Abstract
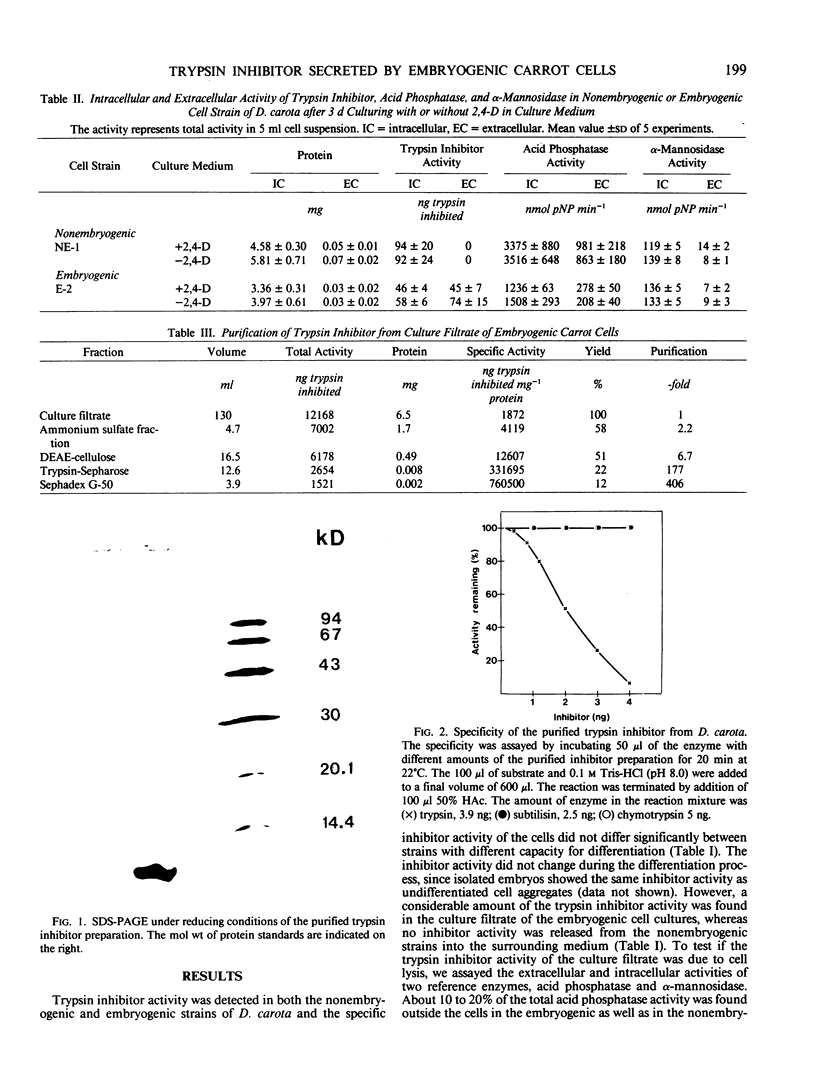
A protease inhibitor with a molecular weight of about 12,800 was purified to electrophoretic homogeneity from Daucus carota cells. The protease inhibitor was heat stable and inhibited trypsin but had no activity toward chymotrypsin or subtilisin. Nonembryogenic as well as embryogenic strains contained the inhibitor in similar amounts, but in the embryogenic strains the trypsin inhibitor was released from the cells and as a result accumulated in high concentrations in the culture medium, whereas no release of the trypsin inhibitor was found during cultivation of the nonembryogenic strains. Very low amounts of acid phosphatase or α-mannosidase activity were found in the culture filtrate of both embryogenic and nonembryogenic strains, which suggest that the release of the inhibitor from embryogenic strains was not due to cell lysis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chase T., Jr, Shaw E. p-Nitrophenyl-p'-guanidinobenzoate HCl: a new active site titrant for trypsin. Biochem Biophys Res Commun. 1967 Nov 30;29(4):508–514. doi: 10.1016/0006-291x(67)90513-x. [DOI] [PubMed] [Google Scholar]
- Feirer R. P., Mignon G., Litvay J. D. Arginine decarboxylase and polyamines required for embryogenesis in the wild carrot. Science. 1984 Mar 30;223(4643):1433–1435. doi: 10.1126/science.223.4643.1433. [DOI] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Montague M. J., Koppenbrink J. W., Jaworski E. G. Polyamine Metabolism in Embryogenic Cells of Daucus carota: I. Changes in Intracellular Content and Rates of Synthesis. Plant Physiol. 1978 Sep;62(3):430–433. doi: 10.1104/pp.62.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitto L., Schiavo F. L., Terzi M. alpha-Amanitin resistance is developmentally regulated in carrot. Proc Natl Acad Sci U S A. 1985 May;82(9):2799–2803. doi: 10.1073/pnas.82.9.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh S., Kamada H., Harada H., Fujii T. Auxin-controlled glycoprotein release into the medium of embryogenic carrot cells. Plant Physiol. 1986 Jul;81(3):931–933. doi: 10.1104/pp.81.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung Z. R. Cycloheximide resistance in carrot culture: a differentiated function. Plant Physiol. 1981 Jul;68(1):261–264. doi: 10.1104/pp.68.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung Z. R., Okimoto R. Embryonic proteins in somatic embryos of carrot. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3683–3687. doi: 10.1073/pnas.78.6.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]