ABSTRACT
At our hospital, we are conducting the “Clinical Study of a Patient-Specific Cardiac Support Net for Dilated Cardiomyopathy (jRCTs042180025)”, a multi-facility clinical study of a customized cardiac support net (CSN). Here, we describe the cardiac rehabilitation (CR) of a heart failure (HF) patient after CSN treatment. The patient was a 65-year-old man who exhibited dilated cardiomyopathy (DCM) because of left ventricular non-compaction; his New York Heart Association status was class III. In November 2019, he received CSN treatment. The early CR program was adapted for this patient, and his postoperative course was uneventful. Functional measurements showed improved leg-muscle strength (before treatment: 61.4% BW; at discharge: 77.3% BW). During long-term follow-up, the patient’s exercise tolerance increased, as shown by 6-minute walk distance (before treatment: 576 m; long-term follow-up: 600 m) and peak oxygen uptake (before treatment: 12.5 mL/kg/min; long-term follow-up: 13.3 mL/kg/min). In the 2 years since discharge, the patient has not been hospitalized for HF. This report is the first to show that the CSN can be used to perform a CR program in a DCM patient without significant functional decline.
Key Words: cardiac rehabilitation, heart failure, cardiac support net, physical function
INTRODUCTION
Heart failure (HF) progresses via heart enlargement (ie, cardiac remodeling), regardless of the original disease. The Acorn Cor-Cap is a cardiac support net (CSN) that was devised to prevent cardiac remodeling by covering both ventricles with a mesh-like bag; this device received the CE-Mark in 2001 and was used in Europe until 2011. Although gradual, sustained improvements in cardiac dimensions have been reported, together with improved functional status,1 left ventricle improvements have been accompanied by right ventricle dysfunction, rather than improved cardiac output.2,3
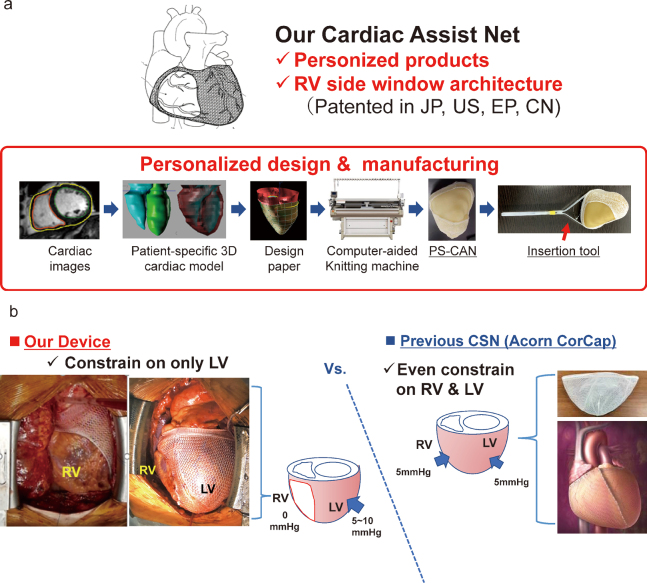
At our hospital, we are conducting the “Clinical Study of a Patient-Specific Cardiac Support Net for Dilated Cardiomyopathy (jRCTs042180025)”, a clinical study of a customized CSN [Figure 1]. Here, we describe a patient who received this treatment followed by cardiac rehabilitation (CR) during the perioperative period; we measured physical function during long-term follow-up.
Fig. 1.
The cardiac support net
Fig. 1a: Customized cardiac support net. The cardiac support net used in this study had a personalized design and manufacturing.
Fig. 1b: Feature of cardiac support net. The cardiac support net was only constrained on the left ventricle.
PS-CAN: patient-specific cardiac assist net
RV: right ventricle
LV: left ventricle
CSN: cardiac support net
CASE REPORT
The patient was a 65-year-old man who exhibited dilated cardiomyopathy (DCM) because of left ventricular non-compaction; his New York Heart Association status was class III. His HF symptoms included shortness of breath after walking rapidly and ascending stairs. The patient had received a cardiac resynchronization therapy defibrillator implant in 2018; his symptoms had not improved after implantation. Echocardiography showed marked heart enlargement with the following parameters: left ventricular end-diastolic diameter, 73.3 mm; left ventricular end-systolic diameter, 68.9 mm; and reduced left ventricular ejection fraction, 24.5%. His serum level of brain natriuretic peptide (BNP) was 254.5 pg/mL. In November 2019, the patient received our CSN treatment [Table 1]. The operation time was 136 min and the intubation time was 3 h; the bleeding volume was 39 mL.
Table 1.
Inclusion and exclusion criteria for cardiac support net
| Inclusion Criteria |
| 1. Patients who sign the consent form of participating clinical study by their free will |
| 2. Patients whose age are 20 years old or more, and 75 years or less at the time of obtaining informed consent |
| 3. Patients with heart failure symptoms in spite of optimal drug oral treatment for heart failure of more than 3 months |
| 4. Patients whom NYHA classification is III or IV, or level of INTERMACS Profile is 4 to 7 |
| 5. Patients with LVEDD more than 60 mm or LVEDDi more than 30 mm/m2 in echocardiography |
| 6. Patients with LVEF less than 35% in echocardiography |
| 7. Patients who have the intention of follow-up examination and observation, and can be admitted to visit the hospital which carries out them |
| Exclusion Criteria |
| 1. Patients with excessively enlarged heart. LVEDD is more than 85 m |
| 2. Patients with extremely low LVEF less than 10% |
| 3. Patients with history of cardiac surgery except pacemaker implantation |
| 4. Patients who are scheduled for other cardiac surgery |
| 5. Patients with the history or the schedule of CABG |
| 6. Patients with the history or candidate of PCI or trans myocardial laser revascularization within 3 months |
| 7. Patients who are implementing the IABP |
| 8. Patients who are adapted to left ventricular assist devices or scheduled heart transplant |
| 9. Patients with the history or schedule for ICD & CRT within 3 months |
| 10. Patients whose life expectancy 1 year or less |
| 11. Heart failure patients in end stage whose surgery risk is unacceptably high |
| 12. Patients who have developed acute myocardial infarction, unstable angina within 3 months |
| 13. Patients with hypertrophic cardiomyopathy |
| 14. Patient with active infection |
| 15. Patients with severe liver dysfunction whose AST or ALT values are more than 5 times of normal range in their institute |
| 16. Patients with poor lung function. FEV1.0 is less than 50% |
| 17. Patients with severe renal failure. Serum creatinine is 3 mg/dL or more or patients with dialysis-dependent |
| 18. Patients with diffuse peripheral vascular disease |
| 19. Patients with history of cerebrovascular disease within 3 months |
| 20. Patients with a high degree of bleeding tendency |
| 21. Patients with blood clotting disorders |
| 22. Patients who refuse blood transfusion |
| 23. Patients with poor prognosis by malignant diseases |
| 24. Patients with severe dementia, drug addiction, alcoholism |
| 25. Patients with severe allergy |
| 26. Pregnant or nursing patients. Patients who do not agree with contraception during the study |
| 27. Patients participating in other studies |
| 28. Patients whom the investigator determine unsuitable for participation |
NYHA: New York Heart Association classification
INTERMACS: interagency registry for mechanically assisted circulatory support
LVEDD: left ventricular end-diastolic diameter
LVDDi: left ventricular end-diastolic diameter index
LVEF: left ventricular ejection fraction
CABG: coronary artery bypass grafting
PCI: percutaneous coronary intervention
IABP: intra-aortic balloon pumping
ICD: implantable cardioverter defibrillator
CRT: cardiac resynchronization therapy
AST: aspartate aminotransferase
ALT: alanine aminotransferase
FEV1.0: forced expiratory volume % in one second
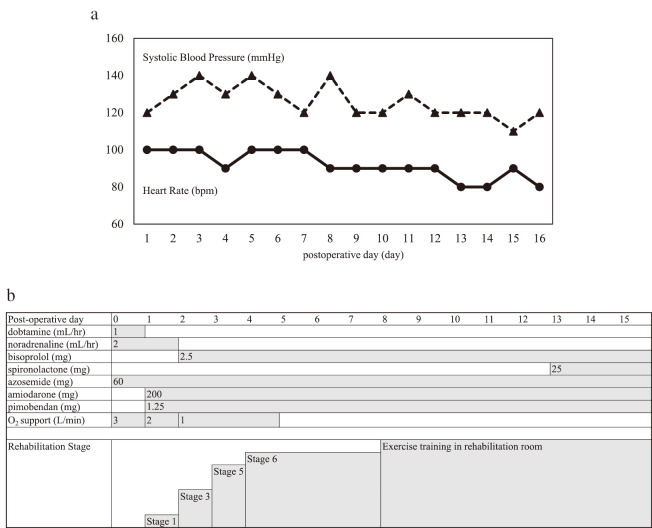
CR was performed in the perioperative period, based on the Standard CR Program for HF formulated by the Japanese Society for CR for use by inpatient acute CR programs.4 To proceed to the next stage of treatment, the patient was required to demonstrate stable hemodynamics in loading tests. The patient exited the surgical-intensive care unit on the first postoperative day and entered the cardiac surgery ward. After the second postoperative day, he advanced through the acute rehabilitation program. The patient received defibrillation treatment for paroxysmal atrial fibrillation on the seventh day. The CR program included early mobilization, aerobic exercise, and resistance training. Exercise guidance comprised an explanation of lifestyle changes commensurate with the patient’s physical capacity. He completed the CR program and was discharged on postoperative day (POD) 15 [Figure 2].
Fig. 2.
The cardiac support net treatment course
Fig. 2a: The trend of heart rate and blood pressure in cardiac-support-net. The dotted line shows the trend in non-invasive systolic blood pressure. The solid line shows the trend in heart rate.
Fig. 2b: Medication and rehabilitation stages after surgery. Stage 1 is sitting on the edge of the bed and maintaining an upright position at the bedside. Stage 3 is a 40-m walk test. Stage 5 is an 80-m walk test, 2–3 times in the cardiac surgery ward. Stage 6 is a 6-minute walk test.
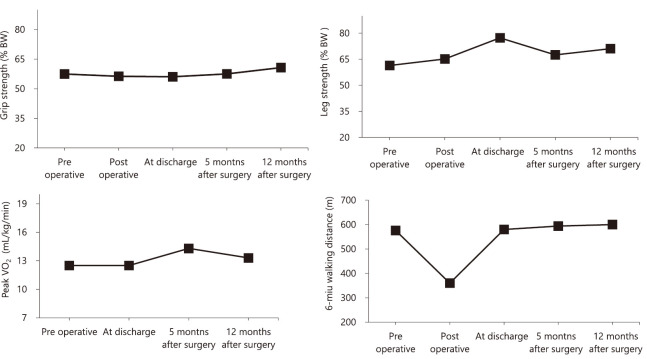
Physical-function measurements were performed before surgery, on the 6th day after surgery, before discharge (14th day after surgery), and during follow up. Physical function measurements were taken and exercise-management guidance was provided during long-term follow-up at the planned educational hospitalization (5 and 12 months after surgery). The maximum values of the grip and leg strength were divided by the body weight [% BW (kgf/kg)]. Supervised physical training was not performed during the long-term follow-up period. To evaluate physical function, we conducted a cardiopulmonary exercise test, a 6-minute walk test, grip-strength assessment, and leg-extensor-muscle strength assessment. At discharge, the patient exhibited a minimal decrease in physical function; his physical function improved during long-term follow-up. Compared to before surgery, at 5 months after surgery he exhibited increases in the 6-minute walk distance (6MD) (from 576 m to 594 m), peak oxygen uptake (peak VO2) (from 12.5 mL/kg/min to 14.3 mL/kg/min), and leg strength (from 61.4% BW to 67.5% BW). At 12 months after surgery, compared to 5 months after surgery, he exhibited increases in the 6MD (from 594 m to 600 m), grip strength (from 57.5% BW to 60.7% BW), and leg strength (from 67.5% BW to 71.1% BW) [Figure 3]. However, his levels of BNP increased from 342.2 at discharge to 489.6 pg/mL at 12 months after surgery [Table 2]. We advised him to avoid excessive physical activity in the long-term follow-up period. The patient was not hospitalized for HF during the observation period (26 months).
Fig. 3.
Changes in physical function
The patient’s grip strength and leg strength did not decrease after surgery, and his 6-minute walk distance was maintained.
Table 2.
Patient characteristic and physical function in each phase
| Pre
operative |
Post
operative |
At
discharge |
5 months
after surgery |
12 months
after surgery |
|
| BW (kg) | 65.6 | 64.3 | 63.5 | 67.1 | 65.4 |
| BMI (kg/m2) | 21.8 | 21.4 | 21.1 | 22.1 | 21.7 |
| Echocardiography | |||||
| LVEF (%) | 24.5 | – | 21.0 | 23.4 | 21.0 |
| LVDd (mm) | 73.3 | – | 70.7 | 73.1 | 73.4 |
| LVDs (mm) | 68.9 | – | 66.9 | 72.4 | 69.1 |
| MR grade | 3 | – | 2 | 3 | 3 |
| E/e′ | 16.3 | – | 7.5 | 21.6 | 11.5 |
| E/A ratio | 0.57 | – | – | 0.92 | 1.34 |
| Laboratory data | |||||
| Serum albumin (g/dL) | 4.0 | 3.0 | 3.4 | 4.4 | 4.4 |
| Serum creatinine (mg/dL) | 1.51 | 1.31 | 1.56 | 1.56 | 1.63 |
| Serum sodium (mEq/L) | 137 | 138 | 134 | 137 | 142 |
| Total bilirubin (mg/dL) | 0.8 | 0.6 | 0.8 | 1.0 | 0.7 |
| C-reactive protein (mg/dL) | 0.08 | 6.59 | 1.99 | 0.11 | 0.24 |
| Plasma BNP (pg/mL) | 254.5 | 1196.0 | 343.2 | 211.3 | 489.6 |
| Physical function | |||||
| Grip strength (% BW) | 57.5 | 56.3 | 56.1 | 57.5 | 60.7 |
| Leg strength (% BW) | 61.4 | 65.2 | 77.3 | 67.5 | 71.1 |
| Peak VO2 (mL/kg/min) | 12.5 | – | 12.5 | 14.3 | 13.3 |
| 6-minute walk distance (m) | 576 | 360 | 580 | 594 | 600 |
BW: body weight
BMI: body mass index
LVEF: left ventricular ejection fraction
LVDd: left ventricular diastolic diameter
LVDs: left ventricular systolic diameter
MR: mitral regurgitation
E/e′: the ratio of early diastolic transmitral flow velocity to early diastolic mitral annular velocity
E/A ratio: the ratio of early to the late transmitral filling velocities
BNP: brain natriuretic peptide
DISCUSSION
In HF patients, exercise ability decreases with disease progression.5,6 The unique myopathy in DCM-induced HF presumably exacerbates exercise intolerance.7 Heart transplantation and ventricular-assist-device (VAD) treatments are necessary for the management of end-stage HF. VAD destination therapy has recently been adapted in Japan for patients aged >65 years. However, VAD surgery for older HF patients is sufficiently invasive that patients’ physical function decreases.8 Thus, in clinical practice, there is a need to perform careful patient selection.
This case was removed from the surgical intensive care unit on POD1 and early mobilization was implemented in the cardiac surgery ward up to POD6. In Europe and the United States, early mobilization includes physical activity performed within 2–5 days.9,10 Early rehabilitation is essential for early recovery of patients, but progression during rehabilitation in an elderly patient with HF is difficult due to hemodynamic problems and the influence of factors such as frailty.
In addition, rehabilitation from HF with the use of an inotropic infusion takes time because hemodynamic stability must be checked.11 The hemodynamics were stable in this case, so we were able to adapt to early rehabilitation. Grip strength, which is an indicator of skeletal muscle wasting,12 was maintained during the perioperative period. Patients with low cardiac function may experience delays in physical function recovery after surgery, but he had no physical decline. Moreover, physical function in HF patients decreases with each hospital readmission. Regardless of whether protein breakdown can be suppressed, protein synthesis cannot be enhanced because muscle training is essential for protein synthesis. Therefore, muscle strength generally does not increase in HF patients during long-term follow-up. In a previous study, muscle strength in VAD patients did not increase after discharge, despite improved cardiac output.13 However, the present patient did not exhibit reduced function in the early postoperative period, and exhibited increased physical capacity during long-term follow-up. The 6MD was 500–600 m from the time of discharge to the remote phase. In international studies, the 6MD is about 400 m at 6 months.14 It was about 350 m at 2 years after VAD in the late 50s.15 In other words, exercise tolerance in this case was maintained at a much better value than the remote stage 6MD of elderly VAD patients.
Our patient’s peak VO2 was 12.5 mL/kg/min before and after surgery; his peak VO2 during long-term follow-up was 13.3–14.3 mL/kg/min. The peak VO2 for transplant recipients is 14 mL/kg/min16; his exercise tolerance was similar to the tolerance in patients with severe HF. The degree of exercise tolerance in our patient indicated an improved probability of not requiring rehospitalization. According to the ROADMAP study, the prevalence of New York Heart Association status class III is 37%.17 The 2-year rate of death or hospitalization was 56.7% in a previous study of HF patients with a reduced left ventricular ejection fraction18; however, our patient did not require readmission for HF after CSN treatment. Sufficient exercise tolerance in patients with HF is effective for avoiding readmission.19
The levels of BNP increased during the long-term follow-up period. A previous study showed that aerobic and resistance training have favorable effects on decreased BNP levels.20 However, strength training induces a significant increase in NT-Pro BNP.21 Our report highlights the need to manage excessive activity in patients after CSN treatment. Although limited outpatient CR is available in Japan,22 inpatient CR centers may be useful for managing excessive activity during long-term follow-up.
This is the first report to show that CSN can be performed during a CR program in a DCM patient without significant functional decline. For patients with low physical reserves who are unlikely to be able to tolerate invasive surgery such as VAD, CSN treatment is suitable because it results in minimal reduction of physical function during the early postoperative period. It is not affected by destination therapy–VAD criteria; it is expected to be much less invasive. For patients with low physical reserves, less-invasive treatments can help to preserve physical ability.
CONCLUSION
We described a patient who received CSN treatment; our patient exhibited improved CR in the perioperative period. Our report may be helpful for the selection of HF patients who cannot tolerate invasive treatment.
ACKNOWLEDGMENTS
We thank Textcheck (textcheck.com) for English language editing.
INFORMED CONSENT
The patient provided permission to publish this case report; his identity has been protected.
CONFLICT OF INTEREST STATEMENT
Dr Toshiaki Akita is employed by iCorNet Co, a venture company of Nagoya University. The other authors have no conflicts of interest to declare.
Abbreviations
- CR
cardiac rehabilitation
- HF
heart failure
- CSN
cardiac support net
- VAD
ventricular-assist-device
REFERENCES
- 1.Acker MA, Jessup M, Bolling SF, et al. Mitral valve repair in heart failure: Five-year follow-up from the mitral valve replacement stratum of the Acorn randomized trial. J Thorac Cardiovasc Surg. 2011;142(3):569–574, 574.e1. doi: 10.1016/j.jtcvs.2010.10.051. [DOI] [PubMed]
- 2.Olsson A, Bredin F, Franco-Cereceda A. Echocardiographic findings using tissue velocity imaging following passive containment surgery with the Acorn CorCap cardiac support device. Eur J Cardiothorac Surg. 2005;28(3):448–453. doi: 10.1016/j.ejcts.2005.05.027. [DOI] [PubMed]
- 3.Elami A. CorCap device in addition to mitral surgery in heart failure: Is it truly beneficial? J Thorac Cardiovasc Surg. 2007;133(2):590. doi: 10.1016/j.jtcvs.2006.09.058. [DOI] [PubMed]
- 4.Izawa H, Yoshida T, Ikegame T, et al. Standard cardiac rehabilitation program for heart failure. Circ J. 2019;83(12):2394–2398. doi: 10.1253/circj.CJ-19-0670. [DOI] [PubMed]
- 5.von Haehling S, Steinbeck L, Doehner W, Springer J, Anker SD. Muscle wasting in heart failure: An overview. Int J Biochem Cell Biol. 2013;45(10):2257–2265. doi: 10.1016/j.biocel.2013.04.025. [DOI] [PubMed]
- 6.Zamboni M, Rossi AP, Corzato F, Bambace C, Mazzali G, Fantin F. Sarcopenia, cachexia and congestive heart failure in the elderly. Endocr Metab Immune Disord Drug Targets. 2013;13(1):58–67. doi: 10.2174/1871530311313010008. [DOI] [PubMed]
- 7.Song T, Manoharan P, Millay DP, et al. Dilated cardiomyopathy-mediated heart failure induces a unique skeletal muscle myopathy with inflammation. Skelet Muscle. 2019;9(1):4. doi: 10.1186/s13395-019-0189-y. [DOI] [PMC free article] [PubMed]
- 8.Ramos Dos Santos PM, Aquaroni Ricci N, Aparecida Bordignon Suster É, de Moraes Paisani D, Dias Chiavegato L. Effects of early mobilisation in patients after cardiac surgery: A systematic review. Physiotherapy. 2017;103(1):1–12. doi: 10.1016/j.physio.2016.08.003. [DOI] [PubMed]
- 9.Hodgson CL, Berney S, Harrold M, Saxena M, Bellomo R. Clinical review: Early patient mobilization in the ICU. Crit Care. 2013;17(1):207. doi: 10.1186/cc11820. [DOI] [PMC free article] [PubMed]
- 10.Cameron S, Ball I, Cepinskas G, et al. Early mobilization in the critical care unit: A review of adult and pediatric literature. J Crit Care. 2015;30(4):664–672. doi: 10.1016/j.jcrc.2015.03.032. [DOI] [PubMed]
- 11.Amiya E, Taya M. Is exercise training appropriate for patients with advanced heart failure receiving continuous inotropic infusion? A review. Clin Med Insights Cardiol. 2018;12:1179546817751438. doi: 10.1177/1179546817751438. [DOI] [PMC free article] [PubMed]
- 12.Fülster S, Tacke M, Sandek A, et al. Muscle wasting in patients with chronic heart failure: Results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur Heart J. 2013;34(7):512–519. doi: 10.1093/eurheartj/ehs381. [DOI] [PubMed]
- 13.Kobayashi K, Mutsuga M, Usui A. Relationship between muscle strength and rehospitalization in ventricular assist device patients. Sci Rep. 2022;12(1):50. doi: 10.1038/s41598-021-04002-3. [DOI] [PMC free article] [PubMed]
- 14.Uriel N, Burkhoff D, Rich JD, et al. Impact of hemodynamic ramp test-guided HVAD speed and medication adjustments on clinical outcomes. Circ Heart Fail. 2019;12(4):e006067. doi: 10.1161/CIRCHEARTFAILURE.119.006067. [DOI] [PubMed]
- 15.McCullough M, Caraballo C, Ravindra NG, et al. Neurohormonal blockade and clinical outcomes in patients with heart failure supported by left ventricular assist devices. JAMA Cardiol. 2020;5(2):175–182. doi: 10.1001/jamacardio.2019.4965. [DOI] [PMC free article] [PubMed]
- 16.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83(3):778–786. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed]
- 17.Estep JD, Starling RC, Horstmanshof DA, et al. Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients: Results from the ROADMAP study. J Am Coll Cardiol. 2015;66(16):1747–1761. doi: 10.1016/j.jacc.2015.07.075. [DOI] [PubMed]
- 18.Lerakis S, Kini AS, Asch FM, et al. Outcomes of transcatheter mitral valve repair for secondary mitral regurgitation by severity of left ventricular dysfunction. EuroIntervention. 2021;17(4):e335–e342. doi: 10.4244/EIJ-D-20-01265. [DOI] [PMC free article] [PubMed]
- 19.Teramatsu H, Shiraishi J, Matsushima Y, Araki M, Okazaki T, Saeki S. Using physical function to predict hospital readmission within 1 year in patients with heart failure. Prog Rehabil Med. 2019;4:20190018. doi: 10.2490/prm.20190018. [DOI] [PMC free article] [PubMed]
- 20.Smart NA, Steele M. Systematic review of the effect of aerobic and resistance exercise training on systemic brain natriuretic peptide (BNP) and N-terminal BNP expression in heart failure patients. Int J Cardiol. 2010;140(3):260–265. doi: 10.1016/j.ijcard.2009.07.004. [DOI] [PubMed]
- 21.Bordbar S, Bigi MA, Aslani A, Rahimi E, Ahmadi N. Effect of endurance and strength exercise on release of brain natriuretic peptide. J Cardiovasc Dis Res. 2012;3(1):22–25. doi: 10.4103/0975-3583.91599. [DOI] [PMC free article] [PubMed]
- 22.Kamiya K, Yamamoto T, Tsuchihashi-Makaya M, et al. Nationwide survey of multidisciplinary care and cardiac rehabilitation for patients with heart failure in Japan: An analysis of the AMED-CHF study. Circ J. 2019;83(7):1546–1552. doi: 10.1253/circj.CJ-19-0241. [DOI] [PubMed]