ABSTRACT
Ustekinumab has recently been approved for the treatment of ulcerative colitis (UC) based on data from clinical trials. However, the effectiveness of ustekinumab in patients with UC in a real-world setting remains unclear. Hence, in this meta-analysis, we aimed to evaluate the effectiveness of ustekinumab in a real-world setting and to investigate the predictors of its effectiveness. A comprehensive literature search was performed to examine the effectiveness of ustekinumab in UC patients admitted between January 2019 and December 2021. Data on clinical remission, response, and corticosteroid-free clinical remission rates were extracted, pooled, and analyzed. Meta-regression analysis was performed to investigate the source of heterogeneity and the impact of moderators on the outcomes of interest. A total of 14 eligible studies were identified. The pooled clinical remission rate was 55.0% at week 8, 36.1% at week 16, 46.6% at month 6, and 38.6% at month 12. The meta-regression analysis showed that prior use of anti-tumor necrosis factor (TNF) agents and vedolizumab and the publication style were significant moderators. Additionally, out of 258 patients, there were 28 adverse events (AEs) (10.9%). The effectiveness of ustekinumab in real-world patients with UC was consistent with the results clinical trials. Moreover, previous treatment with anti-TNF agents and vedolizumab might have affected the effectiveness of ustekinumab.
Key Words: ustekinumab, ulcerative colitis, systematic review, meta-analysis
INTRODUCTION
Ustekinumab (UST) is a monoclonal antibody that acts on the p40 subunit of interleukin-12 and interleukin-23. UST has recently been approved for the treatment of moderate to severe ulcerative colitis (UC) based on the efficacy and safety data derived from the UNIFI clinical trials.1 Although clinical trials showed the efficacy and safety of UST and supported the approval of the use of UST, their results cannot yet be applied to daily clinical practice due to the limitations of the clinical trials’ inclusion and exclusion criteria.2 Since many patients seen in daily clinical practice do not meet the inclusion criteria for randomized controlled trials (RCTs) due to their age, presence of comorbidity, or intake of concomitant therapies, patients enrolled in RCTs are not representative of patients with ulcerative colitis.3 Therefore, real-world evidence is important to complement the results of clinical trials and to guide physicians regarding treatment decisions. Several guidelines on the management of UC recommend the use of different drug classes, such as anti-tumor necrosis factor (TNF) agents, vedolizumab (VDZ), tofacitinib (TOF), or UST, for the induction of remission as a treatment option, especially for patients with moderately to severely active UC with inadequate response or intolerance to conventional therapy.4,5 However, UST has not yet been established as a drug for UC. Real-world evidence investigating the predictors of the effectiveness of UST may be integral in clarifying the clinical value of UST in the management of patients with UC. Furthermore, real-world evidence can clarify the safety profile that is not fully revealed in RCTs. Although some studies reported real-world evidence for UC,6-8 the number of patients included in each study was too small to make any definite conclusions and to investigate the predictors of effectiveness. Therefore, in this systematic review and meta-analysis, we aimed (a) to assess the effectiveness of UST using large real-world data and (b) to investigate the predictors of effectiveness.
MATERIALS AND METHODS
Protocol and registration
The protocol for this study was registered on PROSPERO (CRD42022300184) and was conducted in accordance with the preferred reporting items for systematic review and meta-analysis statements.9
Eligibility criteria
The types of studies included prospective and retrospective observational cohort studies, including conference abstracts. The study participants were adult patients (18 years or older) who underwent UST for UC. Patients who received UST only for maintenance therapy of UC or for treatment of diseases other than UC and patients with a previous colectomy were excluded. Studies reporting the effectiveness or safety outcomes of interest were eligible. Controlled clinical trials, such as RCTs, review articles, case reports, letters to the editor, and studies not published in English or Japanese were excluded from the review.
Information sources and search strategy
A comprehensive literature search was conducted including articles published between January 2019 and December 2021 using PubMed and the Web of Science. To ensure literature saturation, the reference lists of included studies were manually scanned. Conference proceedings for the Digestive Disease Week, United European Gastroenterology Week, European Crohn’s and Colitis Organization, and Japan Digestive Disease Week were also searched. Literature search strategies were developed using the following medical subject headings and text words related to UST in patients with UC: ulcerative colitis, colitis, inflammatory bowel disease, ustekinumab, Stelara, interleukin-12, or interleukin-23. The details of the search strategy are included in the appendices. The titles and abstracts of the results were initially scanned to exclude irrelevant studies. Subsequently, the full texts of the selected articles were screened to determine whether they were eligible. Two authors (GU and MN) independently decided which studies to include. Disagreements were resolved by consensus. Figure 1 shows the flow diagram of the study selection.
Fig. 1.
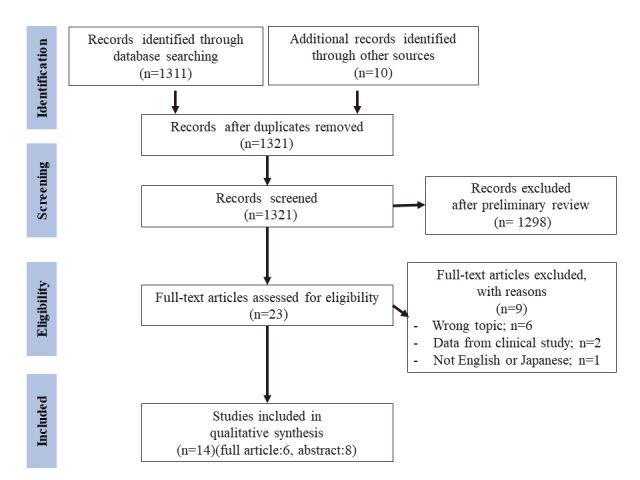
Flow chart of the systematic review
Data extraction
Two reviewers (GU and MN) independently extracted the data using a predesigned form. Data on the study characteristics included the primary author, year of publication, geographic location, and study design. Data on the patient and disease characteristics included age, sex, disease duration, location of disease, and severity. Data on medication history, percentage of patients with concomitant steroid or immunomodulator, or exposure to biologics or TOF were also included. Lastly, data on the outcome assessment, which included the induction and maintenance regimen of UST, total number of patients who received UST therapy, proportion achieving the outcome of interest, and time point when the outcomes were assessed, were also analyzed.
Outcomes assessed
The primary outcome measure was the clinical remission rate after treatment with the UST, which was defined as the proportion of patients with clinical remission at predefined time points. Secondary outcome measures included the clinical response rate, corticosteroid-free remission rate, discontinuation rate of UST, and adverse events (AEs). The definitions of clinical remission and response determined by the authors of each study were used to calculate the outcomes. When studies reported remission and response rates at various time points, we classified the assessment time points as follows: week 8 (4–8 weeks), week 16 (12–20 weeks), month 6 (24–32 weeks), and month 12 (44–56 weeks) after the administration of the initial dose of UST. The severity of disease in each study included in this review was classified into three subgroups: mild, moderate, and severe. The classification was based on the mean or median of disease activity indices, such as the Mayo score,10 simple clinical colitis activity index,11 or clinical activity index.12 The thresholds used to categorize the severity were listed in Supplementary Table 1. When multiple indices were reported in a previous study, a score with a higher severity was used to classify the disease severity.
Assessment of risk of bias in included studies
Two study investigators (GU and MN) independently assessed the risk of bias in individual studies using the Newcastle-Ottawa scale for cohort studies. Three perspectives were considered: the selection of the study groups, comparability of the groups, and ascertainment of either the exposure or outcome of interest for case-control or cohort studies.13 Since studies of interest in this review were uncontrolled cohort studies, the domain ‘comparability’ and ‘selection item 2’ were not applicable.
Statistical analyses
The pooled clinical remission, clinical response, and corticosteroid-free clinical remission rates were appropriately calculated using a random-effect model,14 and a conservative approach was used to account for the between-study variability. Meta-analysis was conducted using a double arcsine transformation with a back-transformation to report the pooled prevalence rates.15 We assessed the statistical heterogeneity using the Q test and inconsistency (I2) test. A P value < 0.10 and an I2 value > 50% indicated significant heterogeneity.16 To examine the impact of the moderators on the study effect size and the source of heterogeneity within the included studies, meta-regression analysis was performed. The omnibus (QM) tests of each moderator were undertaken and used as a basis for model simplification.17 We assessed publication bias by examining the funnel plot symmetry and by conducting the Egger’s regression test.18 All analyses were performed using the R version 4.0.5 (Foundation for Statistical Computing, Vienna, Austria), which was equipped with the “meta” and “metafor” packages.
RESULTS
Study selection
From the 1,311 studies identified using the search strategy, we included 14 studies7,8,19-30 in the quantitative analysis (Figure 1). Of the included studies, six7,8,19-22 were full-text articles, and eight23-30 were conference abstracts. Although the studies by Dalal et al20,24,25 included patients treated in the same institution, each outcome of interest was different and exclusive. Therefore, the data related to the outcome of interest in this review (dose intensification,20 cs-free clinical remission,24 and clinical response25) were separately extracted from each. In addition, although studies by Fumery et al7 and Amiot et al8 included patients from the same cohort, each of the studies analyzed outcomes of different phases: maintenance phase7 and induction phase.8 Therefore, we extracted the data on effectiveness from each study. We extracted the data on optimization, discontinuation, and safety of UST only from the study by Fumery et al.7 Chiappetta et al22 reported that there were 68 patients who received the UST therapy. However, since one patient received UST for the treatment of psoriasis, we included the remaining 67 patients in this meta-analysis. The main characteristics of the studies included in this meta-analysis are summarized in Table 1.
Table 1.
Characteristics of included studies
| Authors, year | Country | Study design | Type of manuscript | Sample size, n | Gender, M, % | Age, years | Disease duration, years | Location of disease (extensive/left sided/ proctitis), n | Severity of disease | Baseline CRP | Prior IM, n, (%) | Concomitant CS, n, (%) | Prior anti-TNFα, n (%) | Prior ≥2 anti-TNFα, n, (%) | Prior VED, n, (%) | Prior TOF, n, (%) | Concomitant IM therapy, n, (%) | Assessment time point, week |
| Fumery M et al,7 2021 | France | R | Full article | 103 | 60.2 | 39.3 (29.1–52.3)b | 7.6 (3.6–12.9)b | 54/43/6 | Moderate | 7.1 (NR)a | 87 (84.5) | 50 (48.5) | 102 (99.0) | 72 (69.9) | 88 (85.4) | 10 (9.71) | 24 (23.3) | 12–16, 26, 52 |
| Chaparro M et al,19 2021 | Spain | P | Full article | 95 | 44.2 | 47 (16)a | NR | 55/37/3 | Moderate | NR | NR | 53 (55.8) | 93 (97.9) | 55 (57.9) | 78 (82.1) | 28 (29.5) | 16 (16.8) | 16, 24, 52 |
| Dalal RS et al,20 2021 | USA | R | Full article | 108 | 43.5 | 39 (30–56)c | 9 (4–16)c | NR/NR/28 | Mild | 3.6 (0.8–12.9)b | 68 (63.0) | 62 (57.4) | 99 (91.7) | 43 (39.8) | NR | NR | 18 (16.7) | 12–16 |
| Amiot A et al,8 2020 | France | P | Full article | 103 | 60.2 | 39.3 (29.1–52.3)c | 7.6 (3.6–12.9)c | 54/43/6 | Moderate | 7.1 (3.1–15.0)c | 85 (82.5) | 50 (48.5) | 102 (99.0) | 72 (69.9) | 88 (85.4) | 10 (9.71) | 24 (23.3) | 12–16 |
| Ochsenkühn T et al,21 2020 | Germany | R | Full article | 19 | 57.9 | 46 (25–81)b | 5 (2–15)b | 11/7/0 | Moderate | NR | NR | 9 (47.4) | 8 (42.1) | NR | 6 (31.6) | NR | 1 (5.26) | 52 |
| Chiappetta MF et al,22 2021 | Italy | R | Full article | 68 | 63.2 | 42 (16–72)b | 8 (NR)b | 41/25/2 | Moderate | NR | NR | 37 (54.4) | 65 (95.6) | 30 (44.1) | 47 (69.1) | NR | 15 (22.1) | 8, 24, 52 |
| Hong S et al,23 2020 | USA | R | Abstract | 19 | 47.4 | 43 (NR)b | 9.6 (NR)b | 10/8/1 | Moderate | 0.48 (0.8)a | 16 (84.2) | NR | 19 (100) | 5 (26.3) | 17 (89.5) | 2 (10.5) | 16 (84.2) | 12, 52 |
| Dalal RS et al,24 2021 | USA | R | Abstract | 81 | 21.0 | 41.7 (11.5)a | 8.6 (NR)a | 22/13/1 | Mild | 1.24 (2.53)a | 28 (34.6) | 23 (28.4) | 34 (42.0) | 17 (21.0) | 34 (42.0) | NR | 8 (9.88) | 12–16, 52 |
| Dalal RS et al,25 2021 | USA | R | Abstract | 108 | 43.5 | 39 (30–56)b | 9 (4–16)b | NR/NR/28 | Mild | 0.36 (0.08–1.29)b | 68 (63.0) | 62 (57.4) | 99 (91.7) | 43 (39.8) | 72 (66.7) | 22 (20.4) | 18 (16.7) | 12–16 |
| Haraikawa M et al,26 2021 | Japan | R | Abstract | 19 | 57.9 | 47.4 (NR)a | NR | NR/NR/NR | Moderate | NR | NR | NR | 6 (31.6) | NR | 11 (57.9) | NR | NR | 8, 24 |
| Yamana Y et al,27 2021 | Japan | R | Abstract | 11 | 27.3 | 40 (24–71)b | NR (2.08–12.7)b | 3/6/0 | NR | NR | NR | NR | 6 (54.5) | NR | NR | NR | NR | 4 |
| Asaeda K et al,28 2021 | Japan | R | Abstract | 20 | 50.0 | 42.9 (20–74)b | NR | 16/NR/NR | Moderate | NR | NR | 4 (20.0) | NR | NR | NR | NR | 6 (30.0) | 8 |
| Ando K et al,29 2021 | Japan | R | Abstract | 71 | NR | NR | NR | NR/NR/NR | NR | NR | NR | 25(35.2) | NR | NR | NR | NR | 27 (38.0) | 8, 16, 32 |
| Dominik E et al,30 2021 | Austria | R | Abstract | 26 | 69.2 | 27 (NR)b | NR | NR/NR/NR | Moderate | NR | NR | NR | NR | NR | NR | NR | NR | 8 |
M: male
R: retrospective
P: prospective
IM: immunomoderator
CS: corticosteroid
TNF: tumor necrosis factor
VED: vedolizumab
TOF: tofacitinib
NR: not reported
a Mean (standard deviation)
b Median (range)
c Median (Interquartile range)
Risk of bias
The quality of the included studies was assessed using the Newcastle-Ottawa Scale (Table 2). The mean of the Newcastle-Ottawa scale among the included studies was 5.57 points out of 6. The ‘outcome item 3’ showed a relatively low score compared to the others. Furthermore, there were some variabilities in the definition of clinical remission and response (Supplementary Table 2, Supplementary Table 3), severity of patients (Table 1), and regimen of UST treatment (Supplementary Table 4), and these differences may be subject to bias. In addition, since there were differences in the prior treatment history and refractory nature of the patients included in each study (Table 1), there might also be a selection bias in the results of the meta-analysis.
Table 2.
Quality assessment with the Newcastle-Ottawa Scale
| Selection | Comparability | Outcome | |||||||
| Author | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow up of cohorts | Total |
| Fumery M et al7 | ★ | NA | ★ | ★ | NA | ★ | ★ | ★ | 6 |
| Chaparro M et al19 | ★ | NA | ★ | ★ | NA | ★ | ★ | ★ | 6 |
| Dalal RS et al20,24,25 | ★ | NA | ★ | ★ | NA | ★ | ★ | 5 | |
| Amiot A et al8 | ★ | NA | ★ | ★ | NA | ★ | ★ | ★ | 6 |
| Ochsenkühn T et al21 | ★ | NA | ★ | ★ | NA | ★ | ★ | ★ | 6 |
| Chiappetta MF et al22 | ★ | NA | ★ | ★ | NA | ★ | ★ | ★ | 6 |
| Hong S et al23 | ★ | NA | ★ | ★ | NA | ★ | ★ | 5 | |
| Haraikawa M et al26 | ★ | NA | ★ | ★ | NA | ★ | ★ | ★ | 6 |
| Yamana Y et al27 | ★ | NA | ★ | ★ | NA | ★ | ★ | ★ | 6 |
| Asaeda K et al28 | ★ | NA | ★ | ★ | NA | ★ | ★ | 5 | |
| Ando K et al29 | ★ | NA | ★ | ★ | NA | ★ | ★ | ★ | 6 |
| Dominik E et al30 | ★ | NA | ★ | ★ | NA | ★ | ★ | 5 | |
NA: not applicable
Primary outcomes
Clinical remission. The clinical remission rate was assessed in 12 studies.7,8,19-21,23-29 The pooled clinical remission rates were 55.0% at week 8 (95% CI, 44.8–65.0%), 36.1% at week 16 (95% CI, 28.4–44.1%), 46.6% at month 6 (95% CI, 32.9–60.5%), and 38.6% at month 12 (95% CI 28.5–49.2%) (Figure 2). There was a significant between-study heterogeneity in the analyses of clinical remission at week 16 and month 6 (I2 = 62%–70%, P = 0.01). Meta-regression analysis was performed to explain the significant between-study heterogeneity in the effectiveness of the UST. The covariates selected in the present meta-regression were the study design; number of centers; prior use of anti-TNF agents, more than two anti-TNF agents, VDZ, or TOF; severity of disease; concomitant use of immunomodulators or steroids; and publication style of studies. The results of the meta-regression analysis showed that prior use of anti-TNF agents and VDZ at week 16 (QM test of moderators: prior anti-TNF agent: QM = 4.1836, P = 0.0408; prior use of VDZ: QM = 4.0142, P = 0.0451), prior use of anti-TNF agents or VDZ, and publication style at month 6 (QM test of moderators: prior anti-TNF agent: QM = 8.2875, P = 0.004; prior use of VDZ: QM = 6.4679, P = 0.011; publication style of studies: QM = 4.6219, P = 0.0316) were significant moderators (Table 3) (Figure 3, 4).
Fig. 2.

Forest plot of the clinical remission rate: (A) at week 8, (B) at week 16, (C) at month 6, and (D) at month 12
Table 3.
All the QM statistics for whether the moderators have a significant effect on clinical remission rate
| (A) Clinical remission week 8 | |||
| Moderator | QM | df | p |
| Number of center | 0.0038 | 1 | 0.9506 |
| Prior anti-TNFα | 0.0884 | 1 | 0.7662 |
| Severity | 0.2327 | 1 | 0.6295 |
| Concomittant IM | 2.8879 | 1 | 0.0892 |
| Concomittant steroid | 3.1418 | 1 | 0.0763 |
| Publish style | 2.8879 | 1 | 0.0892 |
| (B) Clinical remission week 16 | |||
| Moderator | QM | df | p |
| Study design | 0.0183 | 1 | 0.8923 |
| Number of center | 0.0963 | 1 | 0.7563 |
| Prior anti-TNFα | 4.1836 | 1 | 0.0408 |
| Prior more than two anti-TNFα | 0.0006 | 1 | 0.9804 |
| Prior VDZ | 4.0142 | 1 | 0.0451 |
| Prior TOF | 0.1771 | 1 | 0.6739 |
| Severity | 0.9828 | 2 | 0.6118 |
| Concomittant IM | 1.6126 | 1 | 0.2041 |
| Concomittant steroid | 0.3863 | 1 | 0.5343 |
| Publish style | 0.0194 | 1 | 0.8892 |
| (C) Clinical remission month 6 | |||
| Moderator | QM | df | p |
| Study design | 0.3507 | 1 | 0.5537 |
| Number of center | 3.0936 | 1 | 0.0786 |
| Prior anti-TNFα | 8.2875 | 1 | 0.004 |
| Prior VDZ | 6.4679 | 1 | 0.011 |
| Severity | 1.386 | 1 | 0.2391 |
| Concomittant IM | 0.0321 | 1 | 0.8578 |
| Concomittant steroid | 1.3962 | 1 | 0.2374 |
| Publish style | 4.6219 | 1 | 0.0316 |
| (D) Clinical remission month 12 | |||
| Moderator | QM | df | p |
| Number of center | 1.0845 | 2 | 0.5814 |
| Prior anti-TNFα | 3.7936 | 1 | 0.0514 |
| Prior more than two anti-TNFα | 0.3586 | 1 | 0.5493 |
| Prior VDZ | 3.7217 | 1 | 0.0537 |
| Severity | 1.1402 | 1 | 0.2856 |
| Concomittant IM | 1.0255 | 1 | 0.3112 |
| Concomittant steroid | 0.637 | 1 | 0.4248 |
| Publish style | 0.005 | 1 | 0.9434 |
QM: test statistic for the omnibus test of moderators
TNF: tumor necrosis factor
df: degrees of freedom
VDZ: vedolizumab
TOF: tofacitinib
IM: immunomodulator
Fig. 3.
Meta-regression scatter plot of the clinical remission rate based on the prior use of anti-tumor necrosis factor agents
Fig. 4.
Meta-regression scatter plot of the clinical remission rate based on the prior use of vedolizumab
Secondary outcomes
Clinical response. The clinical response rate was assessed in 13 studies.7,8,19-27,29,30 The pooled clinical response rates were 70.2% at week 8 (95% CI, 53.2–85.0%), 56.4% at week 16 (95% CI, 49.7–63.0%), 83.8% at month 6 (95% CI, 74.8–91.3%), and 62.6% at month 12 (95% CI, 16.3–98.1%) (Supplementary Figure 1). There was significant between-study heterogeneity in the analyses of clinical response at week 8 and month 12 (I2 = 76%–92%, P < 0.01). Meta-regression analysis was performed, although the covariates included in the analysis at week 8, month 6, and month 12 were limited because of the lack of studies in which the data were available for the analysis. The meta-regression analysis showed that no covariate was a significant moderator (Table 4).
Table 4.
All the QM statistics for whether the moderators have a significant effect on clinical response rate
| (A) Clinical response week 8 | |||
| Moderator | QM | df | p |
| Number of center | 0.0003 | 1 | 0.9506 |
| Severity | 1.226 | 1 | 0.2682 |
| (B) Clinical response week 16 | |||
| Moderator | QM | df | p |
| Study design | 0.9954 | 1 | 0.3184 |
| Number of center | 1.6779 | 2 | 0.4322 |
| Prior anti-TNFα | 0.3354 | 1 | 0.5625 |
| Prior more than two anti-TNFα | 0.0167 | 1 | 0.8971 |
| Prior VDZ | 0.0059 | 1 | 0.9387 |
| Prior TOF | 0.063 | 1 | 0.8019 |
| Severity | 3.0222 | 2 | 0.2207 |
| Concomittant IM | 0.0733 | 1 | 0.7865 |
| Concomittant steroid | 0.2718 | 1 | 0.6021 |
| Publish style | 0.9954 | 1 | 0.3184 |
| (C) Clinical response month 6 | |||
| Moderator | QM | df | p |
| Severity | 0.0136 | 1 | 0.9073 |
| Publish style | 0.7295 | 1 | 0.3931 |
QM: test statistic for the omnibus test of moderators
TNF: tumor necrosis factor
df: degrees of freedom
VDZ: vedolizumab
TOF: tofacitinib
IM: immunomodulator
Corticosteroid-free clinical remission. The corticosteroid-free clinical remission rate was assessed in 11 studies.7,8,19-25,27,29 The pooled corticosteroid-free clinical remission rates were 29.7% at week 16 (95% CI, 18.1–42.7%), 30.1% at month 6 (95% CI, 24.4–36.0%), and 38.8% at month 12 (95% CI, 28.8–49.2%) (Supplementary Figure 2). There was no data on corticosteroid-free clinical remission at week 8. Significant between-study heterogeneity was observed in the analyses of corticosteroid-free clinical remission at week 14 (I2 = 75%, P = 0.02). The results of the meta-regression analysis showed that no covariate was a significant moderator. Meta-regression analysis at week 8 could not be performed, and covariates included in the analysis at months 6 and 12 were limited due to the lack of data available for the analysis (Table 5).
Table 5.
All the QM statistics for whether the moderators have a significant effect on corticosteroid-free clinical remission rate
| (A) Corticosteroid-free clinical remission week 16 | |||
| Moderator | QM | df | p |
| Study design | 0.2123 | 1 | 0.6449 |
| Number of center | 0.5493 | 1 | 0.4586 |
| Prior anti-TNFα | 0.5493 | 1 | 0.4586 |
| Prior more than two anti-TNFα | 0.5493 | 1 | 0.4586 |
| Prior VDZ | 0.5493 | 1 | 0.4586 |
| Severity | 0.5493 | 1 | 0.4586 |
| Concomittant IM | 0.5493 | 1 | 0.4586 |
| Concomittant steroid | 0.5493 | 1 | 0.4586 |
| Publish style | 0.5493 | 1 | 0.4586 |
| (B) Clinical remission month 6 | |||
| Moderator | QM | df | p |
| Study design | 0.0001 | 1 | 0.9918 |
| Concomittant IM | 0.0049 | 1 | 0.9441 |
| Concomittant steroid | 0.0637 | 1 | 0.8008 |
| (C) Clinical remission month 12 | |||
| Moderator | QM | df | p |
| Prior anti-TNFα | 0.6304 | 1 | 0.4272 |
| Prior VDZ | 2.6149 | 1 | 0.1059 |
| Concomittant IM | 0.5228 | 1 | 0.4697 |
| Concomittant steroid | 0.5238 | 1 | 0.4692 |
| Publish style | 0.3312 | 1 | 0.5649 |
QM: test statistic for the omnibus test of moderators
TNF: tumor necrosis factor
df: degrees of freedom
VDZ: vedolizumab
TOF: tofacitinib
IM: immunomodulator
Optimization of UST interval in maintenance phase. Four studies7,19,20,30 reported that there was an interval shortening of the UST (Table 6). The rate of UST interval shortening ranged from 27.2% to 63.1%. Among the three studies,7,19,20 reporting the outcome after interval shortening, clinical remission, and clinical response was achieved in 5.6%–55% and 30.7%–67.5%, respectively.
Table 6.
Optimization and discontinuation of ustekinumab
| Author | N | Follow up time, week (mean or median) | Shortening of UST interval, n, (%) | Patients with discontinuation, n, (%) | Reason of discontinuation | Interval of UST in maintenance regimen before shortening | Interval of UST in maintenance regimen after shortening | Reason of interval shortening | Outcome after interval shortening |
| Fumery M et al7 | 103 | 48a | 65 (63.1) | 45 (43.7) | Lack of effectiveness (n = 41) Pregnancy (n = 1) Adverse event (n = 1) Personal decision after two episodes of mild skin rash (n=1) | Every 8 weeks | Every 4 weeks | NR | Clinical response (n=20) (30.7%) Clinical remission (n=17) (26.1%) |
| Chaparro M et al19 | 95 | 82b | 18 (27.2) | 34 (36.0) | Primary non-response (n=21) (22%) Loss of response in (n=12) (13%) Adverse event in (n=1) (1%) | Three patients (10%) started the maintenance phase with every-12-week schedule, 24 patients (80%) with every-8-week, and 3 (10%) with intensified schedule (every 6 weeks or every 4 weeks) | Every 4 or 6 weeks | Primary failure (n=10) (55%) Partial response (n=3) (17%) Loss of response (n=5) (28%) | 1 (5.6%) patient who escalated the dose due to loss of response reached remission. |
| Dalal RS et al20,24,25 | 108 | NR | 46 (42.6) | NR | NR | Every 8 weeks | Every 4 or 6 weeks | No initial response (n=22) (47.8%) Loss of response (n=20) (43.5%) | Remission (n=22) (55.0%) Response (n=27) (67.5%) Drug discontinuation or colectomy (n=12) (13%) within 16 weeks after intensification |
| Ochsenkühn T et al21 | 19 | NR | NR | 5 (26.3) | Refractory disease (n=4) (80%) Side effect (n=1) (20%) | Every 8 weeks | NR | NR | NR |
| Chiappetta MF et al22 | 67 | NR | 0 (0) | 9 (13.4) | Primary failure (n=1, 11.1%) Secondary failure (n=7, 77.8%) AEs (n=1, 11.1%) | Every 8 weeks | NR | NR | NR |
| Hong S et al23 | 19 | NR | NR | NR | NR | NR | NR | NR | NR |
| Haraikawa M et al26 | 19 | NR | NR | NR | NR | NR | NR | NR | NR |
| Yamana Y et al27 | 11 | NR | NR | NR | NR | NR | NR | NR | NR |
| Asaeda K et al28 | 20 | NR | NR | NR | NR | NR | NR | NR | NR |
| Ando K et al29 | 71 | NR | NR | NR | NR | NR | NR | NR | NR |
| Dominik E et al30 | 26 | NR | 14 (53.8) | 3 (11.5) | Lack of improvement (n=2) (66.7%) Colorectal cancer (n=1) (33.3%) | Every 8 weeks | Every 4 or 6 weeks | NR | NR |
a median
b mean
NR: not reported
Discontinuation rates. Five studies7,19,21,22,30 reported the discontinuation of UST (Table 6). Discontinuation rates ranged from 11.5% to 43.7%. The most common reason for discontinuation was the lack of the effectiveness of UST, as reported in 28.4% of patients (88 out of 310 patients). Treatment discontinuation due to AEs occurred in 1.29% (4 out of 310 patients) of patients.
Safety. Safety outcomes were reported in five studies.7,19-22 In total, 28 AEs were reported in 258 patients (10.9%). The specific characteristics of an AE are summarized in Table 7. The infection rate was 4.26% (11 out of 243). The most common non-infectious AEs, except inflammatory bowel disease exacerbation, was arthralgia (n = 5, 1.94%), followed by skin rash (n = 4, 1.55%).
Table 7.
Adverse events of ustekinumab treatment for patients with ulcerative colitis
| No. of studies, n | Total patients, n | Patients with AE, n, (%) | Source | |
| Any AEs | 5 | 258 | 28 (10.9) | Fumery M et al,7
Chaparro M et al,19 Dalal RS et al,20 Ochsenkühn T et al,21 Chiappetta MF et al22 |
| Infection | 4 | 11 (4.3) | ||
| Pneumonia | 1 | 1 (0.4) | Fumery M et al7 (n=1) | |
| Dental abscess | 1 | 2 (0.8) | Fumery M et al7 (n=2) | |
| Clostridium difficile infection | 2 | 2 (0.8) | Fumery M et al7 (n=1),
Dalal RS et al20 (n=1) |
|
| Urinary tract infection | 1 | 1 (0.4) | Chaparro M et al19 (n=1) | |
| Rhinopharyngitis | 1 | 1 (0.4) | Fumery M et al7 (n=1) | |
| Lateral pharyngitis | 1 | 1 (0.4) | Ochsenkühn T et al21 (n=1) | |
| Otitis media | 1 | 1 (0.4) | Ochsenkühn T et al21 (n=1) | |
| Covid 19 | 1 | 1 (0.4) | Chaparro M et al19 (n=1) | |
| Malignancies | 1 | 1 (0.4) | ||
| Breast cancer | 1 | 1 (0.4) | Ochsenkühn T et al21 (n=1) | |
| Others | 5 | 17 (6.6) | ||
| Skin rash | 2 | 4 (1.6) | Fumery M et al7 (n=3),
Chaparro M et al19 (n=1) |
|
| Arthralgia | 1 | 5 (1.9) | Fumery M et al7 (n=5) | |
| IBD exacerbation | 2 | 10 (3.9) | Fumery M et al 7 (n=6),
Dalal RS et al 20 (n=4) |
|
| Symptomatic urolithiasis | 1 | 1 (0.4) | Fumery M et al7 (n=1) | |
| Gastroenteritis | 1 | 2 (0.8) | Fumery M et al7 (n=2) | |
| Myocardial infarction | 1 | 1 (0.4) | Fumery M et al7 (n=1) | |
| Fatigue | 1 | 1 (0.4) | Fumery V et al7 (n=1) | |
| Rectal adenoma | 1 | 1 (0.4) | Ochsenkühn T et al21 (n=1) | |
| Hearing loss | 1 | 1 (0.4) | Ochsenkühn T et al21 (n=1) | |
| Atrial fibrillation | 1 | 1 (0.4) | Ochsenkühn T et al21 (n=1) | |
| Pituitary adenoma | 1 | 1 (0.4) | Chiappetta MF et al22 (n=1) | |
| Retinal detachment | 1 | 1 (0.4) | Ochsenkühn T et al21 (n=1) |
AE: adverse events
IBD: inflammatory bowel disease
Publication bias evaluation
We found statistically significant evidence of publication bias using the Egger’s test in the analysis of clinical remission at month 6 and corticosteroid-free clinical remission at month 6 (Figure 5, Supplementary Figures 3 and 4). Although statistical evidence of publication bias was not found in the Egger’s regression test in other analyses, the number of studies included was too small to adequately assess publication bias.18
Fig. 5.
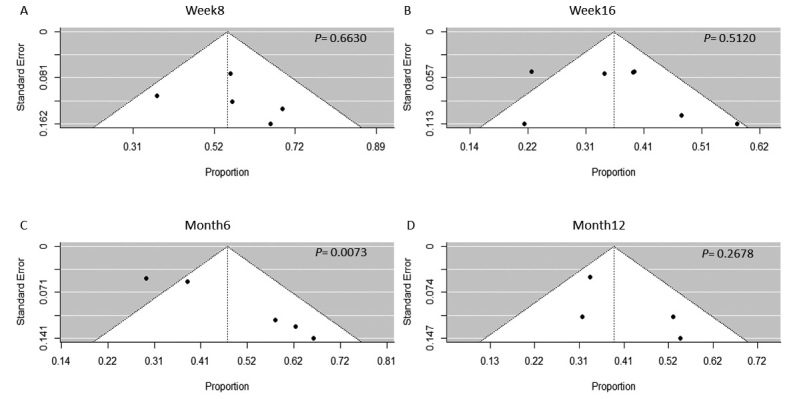
Funnel plot analysis of the clinical remission rate
DISCUSSION
To the best of our knowledge, this was the most comprehensive systematic review and meta-analysis that evaluated the real-world effectiveness of UST for the treatment of UC. Although a systematic review of real-world effectiveness in inflammatory bowel disease was reported recently,6 the study included only three studies on UC. In addition, although the effectiveness of UST for patients with UC was demonstrated in the UNIFI clinical trials,1 the efficacy of RCT was not representative of the real-world population.3 Therefore, the results of this review can provide valuable information for clinicians to develop treatment strategies for patients with UC in daily clinical practice.
This study showed that the pooled clinical remission and response rate at week 8 were 55% and 70.2%, respectively. In addition, the pooled clinical remission, clinical response, and corticosteroid-free clinical remission rate at week 44 in this meta-analysis were 38.6%, 62.6%, and 38.8%, respectively. On the other hand, the UNIFI trial reported that the clinical remission and response rate of patients who received 6 mg/kg of UST on week 8 were 15.5% and 61.8%, respectively, and the clinical remission, response, and corticosteroid-free clinical remission rate at week 44 were 38.4–43.8%, 68.0–71.0%, and 37.8–42.0%, respectively.1 The results from this study were consistent with the results of the induction and maintenance therapy reported in the UNIFI trial, despite the fact that patients in real-world settings could have more complex disease characteristics compared with those in the RCT.
Investigating the predictors of response to UST is important for clinicians in the selection of patients who are eligible for UST treatment. Some studies reported that prior use of anti-TNF-α agents was a predictor of the poor efficacy of UST in patients with Crohn’s disease.31,32 In the UNIFI trial, although the effectiveness of UST was also shown in the subgroup of patients who had previous treatment failure with biologics, the same patients seemed to have a lower response to UST than those who took biologics without failure of treatment.1 However, the impact of previous treatment on the effectiveness of UST in patients with UC has not been well demonstrated. In this study, the results of the meta-regression analysis showed that patients with prior use of anti-TNF-α agents or VDZ had a lower clinical remission rate, although there was no statistically significant correlation at week 8 and month 12. Based on these results, UST might be preferred as an early-line biologic treatment option in patients with UC. However, this result should be interpreted with caution because the effectiveness of anti-TNF-α agents and VDZ in patients with previous UST failure is unknown. Furthermore, since unidentified factors could be responsible for the differential effect sizes across subgroups, the results of the meta-regression analysis could not be interpreted as causal evidence. Therefore, further prospective studies are needed to investigate the predictors of response to biologics and to compare their effectiveness.
Overall, UST was well tolerated. There were no new serious AEs reported in this study, although safety data in real-world practice yielded a lower safety profile lower compared to those in RCTs because of their stricter reporting process of AEs. The reason for discontinuation of UST was lack of effectiveness, whereas the rate of discontinuation due to AEs was low (1.29%) in this study.
Dose escalation or interval shortening of biologics are one of the treatment strategies for the lack of effectiveness of treatments for inflammatory bowel disease.33 There were four studies,7,19,20,30 in which the UST interval was shortened in this study. Of these studies that reported the interval shortening of UST, clinical remission was achieved in 5.6%–55.0% of patients with primary or secondary failure of UST. Although predictors of response to dose intensification are not known, interval shortening of UST can be a useful treatment strategy due to the lack of effectiveness of the UST therapy in patients with UC.
This study has several limitations. First, statistically significant between-study heterogeneity was detected, as shown by the I2 value. Although we performed a meta-regression analysis, the number of studies included in the meta-regression analysis was small. Hence, the statistical power to identify significant factors was limited,34,35 and heterogeneity was not completely controlled. Furthermore, the definitions of clinical remission and response, time points used to evaluate the efficacy of UST, severity of patients, and dosing regimen of UST varied. Thus, these differences could contribute to heterogeneity, and the reliability of the pooled effect sizes is relatively limited. Second, since some retrospective studies or conference abstracts were included in this study, the results could be biased due to incomplete findings. Third, publication bias, which was shown in the results of the funnel plots and Egger’s regression test, could limit the results of this study, although the inclusion of conference abstracts and full papers could minimize the risk of publication bias. Fourth, since data on the endoscopic information were not available in the majority of studies, endoscopic mucosal healing, which was reportedly associated with improved long-term outcomes in patients with UC,36 could not be assessed in this study. Nevertheless, we believe that the real-world effectiveness of UST in heterogeneous and complex patient populations shown in this review could provide important insights into daily clinical practice.
CONCLUSION
Real-world data supported the effectiveness and safety of UST in patients with UC. The early use of UST prior to other biologic agents, such as anti-TNF-α agents or VDZ, might be one of the treatment strategies for patients with UC to maximize the potential of UST. However, further prospective studies investigating the predictors of the effectiveness and long-term outcomes of UST are required.
CONFLICT OF INTEREST
None declared.
Supplementary Materials
Cut-off values used to classify the disease severity
Definition of clinical remission in each studies
Definition of clinical response in each studies
Outcome measures and treatment regimens in each studies
Forest plot of the clinical response rate
Forest plot of the corticosteroid-free clinical remission rate
Funnel plot analysis of the clinical response rate
Funnel plot analysis of the corticosteroid-free clinical response rate
Details of literature search from electronic databases
Abbreviations
- UST
ustekinumab
- UC
ulcerative colitis
- RCTs
randomized controlled trials
- TNF
tumor necrosis factor
- VDZ
vedolizumab
- AEs
adverse events
REFERENCES
- 1.Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2019;381(13):1201–1214. doi: 10.1056/NEJMoa1900750. [DOI] [PubMed]
- 2.Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-World Evidence – What Is It and What Can It Tell Us? N Engl J Med. 2016;375(23):2293–2297. doi: 10.1056/NEJMsb1609216. [DOI] [PubMed]
- 3.Ha C, Ullman TA, Siegel CA, Kornbluth A. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol. 2012;10(9):1002–1007;quiz e1078. doi: 10.1016/j.cgh.2012.02.004. [DOI] [PubMed]
- 4.Feuerstein JD, Isaacs KL, Schneider Y, et al. AGA Clinical Practice Guidelines on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology. 2020;158(5):1450–1461. doi: 10.1053/j.gastro.2020.01.006. [DOI] [PMC free article] [PubMed]
- 5.Raine T, Bonovas S, Burisch J, et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J Crohns Colitis.2022;16(1):2–17. doi: 10.1093/ecco-jcc/jjab178. [DOI] [PubMed]
- 6.Honap S, Meade S, Ibraheim H, Irving PM, Jones MP, Samaan MA. Effectiveness and Safety of Ustekinumab in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2022;67(3):1018–1035. doi: 10.1007/s10620-021-06932-4. [DOI] [PubMed]
- 7.Fumery M, Filippi J, Abitbol V, et al. Effectiveness and safety of ustekinumab maintenance therapy in 103 patients with ulcerative colitis: a GETAID cohort study. Aliment Pharmacol Ther. 2021;54(7):944–951. doi: 10.1111/apt.16544. [DOI] [PubMed]
- 8.Amiot A, Filippi J, Abitbol V, et al. Effectiveness and safety of ustekinumab induction therapy for 103 patients with ulcerative colitis: a GETAID multicentre real-world cohort study. Aliment Pharmacol Ther. 2020;51(11):1039–1046. doi: 10.1111/apt.15717. [DOI] [PubMed]
- 9.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed]
- 10.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317(26):1625–1629. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed]
- 11.Higgins PD, Schwartz M, Mapili J, Krokos I, Leung J, Zimmermann EM. Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut. 2005;54(6):782–788. doi: 10.1136/gut.2004.056358. [DOI] [PMC free article] [PubMed]
- 12.Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989;298(6666):82–86. doi: 10.1136/bmj.298.6666.82. [DOI] [PMC free article] [PubMed]
- 13.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed Feb 10, 2022.
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed]
- 15.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed]
- 17.Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Stat Softw. 2010;36(3):1–48. doi: 10.18637/jss.v036.i03. [DOI]
- 18.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed]
- 19.Chaparro M, Garre A, Iborra M, et al. Effectiveness and Safety of Ustekinumab in Ulcerative Colitis: Real-world Evidence from the ENEIDA Registry. J Crohns Colitis. 2021;15(11):1846–1851. doi: 10.1093/ecco-jcc/jjab070. [DOI] [PMC free article] [PubMed]
- 20.Dalal RS, Esckilsen S, Barnes EL, Pruce JC, Marcus J, Allegretti JR. Predictors and Outcomes of Ustekinumab Dose Intensification in Ulcerative Colitis: A Multicenter Cohort Study. Clin Gastroenterol Hepatol. 2022;20(10):2399–2401.e4. doi: 10.1016/j.cgh.2021.03.028. [DOI] [PMC free article] [PubMed]
- 21.Ochsenkühn T, Tillack C, Szokodi D, Janelidze S, Schnitzler F. Clinical outcomes with ustekinumab as rescue treatment in therapy-refractory or therapy-intolerant ulcerative colitis. United European Gastroenterol J. 2020;8(1):91–98. doi: 10.1177/2050640619895361. [DOI] [PMC free article] [PubMed]
- 22.Chiappetta MF, Viola A, Mastronardi M, et al. One-year effectiveness and safety of ustekinumab in ulcerative colitis: a multicenter real-world study from Italy. Expert Opin Biol Ther. 2021;21(11):1483–1489. doi: 10.1080/14712598.2021.1981855. [DOI] [PubMed]
- 23.Hong S, Zullow S, Axelrad J, Chang S, Hudesman D. P087 Real-world effectiveness of ustekinumab in ulcerative colitis. Gastroenterology. 2020;158(3 Suppl):S120. doi: 10.1053/j.gastro.2019.11.272. [DOI]
- 24.Dalal RS, Mitri J, Goodrick H, Allegretti JR. 24 Real-world comparison of tofacitinib versus ustekinumab among ulcerative colitis patients with prior anti-tumor necrosis factor alpha and anti-integrin treatment failure: a propensity score-adjusted analysis. Gastroenterology. 2021;160(6 Suppl):S5. doi: 10.1016/S0016-5085(21)00761-7. [DOI]
- 25.Dalal RS, Esckilsen S, Barnes EL, Pruce JC, Marcus J, Allegretti JR. Sa555 Colectomy-free drug survival of ustekinumab in ulcerative colitis: a real-world, multicenter cohort study in the United States. Gastroenterology. 2021;160(6 Suppl):S549. doi: 10.1016/S0016-5085(21)02011-4. [DOI]
- 26.Haraikawa M, Shibuya T, Fukuo Y, et al. Effectiveness of ustekinumab in patients with ulcerative colitis [in Japanese]. Jpn J Gastroenterol. 2021;118(S2):A695.
- 27.Yamana Y, Matsubara D, Osada S, et al. Predictor of effectiveness of ustekinumab in patients with refractory ulcerative colitis [in Japanese]. Jpn J Gastroenterol. 2021;118(S2):A691.
- 28.Asaeda K, Uchiyama K, Takagi T, et al. Effectiveness of ustekinumab in patients with ulcerative colitis [in Japanese]. Jpn J Gastroenterol. 2021;118(S2):A729.
- 29.Ando K, Fujiya M, Nakase H. Real-world effectiveness and predictor of short-term outcome of ustekinumab in patients with ulcerative colitis [in Japanese]. Jpn J Gastroenterol. 2021;118(S2):A652.
- 30.Ecker D, Fuchssteiner H, Gregus M, et al. P0395 Ustekinumab for Ulcerative Colitis A real-world experience – retrospective data analysis of the IBD cohort Ordensklinikum Linz. United European Gastroenterol J. 2021;9(S8):490. doi: 10.1002/ueg2.12144. [DOI]
- 31.Iborra M, Beltrán B, Fernández-Clotet A, et al. Real-world long-term effectiveness of ustekinumab in Crohn’s disease: results from the ENEIDA registry. Aliment Pharmacol Ther. 2020;52(6):1017–1030. doi: 10.1111/apt.15958. [DOI] [PubMed]
- 32.Kubesch A, Rueter L, Farrag K, et al. Short and Long-Term Effectiveness of Ustekinumab in Patients with Crohn’s Disease: Real-World Data from a German IBD Cohort. J Clin Med. 2019;8(12):2140. doi: 10.3390/jcm8122140. [DOI] [PMC free article] [PubMed]
- 33.Dalal RS, Cohen RD. What to Do When Biologic Agents Are Not Working in Inflammatory Bowel Disease Patients. Gastroenterol Hepatol (N Y). 2015;11(10):657–665. [PMC free article] [PubMed]
- 34.Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions 4.2.6. In: The Cochrane Library. Issue 4. Chichester, UK: John Wiley & Sons, Ltd; 2006.
- 35.Littell JH, Corcoran J, Pillai V. Systematic Reviews and Meta-Analysis. Oxford: Oxford University Press; 2008.
- 36.Shah SC, Colombel JF, Sands BE, Narula N. Mucosal Healing Is Associated With Improved Long-term Outcomes of Patients With Ulcerative Colitis: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016;14(9):1245–1255.e8. doi: 10.1016/j.cgh.2016.01.015. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cut-off values used to classify the disease severity
Definition of clinical remission in each studies
Definition of clinical response in each studies
Outcome measures and treatment regimens in each studies
Forest plot of the clinical response rate
Forest plot of the corticosteroid-free clinical remission rate
Funnel plot analysis of the clinical response rate
Funnel plot analysis of the corticosteroid-free clinical response rate
Details of literature search from electronic databases




