ABSTRACT
Frailty is considered one of the most important indicators of a patient’s general condition. However, only a few studies have investigated the association between preoperative frailty and postoperative complications in pancreatic cancer. Therefore, this study aimed to examine this association in patients with pancreatic cancer. We retrospectively reviewed 52 consecutive patients who underwent pancreatectomy for pancreatic cancer between July 2019 and March 2021. Patients were classified into two groups according to the presence of postoperative complications. Their characteristics and clinical parameters, including physical function, were analyzed. Patients with postoperative complications had a higher prevalence of frailty (58.8% vs 14.3%, p = 0.003) and a shorter 6-min walk distance (380 m vs 436 m, p = 0.020) than those without postoperative complications. Logistic regression analysis identified preoperative frailty as the only independent risk factor for complications after pancreatectomy (p = 0.002). Preoperative frailty is associated with postoperative complications of pancreatectomy. Since preoperative frailty can be easily evaluated, it is a useful predictor of postoperative complications after pancreatectomy.
Key Words: pancreatic cancer, postoperative complication, frailty
INTRODUCTION
Improvements in the survival rate of patients with pancreatic cancer (PC) have been reported due to advancements in chemotherapy and surgical techniques.1 Pancreatectomy is a highly invasive procedure and has a high incidence rate of postoperative complications, such as pancreatic fistula and subsequent intra-abdominal abscess, ranging from 18% to 60%.2 Therefore, it requires prolonged hospitalization. Cardiopulmonary function and lower limb muscle strength have been reported to significantly decrease 3 months after pancreatectomy.3 Thus, careful attention should be paid to postoperative complications and physical conditions during the perioperative period.
Frailty is defined as a clinical syndrome of progressive decline in physical and mental functioning, regardless of comorbidities,4 and it is a particularly important issue in an aging society. Frailty is a good predictor of postoperative complications after hepatectomy5 and a possible prognostic factor.6 Therefore, in providing perioperative physical therapy, frailty is considered one of the most important indicators to understand the patient’s general condition, such as physical function and exercise tolerance.
Although several studies have investigated the association between preoperative frailty and postoperative complications, only a few have focused exclusively on PC.7-9 Moreover, they focused on relatively severe postoperative complications.8,9 Therefore, this study aimed to investigate whether preoperative frailty is a useful predictor of all postoperative complications, with an exclusive focus on patients with PC.
MATERIALS AND METHODS
Patients
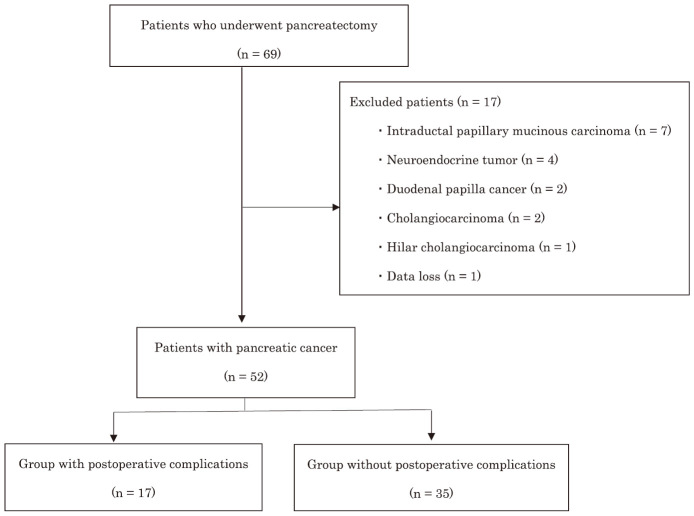
In this retrospective study, patient data were obtained from their medical records. Of the 69 consecutive patients who underwent pancreatectomy at our hospital between July 2019 and March 2021, 52 with PC were included in this study after excluding 17 patients with diseases other than PC and data loss (Fig. 1). According to the Declaration of Helsinki, an opt-out procedure was conducted on our website, and the outline of the study was open to the public so that patients could have the opportunity to refuse to be included in the study. This study was approved by the Institutional Review Board of Kitakyushu Municipal Medical Center (approval number: 202105011).
Fig. 1.
Patient flow diagram
Sixty-nine patients underwent pancreatectomy during the study period. Among them, 52 patients who underwent pancreatectomy for pancreatic cancer were enrolled after excluding 17 patients with other diseases. Finally, the characteristics and clinical parameters of patients with (n = 17) and without (n = 35) postoperative complications were analyzed.
Measurement of frailty
The Clinical Frailty Scale (CFS) was used to evaluate frailty.10 The CFS assesses the health status of the patient over the past 2 weeks. It includes the following four characteristics of the patient: movement, functioning, thinking, and emotions. Each level is scored by comparing a picture with a description.11 The CFS is a combination of clinical judgment and objective measurement, with grades ranging from 1 (very fit) to 9 (terminally ill).12 In this study, a CFS grade ≥ 4 was used to define frailty.5 The use of the CFS was determined by two experienced physical therapists (MO and SK).
Postoperative complications
The Clavien–Dindo (CD) classification, which is categorized into five levels according to the degree of treatment, was used to assess the postoperative complications in this study.13 Grade II or higher level was indicated a complication.14
Measurement items
The variables were as follows: age, sex, body mass index (BMI), Brinkman index score, cancer stage,15 preoperative neoadjuvant chemotherapy (NAC), Geriatric Nutritional Risk Index score, length of hospital stay after surgery, and Charlson Comorbidity Index (CCI) score. Further, CCI score was classified as 0 (low), 1–2 (medium), 3–4 (high), and ≥ 5 (very high).16 Respiratory function variables included vital capacity (VC), predicted VC (%VC), forced expiratory volume in 1 s (FEV1.0), and predicted FEV1.0 (%FEV1.0). Surgical factors included operative time, total fluid volume, and blood loss volume. Preoperative blood data included hemoglobin, serum total protein, and serum albumin (Alb) levels. Preoperative physical function was assessed by measuring the 6-min walk distance (6MWD), grip strength, and knee extensor strength. The 6MWD was measured under maximal effort according to the American Thoracic Society.17 Knee extension muscle strength was assessed using a handheld dynamometer (Anima Co, Ltd, µTas F-1). It was measured with the patient in the sitting position and the knee positioned at approximately 90° flexion. The dynamometer force pad was placed just proximal to the ankle joint and used to measure the force produced during quadriceps muscle contraction. Grip strength was measured twice on each side using a digital grip strength meter (Takei Scientific Instruments Co, Ltd, Grip D) in the standing position, and the maximum value was adopted. Since muscle strength varies with body size, the measurement results were divided by body weight to normalize body size.18
Physical therapy program
Coughing and breathing exercises, training for upper and lower limb muscle strength, and ergometry were performed for preoperative physical therapy from the day of admission to the day before surgery for all patients in this study. Early mobilization and exercise therapy were started on the day after surgery until the day before discharge.
Statistical analyses
Data are presented as median (interquartile range), regardless of normality. Patients were classified into two groups: those with and without postoperative complications. For continuous variables, the unpaired t-test for normality and Mann–Whitney U test for non-normality were used. For categorical variables, the chi-squared test was used. Logistic regression analysis was performed to identify whether preoperative frailty was a useful predictor of postoperative complications.
The dependent variable was the presence or absence of postoperative complications, and the independent variable was preoperative frailty. Age, 6MWD, and Alb level were selected as adjustment variables based on previous studies.14,19-21 To compare postoperative survival in patients with and without preoperative frailty, survival curves were obtained using the Kaplan–Meier method, and log-rank tests were performed to determine differences between the two groups. All statistical analyses were performed using EZR on commander version 1.55 (Saitama Medical Center, Jichi Medical University, Saitama, Japan).
RESULTS
Patients’ basic characteristics
Table 1 shows the basic characteristics of the 52 patients. There were 26 men and 26 women, with a median age of 72 (interquartile range, 69–76) years. Among the 52 patients, 39 (75 %) received NAC. Pancreatoduodenectomy (PD) and distal pancreatectomy (DP) were performed in 29 (55.8 %) and 23 (44.2 %) patients, respectively.
Table 1.
Preoperative characteristics of the study patients
| Age, years | 72 (69–76) |
| Male/female, n (%) | 26/26 (50/50) |
| BMI (kg/m2) | 22.2 (21.3–24.2) |
| Procedure, PD/DP (%) | 29 (55.8)/23 (44.2) |
| Stage, n (%) | |
| 0 | 2 (3.8) |
| IA | 4 (7.7) |
| IB | 0 (0) |
| II A | 20 (38.4) |
| II B | 24 (46.1) |
| III | 1 (1.9) |
| IV | 1 (1.9) |
| NAC (%) | 39 (75) |
| Comorbidities, n (%) | |
| Diabetes | 30 (57.7) |
| Respiratory disease | 9 (17.3) |
| Heart disease | 9 (17.3) |
Values are reported as the median (interquartile range) or number of patients (percentage).
BMI: body mass index
PD: pancreatoduodenectomy
DP: distal pancreatectomy
NAC: neoadjuvant chemotherapy
Postoperative complications
Descriptions of postoperative complications are shown in Table 2. Postoperative complications were observed in 17 (32.7%) patients, including 14 with pancreatic fistula (six with pancreatic fistula only [CD classification, III a for all], three with abscess [II, III a, and III b], two with hemorrhage [II and III b], one with atelectasis [II], one with wound infection [II], one with bile leakage [III a], one with a pulmonary embolus [II], one with atelectasis [II], and one with respiratory failure [IV a]).
Table 2.
Postoperative complications observed in 17 patients after pancreatectomy
| Pancreatic fistula | 14 (82) | |
| Pancreatic fistula only | 6 (35) | |
| + Intra-abdominal abscess | 3 (18) | |
| + Intra-abdominal hemorrhage | 2 (12) | |
| + Bile leakage | 1 (6) | |
| + Atelectasis | 1 (6) | |
| + Wound infection | 1 (6) | |
| Pulmonary embolus | 1 (6) | |
| Atelectasis | 1 (6) | |
| Respiratory failure | 1 (6) |
Data are presented as n (%).
Comparison of factors between groups with and without postoperative complications
The results of the comparison between groups with and without postoperative complications are shown in Table 3. The prevalence of preoperative frailty was significantly higher (58.8 % vs 14.3 %, p = 0.003) and 6MWD was significantly lower (380 m vs 436 m, p = 0.022) in the group with postoperative complications than in the group without postoperative complications. Regarding surgical procedure, there was no significant difference in complication rates between PD and DP (35.3% vs 65.7%, p = 0.073). Respiratory function, surgical information, blood data, and NAC results did not show any significant difference between the two groups. As expected, the length of hospital stay was significantly longer in the group with postoperative complications than in the group without postoperative complications (31 days vs 20 days, p < 0.001).
Table 3.
Comparison of factors between groups with and without postoperative complications
| Variables | With complications
(n=17) |
Without complications
(n=35) |
p-value |
| Age (years) | 73 (71–77) | 72 (66–75) | 0.132 |
| Male/female, n (%) | 10/7 (59/41) | 16/19 (46/54) | 0.555 |
| BMI (kg/m2) | 21.9 (20.7–24.0) | 23.7 (21.8–24.7) | 0.140 |
| Frailty, n (%)
Brinkman index |
10 (58.8)
535 (0–925) |
5 (14.3)
204 (0–735) |
0.003
0.247 |
| GNRI | 96.5 (91.7–102.5) | 94.1 (91–100.5) | 0.788 |
| Cancer stage, n (%) | 0.950 | ||
| 0 | 2 (12) | 0 (0) | |
| IA | 0 (0) | 4 (11) | |
| IB | 0 (0) | 0 (0) | |
| IIA | 6 (35) | 14 (40) | |
| IIB | 8 (47) | 16 (46) | |
| III | 1 (6) | 0 (0) | |
| IV | 0 (0) | 1 (3) | |
| CCI, n (%) | 0.540 | ||
| Low | 6 (35) | 9 (26) | |
| Medium | 8 (47) | 22 (63) | |
| High | 3 (18) | 4 (11) | |
| NAC, n (%) | 12 (70.6) | 27 (77.1) | 0.506 |
| LOS (day) | 31 (28–49) | 20 (18–24) | <0.01 |
| Surgical procedure, PD / DP, n (%) | 6 (35.3) / 11 (64.7) | 23 (65.7) / 12 (34.3) | 0.073 |
| Operative time (min) | 306 (235–382) | 371 (312–462) | 0.106 |
| Infusion volume (mL) | 3220 (2380–3620) | 3255 (2653–5005) | 0.233 |
| Blood loss (mL) | 460 (280–745) | 370 (207–647.5) | 0.470 |
| 6MWD (m) | 380 (345–435) | 436 (408–483) | 0.022 |
| Grip strength (%) | 21.5 (12.3–40.5) | 32.5 (14.5–48.1) | 0.155 |
| KES (%) | 37.2 (24.0–54.8) | 44.2 (32.6–51.0) | 0.135 |
| VC (L) | 2.69 (2.19–3.12) | 2.81 (2.52–3.17) | 0.308 |
| %VC (%) | 101.1 (94.2–110.4) | 111.4 (94.7–117.2) | 0.201 |
| FEV1 (L) | 1.90 (1.71–2.45) | 2.11 (1.77–2.37) | 0.441 |
| FEV1% (%) | 75.3 (66.3–78.6) | 76.9 (67.9–80.9) | 0.565 |
| Hb (g/dL) | 10.9 (10.6–12.5) | 11.7 (11–12.3) | 0.490 |
| TP (g/dL) | 6.3 (6.0–6.9) | 6.2 (5.9–6.6) | 0.532 |
| Alb (g/dL) | 3.4 (3.1–3.6) | 3.6 (3.3–3.9) | 0.074 |
Values are reported as the median (interquartile range) or number of patients (percentage).
BMI: body mass index
GNRI: Geriatric Nutritional Risk Index
CCI: Charlson Comorbidity Index
NAC: neoadjuvant chemotherapy
LOS: length of hospital stay
6MWD: 6-min walk distance
KES: knee extension strength
VC: vital capacity
%VC: percent vital capacity
FEV1: forced expiratory volume in 1 s
FEV1%: percent forced expiratory volume in 1 s
Hb: hemoglobin
TP: serum total protein
Alb: serum albumin
Association between postoperative complications and preoperative frailty
The results of the multivariate analysis are shown in Table 4. Preoperative frailty was used as an independent variable, and age, 6MWD, and Alb level were used as adjustment variables. Therefore, preoperative frailty was an independent risk factor for postoperative complications (p = 0.031), with an odds ratio of 5.950 (95% confidence interval, 1.18–30.00).
Table 4.
Factors associated with postoperative complications
| Odds ratio | 95% CI | p-value | |
| Frailty | 5.950 | 1.18–30.00 | 0.031 |
| Age (year) | 1.070 | 0.96−1.19 | 0.237 |
| 6MWD (m) | 0.997 | 0.99−1.01 | 0.572 |
| Alb (g/dL) | 0.410 | 0.08−2.25 | 0.304 |
6MWD: 6-min walk distance
Alb: serum albumin
CI: confidence interval
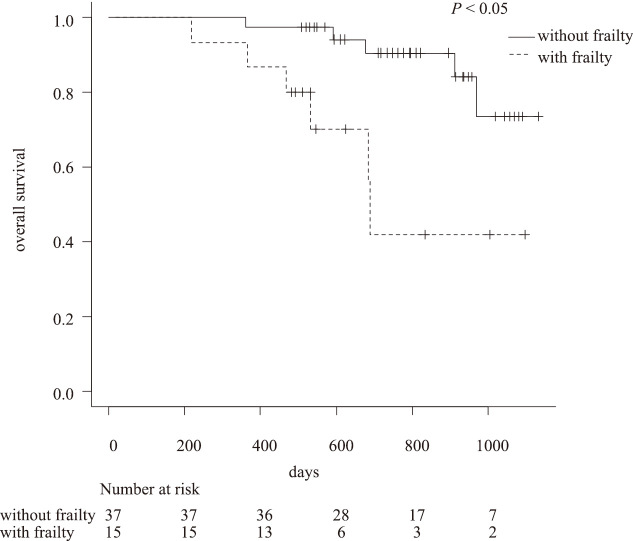
Comparison of postoperative survival in patients with and without frailty
The Kaplan–Meier curves comparing the postoperative survival of patients with PC with and without frailty showed a significant difference (p < 0.05) between the groups (Fig. 2).
Fig. 2.
Kaplan–Meier curves comparing the survival of patients with and without frailty
Patients without frailty had a significantly more favorable prognosis than those with frailty (p < 0.05).
DISCUSSION
In this study, we investigated whether preoperative frailty is associated with postoperative complications in patients with PC. Our study found that preoperative frailty was an independent predictive factor for the occurrence of postoperative complications.
High frailty levels before PD for PC are predictors of postoperative complications and mortality.7 Our study showed similar results for both PD and DP; therefore, the presence of preoperative frailty is an important predictor of pancreatectomy. It remains unclear why preoperative frailty leads to an increased incidence of postoperative complications; however, in general, frailty is characterized by a heterogeneous combination of decreased mobility, weakness, reduced muscle mass, and poor nutritional status.22 These factors are hypothesized to decrease the physiological reserve and increase vulnerability to acute stress7; thus, frailty may contribute to the development of complications.
Recently, NAC has become a standard treatment for PC because it leads to a higher R0 resection rate and a lower positive lymph node rate than upfront surgery.23 NAC did not significantly increase postoperative complications in this study; however, NAC may further reduce the physical function and activity of patients.24 Preoperative physical function has been correlated with postoperative complications, length of hospital stay, and prognosis.25 Several studies have reported that preoperative exercise intervention improves postoperative outcomes in surgical patients,26,27 and preoperative exercise capacity has been reported to predict postoperative physiological reserve by loading the whole-body oxygen delivery system.28 These reports suggest that preventing preoperative physical frailty may reduce the incidence of postoperative complications. Therefore, a combination of NAC and preoperative exercise therapy may be a useful treatment strategy for PC.
Another important predictive factor for postoperative complications is exercise tolerance, as measured using 6MWD. Several studies have reported that 6MWD is a useful predictive factor for postoperative complications in various cancers.14,19,20 Further, 6MWD is associated with maximum oxygen uptake29 and may correlate with loss of muscle mass and tissue fragility; therefore, it may contribute to increased postoperative complications. Cardiopulmonary exercise tests are usually performed to objectively evaluate general exercise tolerance; however, it may be difficult to perform this evaluation in all hospitals or facilities. This study showed that a shorter 6MWD tended to increase postoperative complications, although this was not an independent factor. This may be due to the small sample size, compared with those of other reports. Further studies with larger sample sizes are required to clarify this issue.
A previous study showed that poor nutritional status with low Alb level (< 3.5 g/dL) or low BMI (< 18.5 kg/m2) before surgery for pancreatic head cancer was a predictor of postoperative complications.21 In our study, the group with postoperative complications showed insignificantly lower Alb levels than the group without postoperative complications (3.42 g/dL vs 3.64 g/dL, p = 0.074) (Table 3). The association between preoperative malnutrition and worse postoperative outcomes has not yet been elucidated. However, considering the fragility of tissues, susceptibility to infection, and the possibility of vessel wall fragility,21 preoperative nutritional status might be an important factor. Therefore, the association between exercise tolerance and nutritional status should be investigated in the future.
We used the CFS to evaluate frailty because of the ease and efficacy of using this judgment tool. Other methods used to assess frailty take a longer time to evaluate, such as the modified Frailty Index7 and Edmonton Frail Scale.30 Further studies are required to clarify the most useful preoperative assessment for frailty.
Postoperative complications were defined as CD classification II or higher. In contrast, a previous study that focused on PC defined it as CD classification III or higher.9,19 However, CD classification II or higher, which requires medical treatment, such as antimicrobial agents, should be considered a postoperative complication because it may affect the length of the hospital stay.
Finally, the prognosis of patients with frailty was worse than of that of those without frailty in this study, as reported in a previous study.31As mentioned above, preventing frailty with exercise therapy may contribute to short-term outcomes and prognosis in the era of NAC for PC.
Our study has some limitations. First, this was a retrospective study conducted at a single center involving a small number of patients. Second, the optimal tool for measuring frailty may differ in each study.6 A prospective study with a larger number of patients is required to determine the association between frailty and postoperative complications.
CONCLUSIONS
Frailty assessed using CFS is a useful predictor of postoperative complications in patients with PC.
ACKNOWLEDGMENTS
We wish to acknowledge the rehabilitation staff of Kitakyushu Municipal Medical Center for their assistance in the data collection. We would like to thank Editage (www. editage.com) for the English language editing.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest associated with this manuscript.
Abbreviations
- PC
pancreatic cancer
- CFS
Clinical Frailty Scale
- NAC
neoadjuvant chemotherapy
- Alb
serum albumin
- 6MWD
6-min walk distance
REFERENCES
- 1.Mohammed S, Van Buren G 2nd, Fisher WE. Pancreatic cancer: advanced in treatment. World J Gastroenterol. 2014;20(28):9354–9360. doi: 10.3748/wjg.v20.i28.9354. [DOI] [PMC free article] [PubMed]
- 2.Halloran CM, Ghaneh P, Bosonnet L, Hartley MN, Sutton R, Neoptolemos JP. Complications of pancreatic cancer resection. Dig Surg. 2002;19(2):138–146. doi: 10.1159/000052029. [DOI] [PubMed]
- 3.Clauss D, Tjaden C, Hackert T, et al. Cardiorespiratory fitness and muscle strength in pancreatic cancer patients. Support Care Cancer. 2017;25(9):2797–2807. doi: 10.1007/s00520-017-3694-8. [DOI] [PubMed]
- 4.Fried LP, Tangen CM, Walson J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed]
- 5.Okabe H, Hayashi H, Higashi T, et al. Frailty predicts severe postoperative complication after elective hepatic resection. Gastrointest Tumors. 2019;6(1–2):28–35. doi: 10.1159/000500086. [DOI] [PMC free article] [PubMed]
- 6.Lin HS, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr. 2016;16(1):157. doi: 10.1186/s12877-016-0329-8. [DOI] [PMC free article] [PubMed]
- 7.Mogal H, Vermilion SA, Dodson R, et al. Modified frailty index predicts morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol. 2017;24(6):1714–1721. doi: 10.1245/s10434-016-5715-0. [DOI] [PMC free article] [PubMed]
- 8.Augastin T, Burstein MD, Schneider EB, et al. Frailty predicts risk of life-threatening complications and mortality after pancreatic resection. Surgery. 2016;160(4):987–996. doi: 10.1016/j.surg.2016.07.010. [DOI] [PubMed]
- 9.Nakano Y, Hirata Y, Shimogawara T, et al. Frailty is a useful predictive marker of postoperative complications after pancreaticoduodenectomy. World J Surg Oncol. 2020;18(1):194. doi: 10.1186/s12957-020-01969-7. [DOI] [PMC free article] [PubMed]
- 10.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed]
- 11.Rookwood K, Theou O. Using the clinical frailty scale in allocating scarce health care resources. Can Geristr J. 2020;23(3):210–215. doi: 10.5770/cgj.23.463. [DOI] [PMC free article] [PubMed]
- 12.Church S, Rogers E, Rockwood K, Theou O. A scoping review of the clinical frailty scale. BMC Geriatr. 2020;20(1):393. doi: 10.1186/s12877-020-01801-7. [DOI] [PMC free article] [PubMed]
- 13.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed]
- 14.Inoue T, Ito S, Kanda M, et al. Preoperative six-minute walk distance as a predictor of postoperative complication in patients with esophageal cancer. Dis Esophagus. 2020;33(2):doz050. doi: 10.1093/dote/doz050. [DOI] [PubMed]
- 15.National Comprehensive Cancer Network®. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Pancreatic Adenocarcinoma. https://www2.tri-kobe.org/nccn/guideline/archive/pancreas2020/english/pancreatic.pdf. Accessed December 3, 2021. [DOI] [PubMed]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed]
- 17.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed]
- 18.Jaric S. Muscle strength testing: use of normalisation for body size. Sports Med. 2002;32(10):615–631. doi: 10.2165/00007256-200232100-00002. [DOI] [PubMed]
- 19.Hayashi K, Yokoyama Y, Nakajima H, et al. Preoperative 6-minute walk distance accurately predicts postoperative complications after operations for hepato-pancreato-biliary cancer. Surgery. 2017;161(2):525–532. doi: 10.1016/j.surg.2016.08.002. [DOI] [PubMed]
- 20.Hattori K, Matsuda T, Takagi Y, et al. Preoperative six-minute walk distance is associated with pneumonia after lung resection. Interact CardiovascThorac Surg. 2018;26(2):277–283. doi: 10.1093/icvts/ivx310. [DOI] [PubMed]
- 21.Lee B, Han HS, Yoon YS, Cho JY, Lee JS. Impact of preoperative malnutrition, based on albumin level and body mass index, on operative outcomes in patient with pancreatic head cancer. J Hepatobiliary PancreatSci. 2021;28(12):1069–1075. doi: 10.1002/jhbp.858. [DOI] [PubMed]
- 22.Muscedere J, Waters B, Varambally A, et al. The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive Care Med. 2017;43(8):1105–1122. doi: 10.1007/s00134-017-4867-0. [DOI] [PMC free article] [PubMed]
- 23.Ren X, Wei X, Ding Y, et al. Comparison of neoadjuvant therapy and upfront surgery in resectable pancreatic cancer: a meta-analysis and systematic review. Onco Targets Ther. 2019;12:733–744. doi: 10.2147/OTT.S190810. [DOI] [PMC free article] [PubMed]
- 24.West MA, Loughney L, Barben CP, et al. The effects of neoadjuvant chemoradiotherapy on physical fitness and morbidity in rectal cancer surgery patients. Eur J Surg Oncol. 2014;40(11):1421–1428. doi: 10.1016/j.ejso.2014.03.021. [DOI] [PubMed]
- 25.Patel N, Powell AG, Wheat JR, et al. Cardiopulmonary fitness predicts postoperative major morbidity after esophagectomy for patients with cancer. Physiol Rep. 2019;7(14):e14174. doi: 10.14814/phy2.14174. [DOI] [PMC free article] [PubMed]
- 26.Mikami Y, Kouda K, Kawasaki S, et al. Preoperative in-hospital rehabilitation improves physical function in patients with pancreatic cancer scheduled for surgery. Tohoku J Exp Med. 2020;251(4):279–285. doi: 10.1620/tjem.251.279. [DOI] [PubMed]
- 27.Beggs T, Sepehri A, Szwajcer A, Tangri N, Arora RC. Frailty and perioperative outcomes: a narrative review. Can J Anaesth. 2015;62(2):143–157. doi: 10.1007/s12630-014-0273-z. [DOI] [PubMed]
- 28.Irie M, Nakanishi R, Yasuda M, Fujino Y, Hamada K, Hyodo M. Risk factors for short- term outcomes after thoracoscopic lobectomy for lung cancer. Eur Respir J. 2016;48(2):495–503. doi: 10.1183/13993003.01939-2015. [DOI] [PubMed]
- 29.Cahalin L, Pappagianopoulos P, Prevost S, Wain J, Ginns S. The relationship of the 6-min walk test to maximal oxygen consumption in transplant candidates with end-stage lung disease. Chest. 1995;108(2):452–459. doi: 10.1378/chest.108.2.452. [DOI] [PubMed]
- 30.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35(5):526–529. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed]
- 31.Mima K, Hayashi H, Nagakawa S, et al. Frailty is associated with poor prognosis after resection for pancreatic cancer. Int J Clin Oncol. 2021;26(10):1938–1946. doi: 10.1007/s10147-021-01983-z. [DOI] [PubMed]