Abstract
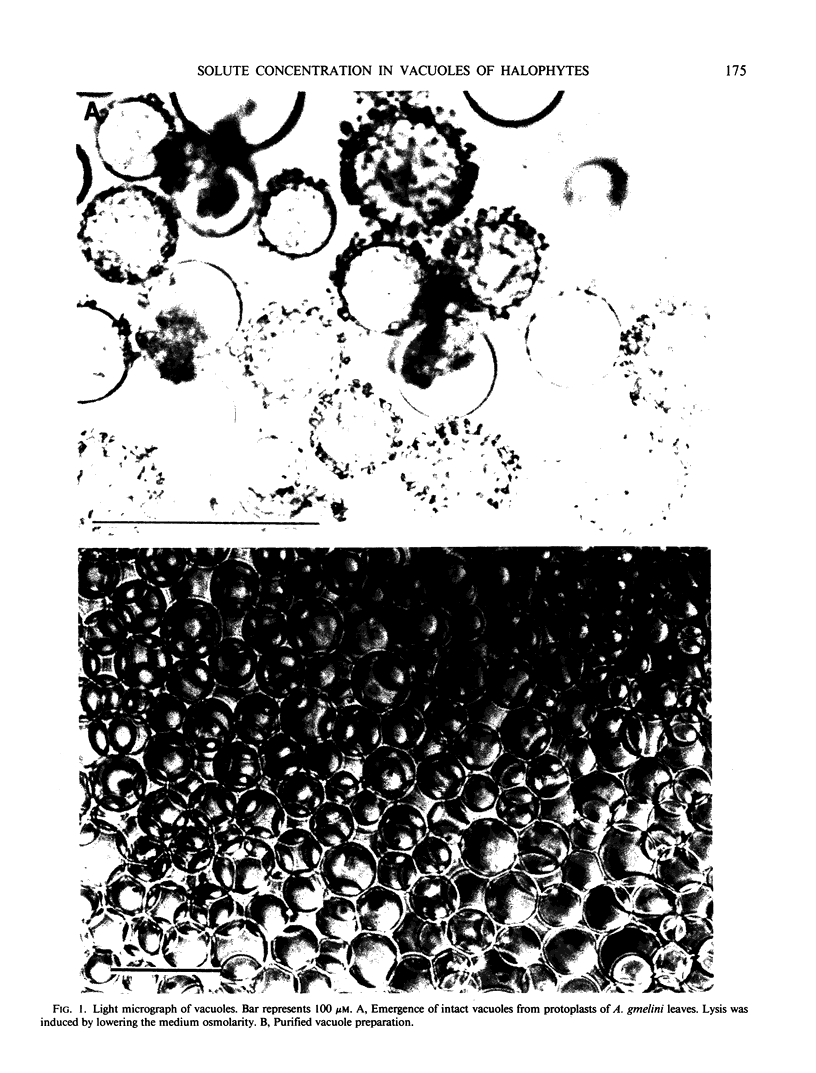
Vacuoles were isolated via protoplasts from the leaves of a halophyte Atriplex gmelini C.A.Mey., grown in culture solution supplemented with 250 millimolar NaCl. Lysis of the protoplasts was induced by lowering the medium osmolarity (1.2 to 1.0 molar sorbitol) and adding a detergent, a synthesized cholate derivative, 3-([3-cholamidopropyl] dimethylammonio)-1-propanesulfonate at a concentration of 0.5 millimolar and the resulting vacuoles were purified by successive dilution and floatation. Isolated vacuoles contained almost the same concentration of sodium (569 millimolar) and chloride (260 millimolar) as recorded in protoplasts (582 and 254 millimolar, respectively), suggesting that the vacuoles are the major sequestration site of NaCl in leaves of halophytes. Betaine concentration in the protoplasts was about 16 millimolar, while that in vacuoles was only about 0.24 millimolar, indicating that betaine is accumulated in the cytoplasm as a compatible solute.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baysdorfer C., Bassham J. A. Spinach pyruvate kinase isoforms : partial purification and regulatory properties. Plant Physiol. 1984 Feb;74(2):374–379. doi: 10.1104/pp.74.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudet A. M., Canut H., Alibert G. Isolation and Characterization of Vacuoles from Melilotus alba Mesophyll. Plant Physiol. 1981 Dec;68(6):1354–1358. doi: 10.1104/pp.68.6.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher H. C., Wagner G. J., Siegelman H. W. Localization of Acid hydrolases in protoplasts: examination of the proposed lysosomal function of the mature vacuole. Plant Physiol. 1977 Jun;59(6):1098–1103. doi: 10.1104/pp.59.6.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway H. Salt responses of enzymes from species differing in salt tolerance. Plant Physiol. 1972 Feb;49(2):256–259. doi: 10.1104/pp.49.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmeland L. M. A nondenaturing zwitterionic detergent for membrane biochemistry: design and synthesis. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6368–6370. doi: 10.1073/pnas.77.11.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinoia E., Schramm M. J., Kaiser G., Kaiser W. M., Heber U. Transport of anions in isolated barley vacuoles : I. Permeability to anions and evidence for a cl-uptake system. Plant Physiol. 1986 Apr;80(4):895–901. doi: 10.1104/pp.80.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettler I. J., Leonard R. T. Isolation and partial characterization of vacuoles from tobacco protoplasts. Plant Physiol. 1979 Dec;64(6):1114–1120. doi: 10.1104/pp.64.6.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Evans H. J. The Influence of Salts on Pyruvate Kinase from Tissues of Higher Plants. Plant Physiol. 1957 Jul;32(4):346–354. doi: 10.1104/pp.32.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M., Maretzki A., Komor E. Vacuoles from Sugarcane Suspension Cultures : I. ISOLATION AND PARTIAL CHARACTERIZATION. Plant Physiol. 1982 Jun;69(6):1315–1319. doi: 10.1104/pp.69.6.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G. J. Vacuolar Deposition of Ascorbate-derived Oxalic Acid in Barley. Plant Physiol. 1981 Mar;67(3):591–593. doi: 10.1104/pp.67.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]