Abstract
An in vitro pharmacokinetic model was used to determine if aztreonam could enhance the pharmacodynamics of cefepime or ceftazidime against an isogenic panel of Pseudomonas aeruginosa 164, including wild-type (WT), partially derepressed (PD), and fully derepressed (FD) phenotypes. Logarithmic-phase cultures were exposed to peak concentrations achieved in serum with 1- or 2-g intravenous doses, elimination pharmacokinetics were simulated, and viable bacterial counts were measured over three 8-h dosing intervals. In studies with cefepime and cefepime-aztreonam against the PD strain, samples were also filter sterilized, assayed for active cefepime, and assayed for nitrocefin hydrolysis activity before and after overnight dialysis. Against WT strains, the cefepime-aztreonam combination was the most active regimen, but viable counts at 24 h were only 1 log below those in cefepime-treated cultures. Against PD and FD strains, the antibacterial activity of cefepime-aztreonam was significantly enhanced over that of each drug alone, with 3.5 logs of killing by 24 h. Hydrolysis and bioassay studies demonstrated that aztreonam was inhibiting the extracellular cephalosporinase that had accumulated and was thus protecting cefepime in the extracellular environment. In contrast to cefepime-aztreonam, the pharmacodynamics of ceftazidime-aztreonam were not enhanced over those of aztreonam alone. Further pharmacodynamic studies with five other P. aeruginosa strains producing increased levels of cephalosporinase demonstrated that the enhanced pharmacodynamics of cefepime-aztreonam were not unique to the isogenic panel. The results of these studies demonstrate that aztreonam can enhance the antibacterial activity of cefepime against derepressed mutants of P. aeruginosa producing increased levels of cephalosporinase. This positive interaction appears to be due in part to the ability of aztreonam to protect cefepime from extracellular cephalosporinase inactivation. Clinical evaluation of this combination is warranted.
Cefepime is the newest of the expanded-spectrum cephalosporins to gain approval from the U.S. Food and Drug Administration for clinical use in the United States. Against genera which characteristically produce Bush group 1 cephalosporinases (7), the intrinsic potency of cefepime surpasses those of ceftazidime and cefotaxime (12, 16). Furthermore, many derepressed mutants of Enterobacter spp., Citrobacter freundii, Serratia sp., and other members of the family Enterobacteriaceae which are resistant to cefotaxime and ceftazidime remain susceptible to cefepime (13). Pseudomonas aeruginosa, however, remains a potential therapeutic problem. Like ceftazidime, cefepime is moderately active against wild-type isolates of P. aeruginosa, with MICs at which 50% of isolates are inhibited generally ranging from 2 to 4 μg/ml (8, 12, 20, 21). When expression of the P. aeruginosa chromosomal enzyme is increased via induction or derepression, susceptibility to both drugs decreases up to 16-fold, often resulting in clinical resistance and possible treatment failures (13).
One approach that has been used to circumvent β-lactamase-mediated resistance is to combine an enzyme-labile drug with an inhibitor of the β-lactamase. However, neither tazobactam, sulbactam, nor clavulanic acid inhibits the chromosomal cephalosporinase of P. aeruginosa sufficiently to be useful in this setting (1). Aztreonam, in contrast, has been shown to be a potent inhibitor of the chromosomal cephalosporinases from Enterobacter cloacae, C. freundii, Serratia spp., and P. aeruginosa (5, 6, 15, 26). Although hydrolysis does eventually occur when aztreonam interacts with these Bush group 1 cephalosporinases, the half-lives of these reactions are long enough that the enzymes remain inactive through several generations of bacterial growth (5). Therefore, aztreonam acts as a competitive inhibitor of Bush group 1 cephalosporinases by serving as a poor substrate. Whether this competitive inhibition can translate into the ability of aztreonam to protect β-lactam antibiotics from these enzymes has not been examined systematically. Therefore, an in vitro pharmacokinetic model was used to determine if aztreonam could enhance the antibacterial activity of cefepime against strains of P. aeruginosa expressing various levels of the chromosomal Bush group 1 cephalosporinase. For comparative purposes, the pharmacodynamics of ceftazidime alone and in combination with aztreonam were also evaluated.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Eight strains of P. aeruginosa were selected for this study. P. aeruginosa 164 was a wild-type clinical isolate. P. aeruginosa 164PD and P. aeruginosa 164FD were partially derepressed (PD) and fully derepressed (FD) isogenic mutants, respectively, selected from wild-type strain 164 in the laboratory (14). Mutant 164PD was selected by single passage of wild-type strain 164 in broth containing 32 μg of cefotaxime per ml and expressed moderate basal levels of its chromosomal cephalosporinase; for this strain cephalosporinase expression could be induced to high levels by cefoxitin (14) (see Table 1). Mutant FD was selected by single passage of wild-type strain 164 in Mueller-Hinton agar (MHA) containing 64 μg of ceftazidime per ml and expressed high basal levels of its chromosomal cephalosporinase in a constitutive manner (see Table 1). P. aeruginosa 111M and P. aeruginosa 113M were mutants selected in the laboratory by single passages of wild-type strains 111 and 113, respectively, in MHA containing 128 μg of cefotaxime per ml. Mutant 111M was similar to 164PD in that it produced moderate basal levels of its chromosomal cephalosporinase, and for this strain cephalosporinase production could be induced to high levels with cefoxitin (see Table 1). Mutant 113M expressed moderate basal levels of the chromosomal cephalosporinase in a constitutive manner (see Table 1). P. aeruginosa GB3, P. aeruginosa GB57, and P. aeruginosa GB66 were all clinical isolates which constitutively expressed high basal levels of their chromosomal cephalosporinases (see Table 1). All isolates were stored at −70°C in brain heart infusion broth (BBL Microbiology Systems, Cockeysville, Md.) supplemented with 50% sterile horse serum (Colorado Serum Company, Denver, Colo.). Strain purity was confirmed by subculturing freezer stocks onto Trypticase soy agar supplemented with 5% sheep blood (Blood Agar Plate [BAP]; BBL).
TABLE 1.
Cephalosporinase activity and susceptibilities of test strains
| Strain | Cephalosporinase activitya
|
MIC (μg/ml)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Uninduced | Induced | Cefepime | Ceftazidime | Cefotaxime | Aztreonam | Aztreonam + cefepimeb | Aztreonam + ceftazidimeb | |
| P. aeruginosa 164 | 5 | 87 | 8 | 4 | 32 | 8 | 8/4 | 4/2 |
| P. aeruginosa 164PD | 38 | 429 | 16 | 32 | >128 | 16 | 32/16 | 32/16 |
| P. aeruginosa 164FD | 1,640 | 1,810 | 128 | >128 | >128 | >128 | 128/64 | 128/64 |
| P. aeruginosa 113M | 70 | 275 | 16 | 32 | >128 | 16 | 32/16 | 32/16 |
| P. aeruginosa 111M | 142 | 169 | 16 | 32 | >128 | 16 | 32/16 | 32/16 |
| P. aeruginosa GB57 | 971 | 1,040 | 64 | >128 | >128 | 128 | 128/64 | 128/64 |
| P. aeruginosa GB66 | 971 | 1,150 | 64 | >128 | >128 | >128 | 128/64 | 128/64 |
| P. aeruginosa GB3 | 1,270 | 1,290 | 64 | 64 | >128 | 64 | 128/64 | 128/64 |
Cephalosporinase activity (nanomoles of cephalothin hydrolyzed per minute per milligram of protein) in sonic extracts of logarithmic-phase cultures treated for 2 h with 25 μg of cefoxitin per ml (induced). Uninduced cultures were supplemented with drug-free broth and were incubated for a similar 2-h period.
Tested at a ratio of 2 parts aztreonam to 1 part cefepime or ceftazidime.
For in vitro pharmacodynamic studies, logarithmic-phase cultures were prepared by inoculating colonies from an overnight BAP culture into 70 ml of Mueller-Hinton broth (MHB; BBL) to equal an optical density at 540 nm of 0.1. The broth culture was then incubated at 37°C with shaking for 2 h until an optical density of 0.4 to 0.5 was achieved and was then diluted 10-fold to a final inoculum concentration of 107 to 108 CFU/ml.
Antibiotic preparations.
Cefepime and aztreonam powders were supplied by Bristol-Myers Squibb Co. (International Health Management Associates, Inc., Chicago, Ill.), and ceftazidime powder was supplied by Glaxo Inc., Hertfordshire, England. Antibiotic powders were reconstituted according to the recommendations of the National Committee for Clinical Laboratory Standards (19), sterilized by passage through a 0.20-μm-pore-size Acrodisc filter membrane syringe (Gelman Sciences, Ann Arbor, Mich.), and diluted to the desired concentrations with the recommended diluents (19).
Antimicrobial susceptibility testing.
Susceptibility testing with cefepime, aztreonam, ceftazidime, cefepime-aztreonam, and ceftazidime-aztreonam was performed by the agar dilution method by the procedure recommended by the National Committee for Clinical Laboratory Standards (19). Since the peak concentrations of aztreonam are generally higher than those of cefepime and ceftazidime with similar doses (18), the combinations of cefepime-aztreonam and ceftazidime-aztreonam were tested at a ratio of 2:1 (2 parts aztreonam to 1 part cefepime or ceftazidime).
β-Lactamase characterization.
For β-lactamase analysis, 5-ml aliquots from overnight MHB cultures were transferred to centrifuge bottles containing 95 ml of sterile MHB, and the bottles were incubated at 37°C with shaking for 1.5 h to achieve logarithmic-phase growth. After 1.5 h of incubation, 1 ml of either sterile normal saline or 5,000 μg of cefoxitin per ml (final concentration, 50 μg/ml) was added to the cultures, and the cultures were allowed to incubate for an additional 2 h with shaking at 37°C. Protein synthesis was halted after 2 h by the addition of 1 ml of 1 mM 8-hydroxyquinoline. The cells were collected by centrifugation at 5,858 × g for 20 min, washed once in 0.1 M phosphate buffer (4 g of KH2PO4, 13.6 g of K2HPO4), and collected by centrifugation as described above. The supernatants were discarded and the pellets were frozen overnight at −20°C. On the following day the bacterial pellets were resuspended in 4 ml of 0.1 M phosphate buffer and were lysed by sonication with an ultrasonic disintegrator (Bronwill Scientific, Rochester, N.Y.). The cellular debris was removed from each sonicate by centrifugation for 1 h at 5,858 × g at 4°C. Immediately after removal of the cellular debris, the sonicates were assayed for protein content (4) and β-lactamase activity was measured spectrophotometrically, with cephalothin (28) or nitrocefin (23) serving as the hydrolysis substrates. In addition, the sonicates were also evaluated for the presence of plasmid-encoded β-lactamases by isoelectric focusing and a nitrocefin overlay method (27).
IVPM.
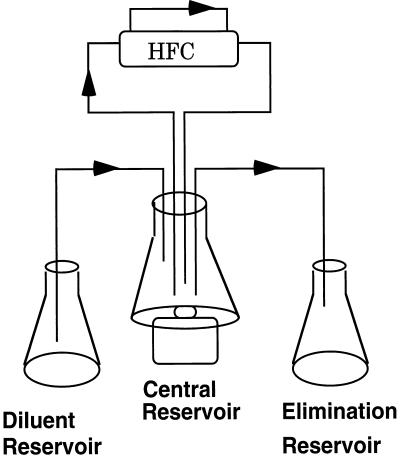
The basics of the in vitro pharmacokinetic model (IVPM) used in these studies have been described in detail previously (2). A schematic representation of the model is presented in Fig. 1. A hollow-fiber cartridge (Unisyn Fibertech, San Diego, Calif.) was connected with a continuous loop of silicone tubing to a central reservoir. Each hollow-fiber cartridge consisted of 2,250 cellulose acetate hollow fibers contained within a polycarbonate housing. At the start of each experiment, peak antibiotic concentrations in MHB in the central reservoir were pumped through the hollow fibers of the cartridge and back into the central reservoir (Fig. 1). As drug-containing MHB passed through the hollow fibers, pores in the fiber walls allowed antibiotic and nutrients to freely diffuse from the lumen of the fibers into the space surrounding the hollow fibers within the cartridge (peripheral compartment) and back into the lumen of the hollow fibers. The exclusion size of the pores in the fiber walls (molecular weight limit, 30,000) prohibited the bacteria introduced into the peripheral compartment from entering the lumen of the hollow fibers. Thus, the drug concentration within the peripheral compartment space could be altered without disrupting bacterial growth. The total surface area of exchange between the lumen of the hollow fibers and the peripheral compartment was 1.5 ft2. The bacterial culture within the peripheral compartment was continuously circulated through a loop of silicone tubing attached to two ports entering and exiting the peripheral compartment, and samples were removed from the peripheral compartment through a three-way stopcock connected within the loop of silicone tubing. The initial volume of culture that circulated through the peripheral compartment and silicone tubing was 30 to 35 ml.
FIG. 1.
Schematic representation of the two-compartment pharmacokinetic model. Each arrow represents a peristaltic pump within the system. Peak concentrations of antibiotic were dosed into the central reservoir and were pumped through the lumens of hollow fibers in the hollow-fiber cartridge (HFC). Pores (molecular weight limit, 30,000) in the fiber walls allowed antibiotic to diffuse freely from the lumen of the hollow fibers into the peripheral compartment of the hollow-fiber cartridge where the bacteria were inoculated. Antibiotic was eliminated from the central reservoir by the addition of drug-free broth from a diluent reservoir and elimination of drug-containing broth into the elimination reservoir. As the antibiotic concentrations in the central reservoir decreased, the antibiotic concentrations within the peripheral compartment also decreased as drug diffused into the lumens of the hollow fibers to maintain equilibrium between the two compartments.
A comparison of antibiotic concentrations between the central reservoir and the peripheral compartment every 15 min after dosing demonstrated that equilibrium was established between the compartments at approximately 0.5 h. After peak concentrations were achieved within the peripheral compartment, the human elimination pharmacokinetics of cefepime, aztreonam, and ceftazidime were simulated by a process of dilution and elimination of drug in the central reservoir. The drug concentrations in the central reservoir (and in the peripheral compartment as equilibrium was maintained) were decreased by the addition of drug-free MHB from a dilution reservoir (Fig. 1). To maintain a constant volume in the central reservoir, drug-containing MHB was pumped from the central reservoir into an elimination reservoir (Fig. 1). The rate at which the drug concentrations in the central reservoir and peripheral compartment were decreased by this method was determined from the flow rate of the peristaltic pumps. This rate was calculated from an equation for clearance by monoexponential decline, based on the serum half-lives of cefepime, aztreonam, and ceftazidime and the volume of medium in the central reservoir. An elimination half-life of 2 h for each of the drugs was simulated by this method (18).
Pharmacokinetics of cefepime, ceftazidime, and aztreonam in the IVPM.
The peak concentrations of cefepime, aztreonam, and ceftazidime were introduced into the central compartment of the IVPM. The peak concentrations targeted for the 2-g intravenous doses were 160 μg/ml for cefepime, 130 μg/ml for ceftazidime, and 210 μg/ml for aztreonam (18). The corresponding peak concentrations for the 1.0-g intravenous doses were 80 μg/ml for cefepime, 60 μg/ml for ceftazidime, and 90 μg/ml for aztreonam (18). In combination studies with the 2-g doses, peak concentrations of aztreonam of 210 μg/ml were dosed simultaneously into the central reservoir with 160 μg of cefepime per ml or 130 μg of ceftazidime per ml. Similarly, for the 1-g dose studies, peak concentrations of aztreonam of 90 μg/ml were dosed simultaneously into the central reservoir with 80 μg of cefepime per ml or 60 μg of ceftazidime per ml. To measure the levels of cefepime, aztreonam, or ceftazidime in the IVPM, samples were removed from the peripheral compartment at 0, 0.5, 1, 2, 4, 6, and 8 h after dosing into the central reservoir. The concentrations of each drug, when dosed alone, were measured by a disk diffusion microbiological assay (11) with a susceptible strain of Klebsiella pneumoniae. When aztreonam was dosed in combination with the cephalosporins, the concentrations of the cephalosporins were measured by a disk diffusion microbiological assay with a susceptible strain of Staphylococcus aureus.
Pharmacodynamics against P. aeruginosa in the IVPM.
Logarithmic-phase cultures (107 to 108 CFU/ml) of each strain were introduced into the peripheral compartment of the IVPM and were exposed to cefepime, aztreonam, and ceftazidime alone or were exposed to the combinations cefepime-aztreonam and ceftazidime-aztreonam. The 1.0-g dose of each drug alone and the two combinations were evaluated against wild-type P. aeruginosa 164 and derepressed mutants P. aeruginosa 164PD, P. aeruginosa, 111M, and P. aeruginosa 113M. The 2.0-g dose of each drug alone and the two combinations were evaluated against derepressed mutants P. aeruginosa 164FD, P. aeruginosa GB3, P. aeruginosa GB57, and P. aeruginosa GB66. To determine if any enhanced pharmacodynamic interactions with the combinations could be due to just the increased total dose of antibiotic and more favorable pharmacokinetics, the pharmacodynamics of a doubled dose of the most active single drug in the combination were evaluated. For example, with the 1-g combination studies, a 2-g dose of the most active single agent was evaluated. For the 2-g combination studies, a 4-g dose of the most active single agent was evaluated. Antibiotic regimens were dosed at 0, 8, and 16 h after introduction of the bacterial culture into the peripheral compartment. At 0, 1, 2, 4, 6, 8, 16, and 24 h, 400-μl samples removed from the peripheral compartment of the IVPM were treated for 15 min at 37°C with 100 μl of type III cephalosporinase from culture supernatants of E. cloacae (BBL) to inactivate residual antibiotic. Viable bacterial counts were measured by plating serial 10-fold dilutions of each sample into MHA (BBL). In the 1-g dose studies, viable bacterial counts were also measured at 10 h. The least-diluted sample plated was 0.1 ml of undiluted sample from the peripheral compartment. Since 30 colonies is the lower limit of accurate quantitation by the pour plate method, the lowest number of bacteria that could be accurately counted was 300 CFU/ml. The lowest level of detection, although actual counts were inaccurate, was 10 CFU/ml. To evaluate for the selection of mutants with decreased susceptibilities to cephalosporins and aztreonam, samples taken at 24 h from experiments with wild-type P. aeruginosa 164 and mutants P. aeruginosa 164PD, P. aeruginosa 111M, and P. aeruginosa 113M were also plated into agar containing antibiotic at a concentration fourfold above the MIC. Antibiotic selection plates were not used in studies with mutants P. aeruginosa 164FD, P. aeruginosa GB3, P. aeruginosa GB57, and P. aeruginosa GB66 due to the high level of resistance that these strains already exhibited.
Extracellular cephalosporinase accumulation during treatment of P. aeruginosa 164PD.
To evaluate the role of aztreonam in inhibiting extracellular cephalosporinase during the treatment of the PD mutant P. aeruginosa 164PD, logarithmic-phase cultures (5 × 107 CFU/ml) were introduced into the peripheral compartment of the IVPM and were exposed to 1-g doses of cefepime alone and to a combination of cefepime-aztreonam at 0 and 8 h. Samples were removed from the peripheral compartment at 0, 1, 2, 4, 6, 8, 8.5, 9, 10, 12, 14, and 16 h and filter sterilized to remove the bacteria. Sterile supernatants were then divided into two aliquots. One aliquot was assayed for active cefepime by a microbiological assay with S. aureus as the indicator organism and was analyzed spectrophotometrically for cephalosporinase activity with nitrocefin as the substrate. The second aliquot was dialyzed against 1,000 ml of phosphate buffer at 4°C. After 24 h of dialysis, the samples were assayed for cephalosporinase activity as described above.
RESULTS
Characterization of test strains and the IVPM.
The susceptibilities of the test strains to cefepime, ceftazidime, cefotaxime, aztreonam, cefepime-aztreonam, and ceftazidime-aztreonam and the levels of uninduced and induced chromosomal cephalosporinase expression are presented in Table 1. No plasmid-mediated β-lactamases were detected in these strains. Mutants P. aeruginosa 164PD, P. aeruginosa 111M, and P. aeruginosa 113M produced moderately increased basal levels of their chromosomal cephalosporinase that were 7 to 30 times greater than those in wild-type P. aeruginosa 164. In the presence of cefoxitin, cephalosporinase expression was increased 10-fold in P. aeruginosa 164PD and 4-fold in P. aeruginosa 113M, whereas cephalosporinase expression in 111M was no longer inducible. The FD mutants P. aeruginosa 164FD, P. aeruginosa GB3, P. aeruginosa GB57, and P. aeruginosa GB66 constitutively produced levels of cephalosporinase that were 200 to 330 times those in wild-type P. aeruginosa 164. As the levels of cephalosporinase production increased, susceptibility to cefepime, aztreonam, and ceftazidime decreased. Aztreonam did not enhance the activity of cefepime or ceftazidime in agar dilution assays, because the MICs obtained with the combinations were similar to those obtained with each drug alone.
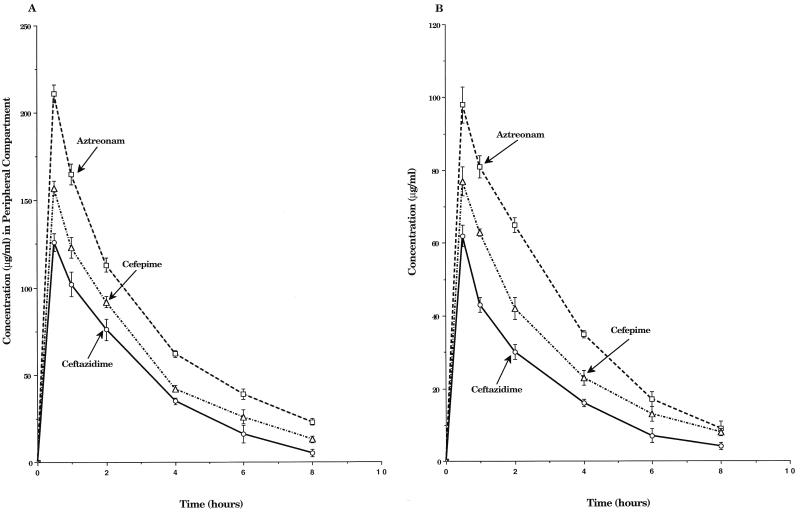
The single-dose pharmacokinetic profiles of cefepime, aztreonam, and ceftazidime in the IVPM are shown in Fig. 2. The peak levels of cefepime, ceftazidime, and aztreonam (mean ± standard deviation [SD]) achieved in the peripheral compartment of the IVPM when 2.0-g doses were simulated were 156 ± 6, 126 ± 8, and 215 ± 10 μg/ml, respectively. Corresponding peak levels for the 1.0-g doses were 77 ± 4 μg/ml for cefepime, 62 ± 6 μg/ml for ceftazidime, and 98 ± 5 μg/ml for aztreonam. Calculated half-lives ranged from 1.9 to 2.1 h for the three drugs. The simultaneous dosing of aztreonam with cefepime or ceftazidime did not substantially alter the pharmacokinetics of either cephalosporin (data not shown).
FIG. 2.
Single-dose pharmacokinetic profiles of 2.0-g (A) and 1.0-g (Panel B) intravenous doses of cefepime, ceftazidime, and aztreonam in the peripheral compartment of the IVPM after dosing peak concentrations into the central reservoir. Drug levels were measured by bioassay. Each datum point represents the mean drug level in the peripheral compartment (in micrograms per milliliter) for duplicate experimental runs. Error bars show SDs.
Pharmacodynamics against P. aeruginosa 164 and its derepressed mutants.
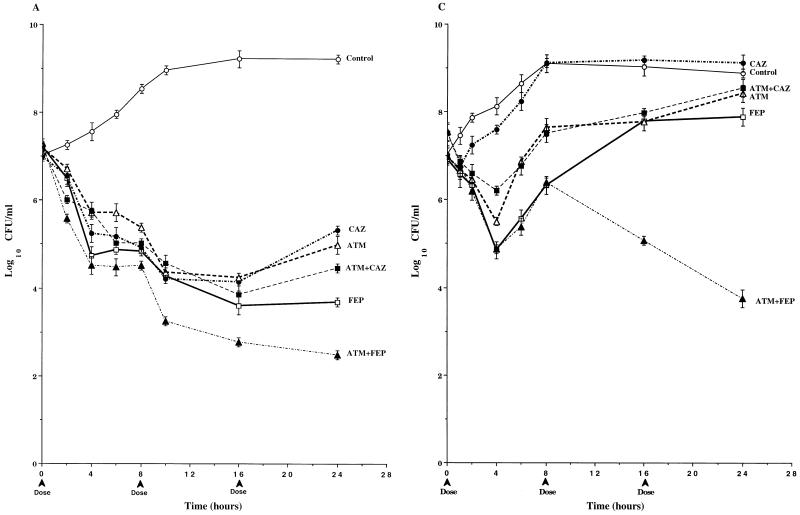
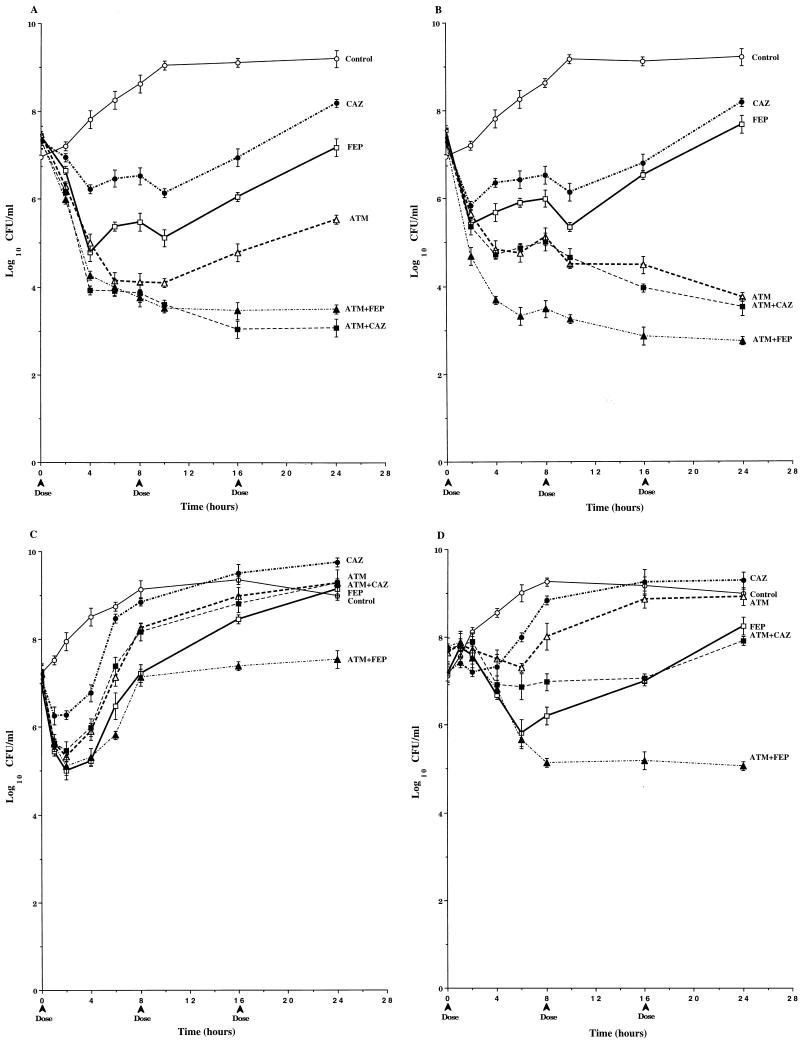
In studies with P. aeruginosa 164 (Fig. 3A), the 1-g dose of cefepime was the most active of the single-drug regimens, with 3.5 logs of killing over three dosing cycles. The pharmacodynamics of 1-g doses of ceftazidime and aztreonam were similar to those of cefepime over the first two dosing cycles. However, the third doses of these drugs were less effective, and bacterial counts at 24 h were about 1 log higher than those at 16 h and were 1.5 logs higher than those in cultures treated with cefepime. The pharmacodynamics of the ceftazidime-aztreonam combination were very similar to those of the individual drugs. The combination of cefepime-aztreonam was the most active of all the regimens tested, with 4.5 logs of killing over three dosing cycles. When a 2-g dose of cefepime was simulated, mean viable counts at 24 h were 1.5 logs above those in cultures treated with cefepime-aztreonam (Table 2). No mutants were detected in any of the drug-treated cultures (Table 2).
FIG. 3.
Time-kill pharmacodynamics of 1.0-g (A and B) and 2.0-g (C) doses of cefepime (FEP), ceftazidime (CAZ), and aztreonam (ATM) alone and combinations of cefepime-aztreonam (FEP+ATM) and ceftazidime-aztreonam (CAZ+ATM) against wild-type P. aeruginosa 164 (A), PD isogenic mutant P. aeruginosa 164PD (B), and FD isogenic mutant P. aeruginosa 164FD (C). Each datum point represents the mean numbers of CFU per milliliter of MHB from the peripheral compartment for duplicate experiments. Error bars show SDs.
TABLE 2.
Viable bacterial counts after three dosing cycles with 1- or 2-g doses of cefepime, ceftazidime, aztreonam, ceftazidime-aztreonam, and cefepime-aztreonam
| Strain | Mean total log10 CFU/ml at 24 h (mean mutant log10 CFU/ml)a
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Drug-free control | Cefepime (1 g) | Cefepime (2 g) | Ceftazidime (1 g) | Ceftazidime (2 g) | Aztreonam (1 g) | Aztreonam (2 g) | Ceftazidime + aztreonamb | Cefepime + aztreonamb | |
| P. aeruginosa 164 | 9.2 | 3.8 (0) | 3.9 (0) | 5.2 (0) | NDc | 5.0 (0) | ND | 4.5 (0) | 2.5 (0) |
| P. aeruginosa 164PD | 9.1 | 9.5 (8.3) | 6.2 (0) | 9.5 (5.6) | ND | 9.3 (5.9) | 5.5 (0) | 8.4 (7.3) | 3.1 (2.3) |
| P. aeruginosa 164FD | 8.9 | ND | 7.9 | ND | 9.0 | ND | 8.3 | 8.4 | 3.8 |
| P. aeruginosa 113M | 9.2 | 7.2 (0) | ND | 8.0 (0) | ND | 5.5 (0) | 4.9 (0) | 3.3 (0) | 3.5 (0) |
| P. aeruginosa 111M | 9.2 | 7.8 (0) | ND | 8.1 (0) | ND | 3.9 (0) | 3.5 (0) | 3.8 (0) | 2.8 (0) |
| P. aeruginosa GB57 | 9.0 | ND | 9.0 | ND | 9.7 | ND | 9.2 | 9.2 | 7.5 |
| P. aeruginosa GB66 | 9.0 | ND | 8.2 | ND | 9.4 | ND | 8.9 | 7.9 | 5.0 |
| P. aeruginosa GB3 | 9.0 | ND | 9.0 | ND | 9.5 | ND | 7.8 | 7.8 | 5.9 |
The number of mutants was measured by plating the samples used to determine total viable bacterial counts into agar containing antibiotic at a concentration fourfold above the MIC.
Simulated 1- or 2-g doses of aztreonam were dosed simultaneously with 1- or 2-g doses of either cefepime or ceftazidime.
ND, not done.
In contrast to the results obtained with P. aeruginosa 164 (Fig. 3A), the single-drug regimens (1-g doses) were relatively ineffective against P. aeruginosa 164PD (Fig. 3B). Viable bacterial counts in cultures treated with the single-drug regimens exceeded 109 CFU/ml by 24 h, despite 1 to 2 logs of killing within the first 4 h after introduction of the initial dose. In addition, mutants with increased levels of resistance to cephalosporins and aztreonam were selected from cultures treated with all three single-drug regimens at a 1-g dose (Table 2). These mutants were similar to P. aeruginosa 164FD in their susceptibility profiles and levels of cephalosporinase expression (data not shown) and reached 2.2 × 108 CFU/ml in cefepime-treated cultures, 3.6 × 105 CFU/ml in ceftazidime-treated cultures, and 7.5 × 105 CFU/ml in aztreonam-treated cultures (Table 2). Similar mutants were also selected in cultures treated with the ceftazidime-aztreonam combination, and these mutants accounted for 1.9 × 107 CFU/ml of the 2.8 × 108 CFU of total viable bacteria per ml at 24 h (Table 2). Although mutants were detected in cultures treated with the cefepime-aztreonam combination, the viable bacterial counts after three dosing cycles were 4 logs below the initial inoculum, with the number of mutants reaching only 2 × 102 CFU/ml (Table 2). Furthermore, at 24 h the viable counts in cultures treated with 1-g doses of cefepime-aztreonam were 2.4 to 3.1 logs below those in cultures treated with 2-g doses of cefepime and aztreonam alone, even though these single drug regimens were able to completely eliminate all of the highly resistant populations.
Due to its high level of resistance to the study drugs, 2-g doses were evaluated against P. aeruginosa 164FD. As seen in Fig. 3C, the 2.0-g dose of ceftazidime demonstrated little if any antibacterial activity, with viable bacterial counts paralleling those in drug-free cultures throughout most of the three dosing cycles. The 2.0-g doses of cefepime and aztreonam decreased viable bacterial counts approximately 2 logs during the first dosing interval. However, by the end of the third dosing cycle, viable counts rose above the initial inoculum in cultures treated with both drugs and surpassed 108 CFU/ml in cultures treated with aztreonam. The pharmacodynamics of the ceftazidime-aztreonam combination were similar to those of aztreonam alone. In contrast, the cefepime-aztreonam combination decreased the viable bacterial counts by 3.5 logs by 24 h, which was at least 4.5 logs below the counts in cultures treated with ceftazidime, aztreonam, and ceftazidime-aztreonam (Table 2) and almost 3 logs below the counts in cultures treated with a 4-g dose of cefepime (data not shown).
Effect of aztreonam on the accumulation of extracellular cephalosporinase during treatment of P. aeruginosa 164PD.
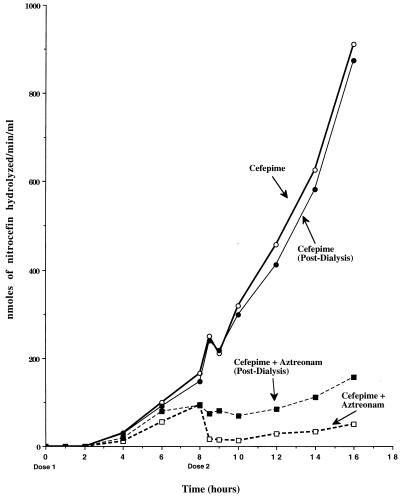
The levels of accumulation of extracellular cephalosporinase in the peripheral compartment of the IVPM during treatment of P. aeruginosa 164PD are shown in Fig. 4. In the cultures treated with cefepime alone, extracellular cephalosporinase activity first became detectable 4 h after introduction of the first dose and gradually increased through 8 h. With the second dose of cefepime at 8 h, a small decrease followed by a rapid increase through 16 h was observed. Furthermore, the levels of cephalosporinase activity after overnight dialysis were not substantially different from those measured immediately after removal of the sample from the peripheral compartment. In cultures treated with cefepime-aztreonam, the kinetics of extracellular cephalosporinase activity were similar to those in cultures treated with cefepime through 6 h. Between 6 and 8 h, however, the level of extracellular cephalosporinase remained relatively constant. The most striking differences between the two regimens were observed over the second dosing interval. After the introduction of the second dose of cefepime-aztreonam, extracellular cephalosporinase activity dropped to undetectable levels and increased only slightly between 10 and 16 h. After overnight dialysis of the samples removed during the second dosing interval, the amount of extracellular cephalosporinase was found to be similar to that observed just prior to introduction of the second dose. The concentrations of active extracellular cefepime 1 h after introduction of the second dose of each regimen were 50 μg/ml in cultures treated with cefepime-aztreonam, whereas they were 15 μg/ml in cultures treated with cefepime alone. By 2 h after introduction of the second dose, active cefepime was undetectable in cultures treated with cefepime alone, whereas the concentration in cultures treated with cefepime-aztreonam was 30 μg/ml.
FIG. 4.
Kinetics of extracellular cephalosporinase accumulation during the treatment of P. aeruginosa 164PD with cefepime and cefepime-aztreonam. Cephalosporinase activity was measured spectrophotometrically at 489 nm with nitrocefin as the substrate. Each datum point represents the nanomoles of nitrocefin hydrolyzed per minute per milliliter of filter-sterilized culture from the peripheral compartment. Samples removed at each time point were divided into two aliquots to measure cephalosporinase activity before and after overnight dialysis against phosphate buffer.
Pharmacodynamics against other derepressed mutants of P. aeruginosa.
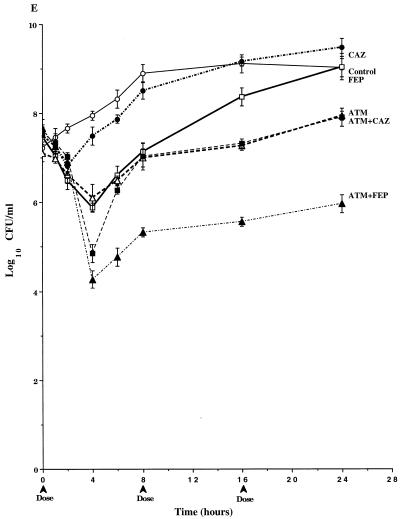
In studies with P. aeruginosa 113M (Fig. 5A), the overall pharmacodynamics of 1-g doses of cefepime and ceftazidime were similar. However, the initial dose of cefepime was more active than ceftazidime, and this difference was reflected throughout the second and third dosing intervals. By the end of the third dosing interval (24 h), viable counts in cefepime-treated cultures approached those in the original inoculum. They surpassed the original inoculum in ceftazidime-treated cultures. The 1-g dose of aztreonam was the most active of the single-drug regimens against P. aeruginosa 113M. In contrast to cefepime and ceftazidime, at 24 h the viable bacterial counts in aztreonam-treated cultures remained 2.0 logs below the initial inoculum. The pharmacodynamics of the cefepime-aztreonam and ceftazidime-aztreonam combinations were very similar, and these combinations were the most active of the regimens tested. Total bacterial killing with these regimens was about 4 logs after three dosing cycles. When a 2-g dose of aztreonam was simulated, mean viable counts at 24 h were 2 logs higher than those in cultures treated with the combinations (Table 2). No mutants were detected in any of the drug-treated cultures (Table 2).
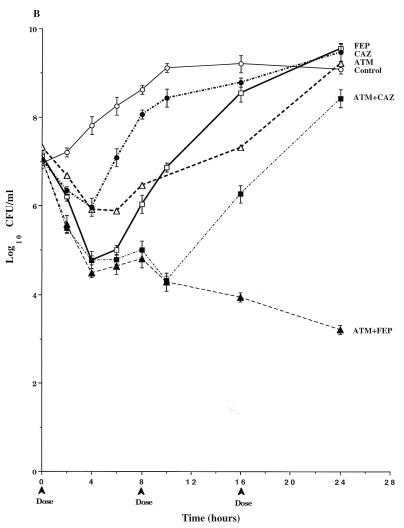
FIG. 5.
Time-kill pharmacodynamics of 1.0- and 2.0-g doses of cefepime (FEP), ceftazidime (CAZ), and aztreonam (ATM) alone and combinations of cefepime-aztreonam (FEP+ATM) and ceftazidime-aztreonam (CAZ+ATM) against derepressed mutants P. aeruginosa 113M (A), P. aeruginosa 111M (B), P. aeruginosa GB57 (C), P. aeruginosa GB66 (D), and P. aeruginosa GB3. Each datum point represents the mean numbers of CFU per milliliter of MHB from the peripheral compartment for duplicate experiments. Error bars show SDs.
In studies with P. aeruginosa 111M (Fig. 5B), the 1-g doses of cefepime and ceftazidime were similar in their pharmacodynamics with a rapid 2-log decrease in viable bacterial counts over the first 2 h, followed by slight increases in viable counts through 8 h. The second and third doses of cefepime and ceftazidime (at 8 h and 16 h) produced less killing than the first doses, with net increases in viable counts between 10 and 24 h. Similar to studies with P. aeruginosa 113M, the 1-g dose of aztreonam was the most active of the single-drug regimens, with almost a 4-log decrease in viable counts by the end of the third dosing cycle. The killing kinetics observed with ceftazidime-aztreonam were similar to those observed with aztreonam alone. The combination of cefepime-aztreonam was the most active regimen over the first dosing interval, with 4 logs of killing by 8 h (Fig. 5B). Viable bacterial counts at 24 h in cultures treated with cefepime-aztreonam were approximately 1 log lower than those in cultures treated with aztreonam or ceftazidime-aztreonam. When a 2-g dose of aztreonam was simulated, mean viable counts at 24 h were still 0.7 log above those in cultures treated with cefepime-aztreonam (Table 2). No mutants were detected in any of the drug-treated cultures (Table 2).
In studies with P. aeruginosa GB57 (Fig. 5C), all of the 2-g single-drug regimens exhibited bacterial killing over the initial 2 h of the first dosing interval, with ceftazidime decreasing viable counts the least. After 2 h, the viable bacterial counts began to increase in all drug-treated cultures. In cultures treated with 2-g doses of ceftazidime, aztreonam, cefepime, and ceftazidime-aztreonam, the viable counts continued to increase until they equaled or surpassed those in drug-free control cultures by 16 to 24 h. In contrast, viable bacterial counts after the second and third dosing cycles with cefepime-aztreonam changed very little from those at 8 h and equaled the initial inoculum. Furthermore, by the end of the third dosing cycle, the viable counts in cefepime-aztreonam-treated cultures were 2 or more logs below those for all the other regimens with 2-g doses (Table 2) as well as that for the regimen simulating a 4-g dose of cefepime alone (data not shown).
In studies with P. aeruginosa GB66 (Fig. 5D), the pharmacodynamics of the 2.0-g doses of ceftazidime and aztreonam were similar, with viable bacterial counts varying little from the initial inoculum over the first 4 to 6 h after dosing. By the end of the second dosing cycles with ceftazidime and aztreonam, viable counts were similar to those in untreated control cultures. In cultures treated with the 2.0-g dose of cefepime, viable counts rapidly decreased 2 logs by 6 h. However, the second and third doses of cefepime were less effective than the first dose, with the viable counts at 24 h reaching the level of the initial inoculum. In cultures treated with the combination of ceftazidime-aztreonam, viable counts varied little over the three dosing cycles. In contrast, the combination of cefepime-aztreonam exhibited bacterial killing throughout the first dosing cycle. Although the viable counts after the second and third dosing cycles did not change from those at 8 h, the viable counts in cultures treated with cefepime-aztreonam remained 2.5 logs below the initial inoculum and approximately 3 logs below those in cultures treated with cefepime alone and the combination of ceftazidime-aztreonam (Table 2). When a 4-g dose of cefepime was simulated, the viable counts after three dosing cycles still remained almost 3 logs above those in cultures treated with cefepime-aztreonam (data not shown).
In studies with P. aeruginosa GB3 (Fig. 5E), the 2.0-g dose of ceftazidime exhibited pharmacodynamics similar to those observed against P. aeruginosa 164FD (Fig. 3C). Although the 2.0-g dose of cefepime exhibited bacterial killing over a portion of the first dosing interval, the viable counts after three dosing cycles equaled those in drug-free control cultures. The pharmacodynamics of the 2.0-g dose of aztreonam were very similar to those of cefepime over the first dosing interval. However, after three dosing cycles with aztreonam alone, the viable counts remained just below 108 CFU/ml. The two aztreonam-containing combinations exhibited the greatest killing activity of all the regimens over the first dosing interval, with 2.5 to 3 logs of killing before regrowth initiated. Inoculum regrowth in cultures treated with ceftazidime-aztreonam was much more rapid than that in cultures treated with cefepime-aztreonam, such that by 8 h the viable bacterial counts were similar to those in cultures treated with aztreonam alone. At the end of the third dosing cycle, the viable counts in cultures treated with ceftazidime-aztreonam were the same as those in cultures treated with aztreonam alone. In contrast, inoculum regrowth in cultures treated with cefepime-aztreonam was diminished, and the viable counts after the second and third dosing cycles were not much different from the viable counts at 8 h. By the end of the third dosing cycle, the viable counts in cultures treated with cefepime-aztreonam were 2 logs below those in cultures treated with 2-g doses of aztreonam or ceftazidime-aztreonam and more than 3 logs below those in cultures treated with cefepime or ceftazidime alone (Table 2). When a 4-g dose of aztreonam was simulated, viable counts after three dosing cycles were not much different from those in cultures treated with a simulated 2-g dose of aztreonam (data not shown).
DISCUSSION
The results of this study suggest that combinations of cefepime-aztreonam are, in general, the most effective β-lactam regimens against strains of P. aeruginosa producing elevated levels of Bush group 1 chromosomal cephalosporinases, i.e., PD and FD mutants of P. aeruginosa. The explanation for the improved pharmacodynamics appears to be related to aztreonam’s ability to inhibit the Bush group 1 cephalosporinase and to enhance cefepime’s activity against P. aeruginosa at pharmacologically attainable levels.
Aztreonam has been shown in a number of studies to be a potent competitive inhibitor of the Bush group 1 chromosomal cephalosporinases of members of the family Enterobacteriaceae and P. aeruginosa (5, 6, 15, 26). Although aztreonam is eventually hydrolyzed during interactions with these enzymes, the deacylation half-lives are long enough that the enzymes remain inactive through several generations of bacterial growth (5). Whether aztreonam can protect β-lactam antibiotics from Bush group 1 cephalosporinases has not been systematically ascertained. Synergy between cefepime and aztreonam has not been consistently observed in previous studies (3, 17). However, the interaction between cefepime and aztreonam may not fulfill the strict criteria for synergy required for many of these tests. In one study, Sakurai et al. (26) observed ambiguous results when aztreonam was combined with ampicillin, cephalothin, or cephaloridine against a strain of C. freundii. These results were not surprising, however, since the potency of aztreonam alone against wild-type C. freundii would have made it difficult to show any enhancement of activity with the combinations. Therefore, the potential use of aztreonam as a β-lactamase inhibitor needs to be studied against bacterial species, such as P. aeruginosa, which are intrinsically less susceptible to aztreonam and its companion drug and in which susceptibility is related to the basal level of β-lactamase expression. These criteria were fulfilled by the current study.
In the first phase of this study, the pharmacodynamics of cefepime, aztreonam, and ceftazidime alone and combinations of cefepime-aztreonam and ceftazidime-aztreonam were evaluated against an isogenic panel of P. aeruginosa strains producing various levels of chromosomal cephalosporinase. Against wild-type P. aeruginosa 164, cefepime-aztreonam was the most active regimen evaluated. However, the pharmacodynamics and level of total killing observed with cefepime-aztreonam were not substantially different from those observed with cefepime alone. Similar to the difficulty encountered previously with C. freundii (26), this wild-type strain of P. aeruginosa may have been too susceptible to cefepime alone to observe any enhancement of activity with cefepime-aztreonam. As the level of basal cephalosporinase production increased and the level of susceptibility decreased with the derepressed isogenic mutants, positive interactions between aztreonam and cefepime became apparent. In studies with the PD and FD mutants P. aeruginosa 164PD and P. aeruginosa 164FD, respectively, cefepime-aztreonam was the only regimen that produced killing over all three dosing intervals. Furthermore, in studies with P. aeruginosa 164PD, cefepime-aztreonam was able to suppress the outgrowth of an FD mutant population similar to P. aeruginosa 164FD. Although no FD mutants were detected in cultures of P. aeruginosa 164PD treated with the 2-g doses of cefepime and aztreonam alone, viable counts remained well above those in cultures treated with the 1-g combination of cefepime-aztreonam. From these data, it appears that aztreonam can enhance the pharmacodynamics of cefepime against P. aeruginosa, especially against strains producing increased levels of their chromosomal cephalosporinase. Furthermore, this positive interaction extended to other derepressed mutants of P. aeruginosa unrelated to P. aeruginosa 164.
The enhanced pharmacodynamic interactions between cefepime and aztreonam against derepressed mutants of P. aeruginosa were most obvious over the second and third dosing intervals, because cefepime-aztreonam did not consistently exhibit enhanced pharmacodynamics over the first dosing interval. For example, during the first dosing cycle against P. aeruginosa 164PD and P. aeruginosa 164FD, both the rates of killing and the levels of killing observed with cefepime-aztreonam were similar to those observed with the most active agent in the combination. However, over the second and third dosing cycles, differences between cefepime-aztreonam and the single-drug regimens became apparent. Further investigation into the effects of aztreonam on the accumulation of extracellular cephalosporinase suggested that the enhanced pharmacodynamics of cefepime-aztreonam over the second and third dosing cycles was the result of aztreonam’s inhibition of extracellular cephalosporinase and protection of cefepime in the extracellular environment. With the level of extracellular inactivation of cefepime diminished, more active cefepime would gain access to the periplasmic space where additional aztreonam could provide protection as well. This is in contrast to the situation with the regimen of cefepime alone, in which the accumulation of extracellular cephalosporinase resulted in the rapid inactivation of cefepime.
In contrast to studies with P. aeruginosa 164PD, in which rapid increases in viable counts were associated with the outgrowth of a more resistant mutant population, net increases in the viable counts of P. aeruginosa 164, P. aeruginosa 111M, and P. aeruginosa 113M over the second and third dosing intervals were not due to the selection of a more resistant mutant population. Rather, the decreased antibacterial activity observed with the second and third doses of some regimens was likely due to adaptive resistance or the reversible decrease in susceptibility after the first exposure to an antibiotic (9, 10, 17). This phenomenon is reversible upon removal of antibiotic from the environment and has been observed with aminoglycosides as well as β-lactam antibiotics. The mechanism of adaptive resistance appears to involve a permeability change in the target bacteria in response to their initial exposure to the antibiotic.
In this study, the enhanced antibacterial activity observed with cefepime-aztreonam was not always observed with ceftazidime-aztreonam. This most likely reflects differences between ceftazidime and cefepime in their pharmacodynamic interactions with the target bacteria. Three factors affect the pharmacodynamics of β-lactam antibiotics against gram-negative bacteria: the ability of the drug to reach the periplasmic space (penetration of outer membrane), the affinity of penicillin-binding proteins for the drug, and the interaction of the drug with β-lactamases. In comparison to cefepime, ceftazidime is a much slower penetrator of the outer membranes of gram-negative bacteria (22). Once inside the periplasmic space, cefepime has another advantage over ceftazidime in that it binds with a higher affinity to penicillin-binding proteins 1B and 1C, which are involved in cell lysis (25). Furthermore, the affinities of the group 1 chromosomal cephalosporinases for cefepime have been shown to be 10 to 100 times lower than the affinities of these enzymes for ceftazidime (22, 24). Taken together, these three factors combined with the higher levels achieved pharmacologically provide cefepime with a pharmacodynamic advantage over ceftazidime against P. aeruginosa, even though their MICs are often similar. In this study, the MICs of cefepime were within a range of ±1 twofold dilution of those of ceftazidime for five of the eight strains. However, the pharmacodynamics of cefepime were enhanced over those of ceftazidime, even against P. aeruginosa 164, which appeared to be more susceptible to ceftazidime by the MIC method. The lack of a correlation between the MICs and the pharmacodynamics observed in this study is not surprising, however, considering that MIC assays measure only the suppression of visible bacterial growth over 18 to 24 h and provide no insight into the pharmacodynamic differences between drugs. A bacteriostatic drug and a bactericidal drug can have the same potency according to their MICs, yet they can be strikingly different in their pharmacodynamic interactions. Therefore, data from this study demonstrate that the pharmacodynamics of cefepime against P. aeruginosa are enhanced over those of ceftazidime when their pharmacokinetics in serum are simulated, despite the similarity in their potencies according to their MICs.
If aztreonam enhances the activity of cefepime by inactivation of extracellular β-lactamase, does this mechanism carry any clinical significance? Does extracellular cephalosporinase accumulate in situ in infected tissues and diminish drug efficacy at the site of infection? The answers to these questions appear to be affirmative from studies with cystic fibrosis patients by Giwercman et al. (15). In a study of cephalosporinase activity in the sputum of 43 cystic fibrosis patients infected with P. aeruginosa, Giwercman et al. (15) observed that extracellular cephalosporinase activity in sputum increased significantly in patients treated with piperacillin, imipenem, ceftazidime, or cefsulodin for 15 days. In contrast, cephalosporinase activity decreased significantly or actually disappeared in 21 of 21 patients treated for 15 days with aztreonam (15). This occurred despite the presence of stably derepressed mutants of P. aeruginosa in some of the patients. When sputum samples from aztreonam-treated patients were dialyzed overnight against phosphate buffer, a significant increase in cephalosporinase activity was observed, suggesting that aztreonam was inhibiting cephalosporinase activity in situ. Therefore, the in vitro kinetic interactions observed between aztreonam and extracellular cephalosporinase in this study correlate with those observed in patients infected with P. aeruginosa. Perhaps a therapeutic benefit could be derived by using cefepime in combination with aztreonam in these cystic fibrosis patients. The results of the current study indicate that such a clinical trial is warranted.
REFERENCES
- 1.Akova M, Yang Y, Livermore D M. Interactions of tazobactam and clavulanate with inducibly- and constitutively-expressed class I β-lactamase. J Antimicrob Chemother. 1990;25:199–208. doi: 10.1093/jac/25.2.199. [DOI] [PubMed] [Google Scholar]
- 2.Blaser, J., B. B. Stone, and S. H. Zinner. 1985. Two compartment kinetic model with multiple artificial capillary units. J. Antimicrob. Chemother. 15(Suppl. A):131–137. [DOI] [PubMed]
- 3.Bosso J A, Saxon B A, Maxtsen J M. Comparative activity of cefepime, alone and in combination, against clinical isolates of Pseudomonas aeruginosa and Pseudomonas cepacia from cystic fibrosis patients. Antimicrob Agents Chemother. 1991;35:783–784. doi: 10.1128/aac.35.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Bush K. β-Lactamase inhibitors from laboratory to clinic. Clin Microbiol Rev. 1988;1:109–123. doi: 10.1128/cmr.1.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bush K, Freudenberger J S, Sykes R B. Interaction of azthreonam and related monobactams with β-lactamases from gram-negative bacteria. Antimicrob Agents Chemother. 1982;22:414–420. doi: 10.1128/aac.22.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conrad D A, Scribner R K, Weber A H, Marks M I. In vitro activity of BMY-28142 against pediatric pathogens, including isolates from cystic fibrosis sputum. Antimicrob Agents Chemother. 1985;28:58–63. doi: 10.1128/aac.28.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daikos G L, Jackson G G, Lolans V T, Livermore D M. Adaptive resistance to aminoglycoside antibiotics from first-exposure down-regulation. J Infect Dis. 1990;162:414–420. doi: 10.1093/infdis/162.2.414. [DOI] [PubMed] [Google Scholar]
- 10.Daikos G L, Lolans V T, Jackson G G. First-exposure adaptive resistance to aminoglycoside antibiotics in vivo with meaning for optimal clinical use. Antimicrob Agents Chemother. 1991;35:117–123. doi: 10.1128/aac.35.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edberg S C. The measurement of antibiotics in human fluids: techniques and significance. In: Lorian V, editor. Antibiotics in laboratory medicine. 2nd ed. Baltimore, Md: The Williams & Wilkins Co.; 1986. pp. 381–476. [Google Scholar]
- 12.Fuchs P C, Jones R N, Barry A L, Thornsberry C. Evaluation of the in vitro activity of BMY-28142, a new broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1985;27:679–682. doi: 10.1128/aac.27.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fung-Tomc J, Dougherty T J, DeOrio F J, Simich-Jacobson V, Kessler R E. Activity of cefepime against ceftazidime- and cefotaxime-resistant gram-negative bacteria and its relationship to β-lactamase levels. Antimicrob Agents Chemother. 1989;33:498–502. doi: 10.1128/aac.33.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gates M L, Sanders C C, Goering R V, Sanders W E., Jr Evidence of multiple forms of type I chromosomal β-lactamase in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1986;30:453–457. doi: 10.1128/aac.30.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giwercman B, Meyer C, Lambert P A, Reinert C, Høiby N. High-level β-lactamase activity in sputum samples from cystic fibrosis patients during antipseudomonal treatment. Antimicrob Agents Chemother. 1992;36:71–76. doi: 10.1128/aac.36.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kessler R E, Bies M, Buck R E, Chisholm D R, Pursiano T A, Tsai Y H, Misiek M, Price K E, Leitner F. Comparison of a new cephalosporin, BMY 28142, with other broad-spectrum β-lactam antibiotics. Antimicrob Agents Chemother. 1985;27:207–216. doi: 10.1128/aac.27.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGrath B J, Bailey E M, Lamp K C, Rybak M J. Pharmacodynamics of once-daily amikacin in various combinations with cefepime, aztreonam, and ceftazidime against Pseudomonas aeruginosa in an in vitro infection model. Antimicrob Agents Chemother. 1992;36:2741–2746. doi: 10.1128/aac.36.12.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medical Economics Data. Physician’s desk reference. Montvale, N.J: Medical Economics Data; 1996. [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A3. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 20.Neu H C, Chin N-X, Jules K, Labthavikul P. The activity of BMY 28142 a new broad spectrum β-lactamase stable cephalosporin. J Antimicrob Chemother. 1986;17:441–452. doi: 10.1093/jac/17.4.441. [DOI] [PubMed] [Google Scholar]
- 21.Neu H C, Chin N-X, Novelli A. In vitro activity of E-1040, a novel cephalosporin with potent activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1988;32:1666–1675. doi: 10.1128/aac.32.11.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikaido H, Liu W, Rosenberg E Y. Outer membrane permeability and β-lactamase stability of dipolar ionic cephalosporins containing methoxyimino substituents. Antimicrob Agents Chemother. 1990;34:337–342. doi: 10.1128/aac.34.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Callaghan C H, Morris A, Kirby S M, Shingler A H. Novel method for detection of β-lactamase by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972;1:283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phelps D J, Carlton D D, Farrell C A, Kessler R E. Affinity of cephalosporins for β-lactamases as a factor in antibacterial activity. Antimicrob Agents Chemother. 1986;29:845–848. doi: 10.1128/aac.29.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pucci M J, Boice-Sowek J, Kessler R E, Dougherty T J. Comparison of cefepime, cefpirome, and cefaclidine binding affinities for penicillin-binding proteins in Escherichia coli K-12 and Pseudomonas aeruginosa SC8329. Antimicrob Agents Chemother. 1991;35:2312–2317. doi: 10.1128/aac.35.11.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakurai Y, Yoshida Y, Saitoh K, Nemoto M, Yamaguchi A, Sawaii T. Characteristics of aztreonam as a substrate inhibitor and inducer for β-lactamases. J Antibiot. 1990;XLIII:403–410. doi: 10.7164/antibiotics.43.403. [DOI] [PubMed] [Google Scholar]
- 27.Sanders C C, Sanders W E, Jr, Moland E S. Characterization of β-lactamases in situ on polyacrylamide gels. Antimicrob Agents Chemother. 1986;30:951–952. doi: 10.1128/aac.30.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanders C C, Sanders W E., Jr Emergence of resistance to cefamandole: possible role of cefoxitin-inducible β-lactamases. Antimicrob Agents Chemother. 1979;15:792–797. doi: 10.1128/aac.15.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]