.1038/s41581-020-00349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hilbrands LB, Duivenvoorden R, Vart P, et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35:1973–1983. doi: 10.1093/ndt/gfaa261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu CM, Weiner DE, Aweh G, et al. COVID-19 among US dialysis patients: risk factors and outcomes from a national dialysis provider. Am J Kidney Dis. 2021;77:748–756. doi: 10.1053/j.ajkd.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salerno S, Messana JM, Gremel GW, et al. COVID-19 risk factors and mortality outcomes among Medicare patients receiving long-term dialysis. JAMA Netw Open. 2021;4:e2135379. doi: 10.1001/jamanetworkopen.2021.35379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu J, Li J, Zhu G, et al. Clinical features of maintenance hemodialysis patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Clin J Am Soc Nephrol. 2020;15:1139–1145. doi: 10.2215/CJN.04160320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talic S, Shah S, Wild H, et al. Effectiveness of public health measures in reducing the incidence of COVID-19, SARS-CoV-2 transmission, and COVID-19 mortality: systematic review and meta-analysis. BMJ. 2021;375:e068302. doi: 10.1136/bmj-2021-068302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yau K, Muller MP, Lin M, et al. COVID-19 outbreak in an urban hemodialysis unit. Am J Kidney Dis. 2020;76:690–695. doi: 10.1053/j.ajkd.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azmandian J, Shafii Z, Ezzatzadegan Jahromi S, et al. The epidemiological characteristics and outcome of COVID-19 in patients undergoing peritoneal dialysis: a multi-center study in Iran. Iran J Kidney Dis. 2022;16:280–283. [PubMed] [Google Scholar]
- 15.Ghonimi TA, Alkad MM, Abuhelaiqa EA, et al. Mortality and associated risk factors of COVID-19 infection in dialysis patients in Qatar: a nationwide cohort study. PLoS One. 2021;16:e0254246. doi: 10.1371/journal.pone.0254246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perl J, Thomas D, Tang Y, et al. COVID-19 among adults receiving home versus in-center dialysis. Clin J Am Soc Nephrol. 2021;16:1410–1412. doi: 10.2215/CJN.04170321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilkie M, Davies S. Peritoneal dialysis in the time of COVID-19. Perit Dial Int. 2020;40:357–358. doi: 10.1177/0896860820921657. [DOI] [PubMed] [Google Scholar]
- 18.Akalin E, Azzi Y, Bartash R, et al. COVID-19 and kidney transplantation. N Engl J Med. 2020;382:2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97:1083–1088. doi: 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson BM, Guedes M, Alghonaim M, et al. Worldwide early impact of COVID-19 on dialysis patients and staff and lessons learned: a DOPPS roundtable discussion. Kidney Med. 2021;3:619–634. doi: 10.1016/j.xkme.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajatanavin N, Tuangratananon T, Suphanchaimat R, Tangcharoensathien V. Responding to the COVID-19 second wave in Thailand by diversifying and adapting lessons from the first wave. BMJ Glob Health. 2021;6:e006178. doi: 10.1136/bmjgh-2021-006178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Worldometer . Worldometer; Thailand COVID - Coronavirus statistics [Internet] 2022 [cited 2022 Oct 24]. Available from: https://www.worldometers.info/coronavirus/country/thailand/ [Google Scholar]
- 23.Marome W, Shaw R. COVID-19 response in Thailand and its implications on future preparedness. Int J Environ Res Public Health. 2021;18:1089. doi: 10.3390/ijerph18031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ikizler TA. COVID-19 and dialysis units: what do we know now and what should we do? Am J Kidney Dis. 2020;76:1–3. doi: 10.1053/j.ajkd.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kliger AS, Silberzweig J. Mitigating risk of COVID-19 in dialysis facilities. Clin J Am Soc Nephrol. 2020;15:707–709. doi: 10.2215/CJN.03340320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaoka K, Nakagawa T, Uno T. Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978;6:165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]
- 27. Department of Administration, Division of Registration Management, Registration Technology Management and Development. Official population statistics from the civil registration (monthly) [Internet]. Registration Statistics System: 2022 [cited 2022 Oct 24]. Available from: https://stat.bora.dopa.go.th/stat/statnew/statMONTH/statmonth/#/mainpage.
- 28.Ortiz AM, Sepúlveda RA, Torres R, et al. Survival study and factors associated with mortality in Chilean patients on peritoneal dialysis infected with SARS-CoV-2. Perit Dial Int. 2022;42:535–539. doi: 10.1177/08968608221087794. [DOI] [PubMed] [Google Scholar]
- 29.Weinhandl ED, Liu J, Gilbertson DT, Wetmore JB, Johansen KL. Associations of COVID-19 outcomes with dialysis modalities and settings. Clin J Am Soc Nephrol. 2022;17:1526–1534. doi: 10.2215/CJN.03400322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Department of Disease Control, Ministry of Public Health of Thailand. Thailand COVID-19 situation report [Internet]. Ministry of Public Health of Thailand: 2020 Dec 23 [cited 2022 Oct 24]. Available from: https://ddc.moph.go.th/viralpneumonia/
- 31.Maldonado M, Ossorio M, Del Peso G, et al. COVID-19 incidence and outcomes in a home dialysis unit in Madrid (Spain) at the height of the pandemic. Nefrologia (Engl Ed) 2021;41:329–336. doi: 10.1016/j.nefroe.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gursu M, Ozturk S, Arici M, et al. Characteristics and survival results of peritoneal dialysis patients suffering from COVID-19 in Turkey: a multicenter national study. Kidney Blood Press Res. 2022;47:605–615. doi: 10.1159/000526909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maimouna M, Menye HF, Nzana V, et al. Burden of COVID-19 in a hemodialysis center in Sub-Saharan Africa. Open J Nephrol. 2022;12:293–302. [Google Scholar]
- 34.Park HC, Lee YK, Ko E, et al. COVID-19-related clinical outcomes among Korean hemodialysis patients. Kidney Res Clin Pract. 2022;41:591–600. doi: 10.23876/j.krcp.22.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anand S, Montez-Rath ME, Han J, et al. Antibody response to COVID-19 vaccination in patients receiving dialysis. J Am Soc Nephrol. 2021;32:2435–2438. doi: 10.1681/ASN.2021050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anand S, Montez-Rath ME, Han J, et al. SARS-CoV-2 vaccine antibody response and breakthrough infection in patients receiving dialysis. Ann Intern Med. 2022;175:371–378. doi: 10.7326/M21-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia P, Anand S, Han J, et al. COVID-19 vaccine type and humoral immune response in patients receiving dialysis. J Am Soc Nephrol. 2022;33:33–37. doi: 10.1681/ASN.2021070936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia P, Han J, Montez-Rath ME, et al. SARS-CoV-2 booster vaccine response among patients receiving dialysis. Clin J Am Soc Nephrol. 2022;17:1036–1038. doi: 10.2215/CJN.00890122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.