Abstract
The overall survival of melanoma patients has improved using antibodies targeting immune checkpoints (anti-PD-1, anti-CTLA-4 and anti-LAG-3). Systemic chemotherapy was administered in melanoma for many years with limited effectiveness. Here we report a case of a patient who experienced immune-mediated adverse effects from checkpoint blockade therapy and subsequently responded to chemotherapy. The patient presented with melanoma and paraneoplastic digital ischemia. She received a combination of ipilimumab/nivolumab and experienced G3 myocarditis, followed by melanoma progression after a steroid taper. This patient achieved a partial and durable response with platinum and taxane-based chemotherapy. This report suggests the possibility of a subset of patients who experience progression after immune-based side effects where chemotherapy may be effective in the modern age of melanoma treatment.
Keywords: chemotherapy, immune-mediated adverse events, immunotherapy, melanoma, myocarditis, paraneoplastic acral vascular syndrome
Plain language summary
We describe a patient with a type of skin cancer called melanoma. At first, we tried to treat it with a medication that made the patient's body's defense system fight against it, but it caused problems with her heart so we had to stop it. Instead, we used another type of treatment called chemotherapy, which worked. The patient remains off therapy 4.5 years later. The lesson learned from this case is that chemotherapy is still helpful in certain situations. The initial treatment did not stop the melanoma growth. In addition, the patient had life-threatening toxicity.
Tweetable abstract
Chemotherapy may be useful for melanoma patients intolerant to checkpoint blockade therapy. Written informed consent was obtained from the patient to publish a case report.
Treatment of metastatic melanoma has improved the survival of melanoma patients with the use of immune checkpoint inhibitors (ICIs) [1,2]. Immunological side effects occur more frequently with combination ipilimumab/nivolumab (ipi/nivo) than with single-agent nivolumab. A total of 59% of the patients who received combination ipi/nivo experienced grade 3 or 4 immune-related adverse events (irAEs) as defined by Common Terminology Criteria for Adverse Events (CTCAE), whereas only 23% of the patients treated with nivolumab experienced such toxicity [1,2]. Dermatologic toxicities and thyroid dysfunction are the most common irAE elsewhere [3,4]. Myocarditis is less common for combination therapy but is still seen in clinical practice (∼0.27%) [5–7]. Fortunately, this does not happen more in clinical practice as knock-out of the PD1 gene in BALB/c but not C75BL/6 murine models leads to cardiomyopathy [8–11]. However, murine models differ from the human condition as there is an autoantibody to cardiac troponin rather than symptomatic myocarditis [11]. Resolving the pathophysiology of irAEs has been extraordinarily difficult, given the physiological difference between murine models and people. Whatever the underlying pathophysiology, irAEs have recently been reported to be associated with a high disease control rate in patients with metastatic melanoma [12]. In addition, recent guidelines have been published by the National Comprehensive Cancer Network for the management of irAEs [13].
Here we present an unusual case presentation of stage IV melanoma with paraneoplastic digital ischemia (necrotic bilateral index fingers), where the patient experienced a long clinical response to chemotherapy after myocarditis experienced from combination immunotherapy. Paraneoplastic acral vascular syndrome is an unusual presentation of a melanoma patient and digital ischemia has been reported in melanoma patients after treatment with immunotherapy [14,15]. The patient experienced myocarditis 20 days after the first infusion of combination immunotherapy with ipi/nivo. Systemic platinum and taxane-based chemotherapy was administered after the myocarditis resolved. The patient achieved a durable partial response (PR) of 3.5 years. In this case, the learning objective is that a partial and durable response can be achieved with chemotherapy after serious immune-related adverse events with immunotherapy and progression of melanoma.
Case presentation
A 33-year-old woman presented with digital ischemia to an outside facility. A left leg biopsy was consistent with a 1.4 mm-thickness melanoma with ulceration. Positron emission tomography and computed tomography (PET/CT) demonstrated extensive hypermetabolic masses throughout the anterior medial left thigh subcutaneous tissue, right lower quadrant mesenteric, left inguinal, external iliac and internal iliac hypermetabolic lymphadenopathy, T10 vertebral body lesion, and left lower lobe pulmonary nodules. Her recent medical history was significant for pulmonary embolism, for which she was started on anticoagulation. She was worked up for vasculitis at an outside facility and placed on amlodipine for presumed rheumatological hand pain. There were no other risk factors, such as diagnosis of diabetes mellitus, hypertension or smoking, in her past medical history. Anti-nuclear antibody (ANA), anti-neutrophil cytoplasmic antibodies (ANCA), antimyeloperoxidase (MPO) and antiproteinase 3 (PR3) were negative. Urinalysis was normal. Routine serologies over the course of treatment are summarized in Table 1. The review of systems was otherwise negative, with an Eastern Cooperative Oncology Group performance status of 1. Upon physical examination she had swelling on her left leg (from thigh to foot), obesity, discoloration of the hand and fingers, and bilateral index finger infection with distal tip necrosis.
Table 1. . Routine serologies measured on patient pre-ipilimumab/nivolumab, pre-radiation therapy, pre-chemotherapy, and 3 months after the start of chemotherapy.
| Preimmunotherapy | Preradiotherapy | Prechemotherapy | 3 months after chemotherapy start | |
|---|---|---|---|---|
| WBC | 5.68 | 5.38 | 8.34 | 5.52 k/µl |
| Hgb | 8.6 | 10.7 | 9.8 | 9.7 g/dl |
| Plts | 203 | 189 | 218 | 169 k/µl |
| Absolute neutrophil | 4.00 | 3.26 | 6.35 | 3.98 k/µl |
| Relative neutrophil | 70.3 | 60.5 | 76.1 | 72.0% |
| Absolute eosinophil | 0.09 | 0.09 | 0.20 | 0.14 k/µl |
| Relative eosinophil | 1.6 | 1.7 | 2.4 | 2.5% |
| Absolute monocyte | 0.35 | 0.44 | 0.50 | 0.39 k/µl |
| Relative monocyte | 6.2 | 8.2 | 6.0 | 7.1% |
| Absolute lymphocyte | 1.18 | 1.57 | 1.24 | 0.98 k/µl |
| Relative lymphocyte | 20.8 | 29.2 | 14.9 | 17.8% |
| Erythrocyte sedimentation rate | 68 | 19 | 30 | |
| LDH | 333 | 425 | 226 | 172 U/l |
| ALT | 19 | 12 | 14 | 38 U/l |
| AST | 24 | 17 | 15 | 18 U/l |
| Alkaline phosphatase | 55 | 46 | 51 | 62 U/l |
| T Bili | 0.50 | 0.30 | 0.64 | 0.40 mg/dl |
| Cr | 0.5 | 0.6 | 0.5 | 0.5 mg/dl |
| BUN | 8 | 15 | 11 | 10 mg/dl |
| Na | 139 | 140 | 139 | 139 mmol/l |
| K | 4.3 | 4.2 | 4.4 | 4.2 mmol/l |
| Cl | 100 | 102 | 105 | 102 mmol/l |
Bold values represent the inflammatory state of the patient. ESR was elevated, and LDH was elevated before treatment.
LDH, a marker of inflammation decreased 3 months after the start of chemotherapy.
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; BUN: Blood urea nitrogen; Cl: Chloride; Cr: Creatinine; Hgb: Hemoglobin; K: Potassium; LDH: Lactate dehydrogenase; Na: Sodium; Plt: Platelet; T bili: Total bilirubin; WBC: White blood cell.
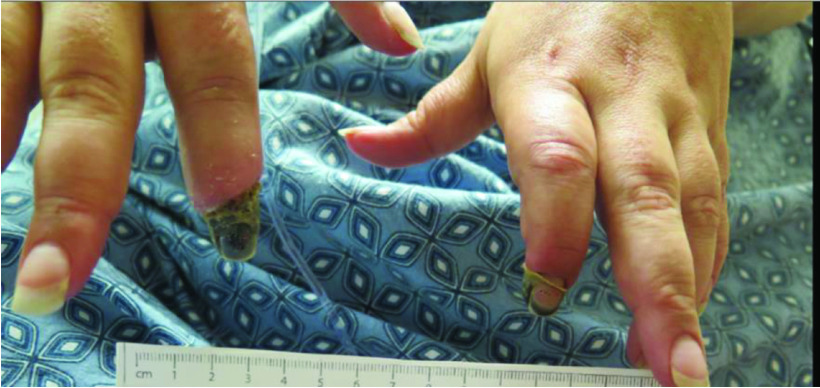
Incision and drainage of the right index finger were followed by empiric treatment with clindamycin. No further purulent drainage from this area was noted. The left distal index finger resolved, but the right distal index finger eventually self-amputated (Figure 1). However, from the time course, it is possible that paraneoplastic digital ischemia secondary to emboli caused infection in the distal digit.
Figure 1. . Resolving necrotic bilateral index fingers.
At this point, the patient was diagnosed with a BRAF wildtype stage IV m1c metastatic melanoma and suspected paraneoplastic acral vascular syndrome secondary to melanoma with necrotic bilateral index fingers. If the acral vascular syndrome was paraneoplastic in origin, then the primary treatment of melanoma would be the treatment of choice. The patient started on her first cycle of combination immunotherapy with the ipi/nivo combination. A total of 5 days later, she experienced grade 2 morbilliform drug eruption, for which she began a 0.5 mg/kg prednisone taper with rash resolution.
20 days after treatment with ipi/nivo, she was admitted to the hospital with shortness of breath and atypical chest pain. Admission labs were significant for Troponin-T at 0.177 ng/ml, and an electrocardiogram demonstrated left axis deviation, poor R-wave progression and a QTc of 0.454 s. The differential diagnosis was myocarditis, coronary vasculitis and worsening pulmonary embolism.
CT pulmonary angiography revealed no central pulmonary embolism but did demonstrate cardiomegaly, small pericardial effusion and multiple bilateral pulmonary nodules. Cardiac MRI was consistent with acute myocarditis with a nondepressed left ventricular ejection fraction (59%). To treat grade 3 myocarditis, a methylprednisolone taper was started at 2 mg/kg/day and tapered with no subsequent cardiac issues. Since the patient responded well to steroid treatment and had confirmed myocarditis on MRI, it was decided not to transfer her to an outside facility for cardiac catheterization.
2 months after her first cycle of ipi/nivo and while on low doses of steroids, CT thorax, abdomen and pelvis (TAP) revealed bulky left pelvic and inguinal lymphadenopathy encasing and compressing the left external iliac and common femoral vessels with asymmetric left thigh edema and skin thickening, likely related to venous compression by tumor with associated pain. Palliative radiotherapy (30 gray/10 fractions) was delivered to the left inguinal area to immediately relieve venous compression. However, postradiation CT TAP demonstrated a new paraaortic lymphadenopathy and mesenteric mass with reduction of the left inguinal and external iliac lymph nodes (Figure 2). Clinically, this was felt to be an actual progression as there were multiple new symptomatic melanoma lesions in the lymph node and mesenteric mass. Whether this is secondary to progression while on immunotherapy or progression due to insufficient treatment secondary to intolerance to immunotherapy can be debated. The diagnosis of pseudoprogression was also entertained but felt less likely owing to the clinical presentation. However, with disease progression and significant irAEs, radiation followed by chemotherapy was initiated for this patient. Biopsy was not performed for the mesenteric mass and the lymph nodes responded to radiation therapy. Palliative radiotherapy (30 gray/10 fractions) was delivered to the left inguinal area for symptom control.
Figure 2. . Computed tomography images (preimmunotherapy, postimmunotherapy, prechemotherapy and postchemotherapy).
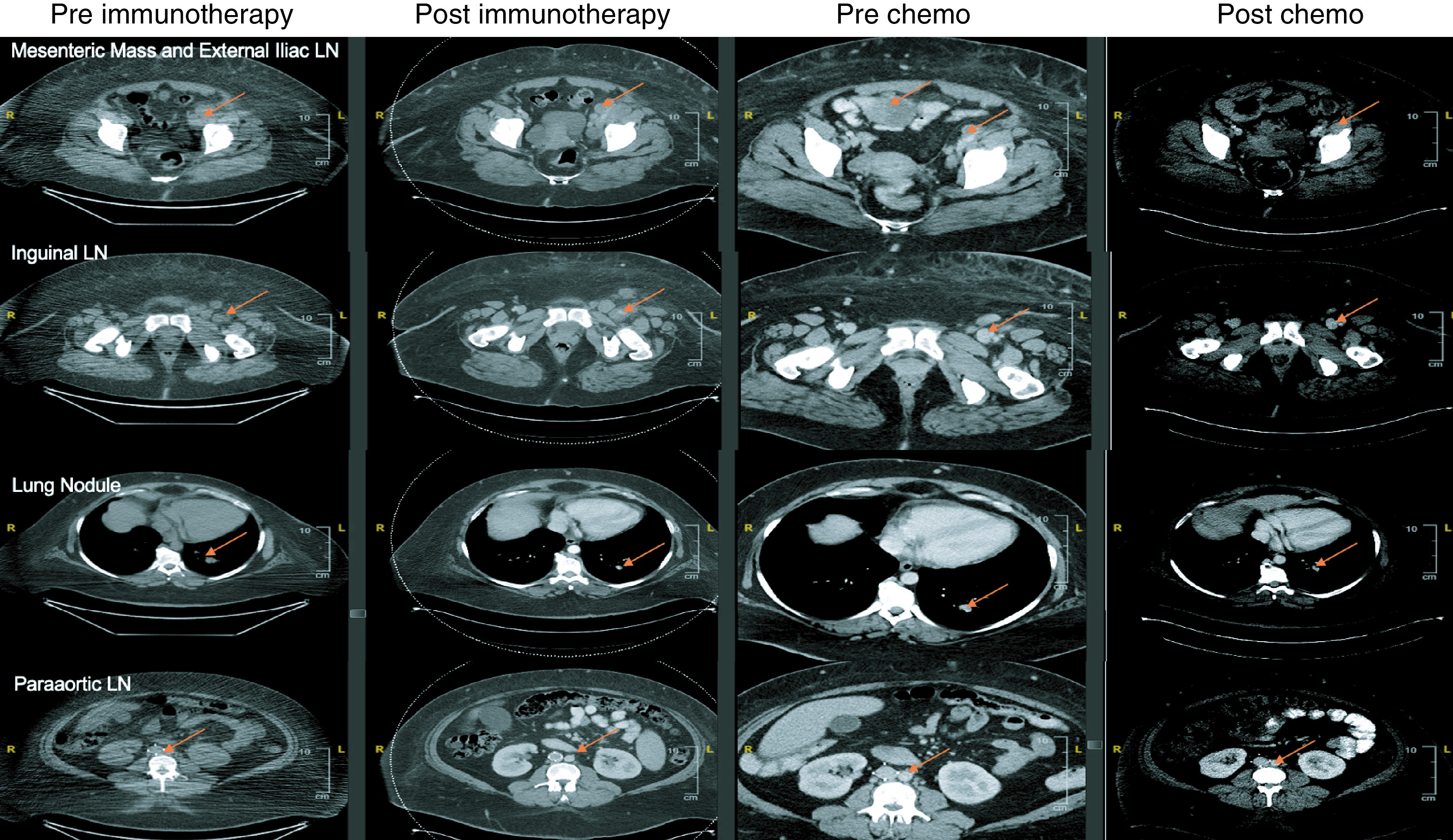
Computed tomography findings in four key regions (mesenteric mass, inguinal lymph nodes, lung nodule and paraaortic lymph node) at four time points (pre-anti-PD1/CTLA4, postimmunotherapy, prechemotherapy/post-radiation therapy and postchemotherapy). After immunotherapy, the inguinal lymph nodes and mesenteric mass increased in size, even with steroids. The mesenteric mass significantly increased in size postradiation therapy as this was outside the radiation field. After chemotherapy, all areas decreased in size.
Subsequently, carboplatin/paclitaxel and zoledronic acid were initiated. Chemotherapy was well tolerated with G1-2 nausea/vomiting/diarrhea/muscle cramps/joint pain that was controlled with the adjustment of medications. After cycle 3 of chemotherapy, the index fingertip self-amputated. There was no further clinical evidence of autoimmune phenomena. After 3 months of therapy, CT demonstrated a partial response. After 11 cycles of treatment with carboplatin/paclitaxel, PET/CT illustrated multiple metabolically inactive lymph nodes/pulmonary nodules and one metabolically active pulmonary nodule. Subsequent biopsy of the metabolically active nodule was negative for melanoma, and LDH and ESR normalized. This patient has a durable partial response for more than 4.5 years with no subsequent therapy.
Discussion & conclusion
Herein, we present a case of metastatic melanoma who responded to chemotherapy after progression following an irAE with immunotherapy treatment. We describe a melanoma patient who presented with manifestations of digital ischemia. She experienced myocarditis with subsequent combination immune therapy (ipi/nivo). Following irAEs to immune therapy, she experienced a prolonged clinical response to chemotherapy.
Paraneoplastic digital ischemia, as the first presentation in melanoma, is a rare condition and is a diagnosis of exclusion [16]. Digital ischemia has been previously reported in two patients with melanoma after treatment with single-agent ipilimumab [15] and also with combination ipilimumab/nivolumab [14]. Mechanisms of paraneoplastic digital ischemia are not well understood. There may be embolization by microfragments of the tumor, increased viscosity of the blood, sympathetic nervous system involvement by the tumor and vasoconstriction secondary to cytokine secretion by immune checkpoint blockade [14,17,18]. Given the concurrent presentation of digital ischemia and melanoma, without any other apparent comorbidities that could have caused it, it is possible that the patient experienced acral vascular syndrome. The etiology of myocarditis is easier to explain. Myocarditis is a rare side effect of ICIs, with a reported incidence of 0.04–1.14% and a 25–50% mortality rate [7,19,20]. There are three hypotheses: 1) involvement of a common antigen in both tumor cell and cardiac muscle; 2) T cell receptor-targing muscle antigen that is similar to the tumor antigen; and, 3) targeting of different antigens by certain T cell receptors. [5]. As noted in the introduction, these mechanisms are different to the mechanisms of loss of PD1 activity in murine models. Cardiovascular toxicities of ICIs include myocarditis, pericarditis and vasculitis. Myocarditis and vasculitis are known side effects of melanoma, whereas pericarditis is more often reported in patients with lung cancer [21]. A review of 87 patients with myocarditis showed that they were diagnosed at a median of 16 days (range: 1–196 days) [6]. Our patient presented with myocarditis symptoms on the 20th day of the first dose of combination therapy and fell within the range of expected toxicities and treatment with high-dose corticosteroids at a tertiary care center.
Chemotherapy efficacy after immune therapy is controversial. Maeda et al. reported platinum-based chemotherapy results in seven patients with ICI-resistant advanced melanoma – three out of seven achieved a partial response (PR) [22]. In a second retrospective study of 463 patients, progression-free survival (PFS) was not favorable (2.5 months), with overall survival (OS) from the start of chemotherapy of 7.1 months [23]. In a smaller cohort of patients, the mean PFS was 1.7 months in patients treated with chemotherapy after immunotherapy [24]. In a group of 18 patients, the disease control rate of chemotherapy after immune-based therapy was reported to be approximately 25%, with 12-month overall survival suggestive of a continued immune-based effect with chemotherapy [25]. To put this into perspective, a review from 2010 indicated that survival times of front-line metastatic-treated melanoma patients were between 6 and 12 months [26]. In some cases, chemo-immunotherapy after PD1 inhibitor failure may be beneficial and CX3CR1+CD8+ T cells may indicate the level of activity [27]. Therefore, we are achieving in the second-line setting with chemotherapy what was seen in the first-line setting in 2010, which suggests that the use of chemotherapy is beneficial in select cases. A recent retrospective review compared 11 patients who received chemotherapy post-immunotherapy to those without prior immunotherapy. In the group of post-immunotherapy-treated patients, the median PFS was higher than the group of preimmunotherapy patients (5.2 vs 2.5 months) [28]. These findings also suggest that immunotherapy may increase the effectiveness of chemotherapy. It is known that patients with grade 3 or 4 immune-based adverse events tend to have favorable outcomes [12]. The patients progressing after discontinuing because of serious immune-based adverse events represent a small group of patients, and none of these studies substratify for these patients. Hyperprogressive disease is an aggressive pattern of progression (>50% increase in volume) reported for patients treated with ICIs [29]. Our patient received a single dose of combination immunotherapy and progressed after discontinuation due to side effects. It is unclear whether this early progression was due to insufficient treatment or immunotherapy failure. However, given her myocarditis, it was not prudent to continue combination immunotherapy.
Preclinical models also substantiate these results. For example, a murine melanoma model study carried out in 2013 demonstrated that paclitaxel, at a noncytotoxic dose, can suppress the accumulation of myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment, which in turn increases the number of effector CD8+ T cells and promotes anti-tumor activity [30]. MDSCs are immature cells of myeloid origin that accumulate in response to chronic inflammation and promote tumor growth by suppressing the immune response to the tumor [31,32]. They stimulate the production of proinflammatory cytokines such as TNFα, IL-1β, IL-10, IL-6 and TGF-β [33,34], and reduce the recruitment of dendritic cells and T cells through the p38 MAPK pathway [35]. MDSCs can further differentiate into S100A9 suppressive macrophages to promote tumor growth [36]. S100A9 together with S100A8 is called calprotectin and is upregulated in inflammatory diseases to the point where it can be used as a clinical biomarker in colitis [37,38]. Such abnormal increases in the immune response are usually characterized by abnormally elevated MDSC. It has also been shown that prolonged exposure to reactive oxygen and nitrogen metabolites resulting in post-translational modifications of proteins may interfere with checkpoint blockade, and MDSCs are one such source of these metabolites [39–42]. Recently, an article was also published outlining the preclinical and clinical trial efforts of inhibiting nitric oxide synthase [43]. Therefore, it is at least plausible that chemotherapy can mitigate aberrant immune reactions when there is progression with immune-related adverse events. There is ongoing research to study abnormal immune responses in cancer.
This discussion suggests that some patients may benefit from chemotherapy postgrade 3–4 immune-mediated toxicity from checkpoint blockade. Further work is indicated to study this effect.
Executive summary.
Melanoma has multiple presentations and, in complex cases, should be managed in collaboration with a tertiary medical center.
Rare symptoms such as paraneoplastic acral vascular syndrome can be a presenting symptom of melanoma.
Chemotherapy is generally considered ineffective for melanoma, but a few patients benefit. Based on this case, it is hypothesized that chemotherapy may be helpful in cases of aberrant immune stimulation from combination immunotherapy.
Animal studies suggest that immune suppressor cells can be controlled with chemotherapy, which provides insight for future studies in this area.
The pathophysiology of myocarditis from immune checkpoint inhibitors deserves further study.
The presence of immune-related adverse events has a favorable prognosis, but these patients should be watched closely as there are melanoma patients who will progress quickly.
Footnotes
Financial & competing interests disclosure
J Markowitz is NIH funded; grant number KO8CA252164. Markowitz has received industry funding to the Moffitt Cancer Center unrelated to this manuscript from Morphogenesis, Jackson Laboratories, Microba, Idera Pharmaceuticals and Merck within the past 2 years. Moffitt Cancer Center has submitted a patent application on behalf of J Markowitz on work unrelated to this manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained verbal and written informed consent from the patient/patients for the inclusion of their medical and treatment history within this case report.
References
Papers of special note have been highlighted as: • of interest
- 1.Wolchok JD, Chiarion-Sileni V, Gonzalez R et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 377(14), 1345–1356 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larkin J, Chiarion-Sileni V, Gonzalez R et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 381(16), 1535–1546 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378(2), 158–168 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Zhou S, Yang F et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol. 5(7), 1008–1019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson DB, Balko JM, Compton ML et al. Fulminant myocarditis with combination immune checkpoint blockade. N. Engl. J. Med. 375(18), 1749–1755 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes myocarditis in the melanoma population treated with combination checkpoint blockade.
- 6.Matzen E, Bartels LE, Logstrup B, Horskaer S, Stilling C, Donskov F. Immune checkpoint inhibitor-induced myocarditis in cancer patients: a case report and review of reported cases. Cardiooncology 7(1), 27 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiménez-Alejandre R, Ruiz-Fernández I, Martín P. Pathophysiology of immune checkpoint inhibitor-induced myocarditis. Cancers (Basel) 14(18), (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11(2), 141–151 (1999). [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T. Establishment of NOD-Pdcd1-/- mice as an efficient animal model of type I diabetes. Proc. Natl Acad. Sci. USA 102(33), 11823–11828 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishimura H, Okazaki T, Tanaka Y et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291(5502), 319–322 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Okazaki T, Tanaka Y, Nishio R et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat. Med. 9(12), 1477–1483 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Serna-Higuita LM, Amaral T, Forschner A et al. Association between immune-related adverse events and survival in 319 stage IV melanoma patients treated with PD-1-based immunotherapy: an approach based on clinical chemistry. Cancers (Basel) 13(23), (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson JA, Schneider BJ, Brahmer J et al. NCCN Guidelines Insights: Management of Immunotherapy-Related Toxicities, Version 1.2020. J. Natl Compr. Canc. Netw. 18(3), 230–241 (2020). [DOI] [PubMed] [Google Scholar]; • Describes how to treat immune-related adverse events.
- 14.Gambichler T, Strutzmann S, Tannapfel A, Susok L. Paraneoplastic acral vascular syndrome in a patient with metastatic melanoma under immune checkpoint blockade. BMC Cancer 17(1), 327 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zenati N, Charles J, Templier I, Blaise S. Digital ischaemia with fingertip ulcers during ipilimumab therapy. Ann. Dermatol. Venereol. 147(3), 212–216 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Poszepczynska-Guigne E, Viguier M, Chosidow O, Orcel B, Emmerich J, Dubertret L. Paraneoplastic acral vascular syndrome: epidemiologic features, clinical manifestations, and disease sequelae. J. Am. Acad. Dermatol. 47(1), 47–52 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Jud P, Raggam RB, Hafner F. Paraneoplastic acral vascular syndrome. CMAJ 192(46), E1470 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J. Thromb. Haemostas. 11(2), 223–233 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Makunts T, Saunders IM, Cohen IV et al. Myocarditis occurrence with cancer immunotherapy across indications in clinical trial and post-marketing data. Sci. Rep. 11(1), 17324 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palaskas N, Lopez-Mattei J, Durand JB, Iliescu C, Deswal A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J. Am. Heart Assoc. 9(2), e013757 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salem JE, Manouchehri A, Moey M et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 19(12), 1579–1589 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maeda T, Yoshino K, Nagai K et al. The efficacy of platinum-based chemotherapy for immune checkpoint inhibitor-resistant advanced melanoma. Acta Oncol. 58(3), 379–381 (2019). [DOI] [PubMed] [Google Scholar]; • Describes how to treat immune-related adverse events.
- 23.Goldinger SM, Buder-Bakhaya K, Lo SN et al. Chemotherapy after immune checkpoint inhibitor failure in metastatic melanoma: a retrospective multicentre analysis. Eur. J. Cancer 162, 22–33 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Gaughan EM, Horton BJ. Outcomes from cytotoxic chemotherapy following progression on immunotherapy in metastatic melanoma: an institutional case-series. Front. Oncol. 12, 855782 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saint-Jean M, Fronteau C, Peuvrel L et al. Chemotherapy efficacy after first-line immunotherapy in 18 advanced melanoma patients. Medicine (Baltimore) 99(29), e21329 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orouji A, Goerdt S, Utikal J. Systemic therapy of non-resectable metastatic melanoma. Cancers (Basel) 2(2), 955–969 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vera Aguilera J, Paludo J, Mcwilliams RR et al. Chemo-immunotherapy combination after PD-1 inhibitor failure improves clinical outcomes in metastatic melanoma patients. Melanoma Res. 30(4), 364–375 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hadash-Bengad R, Hajaj E, Klein S et al. Immunotherapy potentiates the effect of chemotherapy in metastatic melanoma – a retrospective study. Front. Oncol. 10, 70 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kas B, Talbot H, Ferrara R et al. Clarification of definitions of hyperprogressive disease during immunotherapy for non-small cell lung cancer. JAMA Oncol. 6(7), 1039–1046 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sevko A, Michels T, Vrohlings M et al. Antitumor effect of paclitaxel is mediated by inhibition of myeloid-derived suppressor cells and chronic inflammation in the spontaneous melanoma model. J. Immunol. 190(5), 2464–2471 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 21(8), 485–498 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9(3), 162–174 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim C, Sano Y, Todorova K et al. The kinase p38 alpha serves cell type-specific inflammatory functions in skin injury and coordinates pro- and anti-inflammatory gene expression. Nat. Immunol. 9(9), 1019–1027 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin. Cancer Res. 13(18 Pt 1), 5256–5261 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Zhao F, Falk C, Osen W, Kato M, Schadendorf D, Umansky V. Activation of p38 mitogen-activated protein kinase drives dendritic cells to become tolerogenic in ret transgenic mice spontaneously developing melanoma. Clin. Cancer Res. 15(13), 4382–4390 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Kwak T, Wang F, Deng H et al. Distinct populations of immune-suppressive macrophages differentiate from monocytic myeloid-derived suppressor cells in cancer. Cell. Rep. 33(13), 108571 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S, Song R, Wang Z, Jing Z, Wang S, Ma J. S100A8/A9 in inflammation. Front. Immunol. 9, 1298 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inciarte-Mundo J, Frade-Sosa B, Sanmarti R. From bench to bedside: calprotectin (S100A8/S100A9) as a biomarker in rheumatoid arthritis. Front. Immunol. 13, 1001025 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garg SK, Sun J, Kim Y et al. Dichotomous nitric oxide-dependent post-translational modifications of STAT1 are associated with ipilimumab benefits in melanoma. Cancers (Basel) 15(6), (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Markowitz J, Wang J, Vangundy Z et al. Nitric oxide mediated inhibition of antigen presentation from DCs to CD4(+) T cells in cancer and measurement of STAT1 nitration. Sci. Rep. 7(1), 15424 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yarlagadda K, Hassani J, Foote IP, Markowitz J. The role of nitric oxide in melanoma. Biochim. Biophys Acta Rev. Cancer 1868(2), 500–509 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou J, Nefedova Y, Lei A, Gabrilovich D. Neutrophils and PMN-MDSC: their biological role and interaction with stromal cells. Semin. Immunol. 35, 19–28 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reddy TP, Glynn SA, Billiar TR, Wink DA, Chang JC. Targeting nitric oxide: say NO to metastasis. Clin. Cancer Res. 29(10), 1855–1868 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]