This cohort study examines data from the Collaborative Study on the Genetics of Alcoholism to assess whether specific diagnostic criteria for alcohol use disorder that are indicative of greater severity could help detect a greater likelihood of progression.
Key Points
Question
Does emphasis on specific criteria for alcohol use disorder (AUD) improve the identification of individuals at risk for developing more severe AUD?
Findings
In this cohort study, cross-sectional and longitudinal multimodal secondary analyses involving a combined 15 928 individuals indicated that endorsement of criteria empirically designated as representing greater severity of AUD was significantly associated with 2-fold increased likelihood of progression from mild-to-moderate AUD to severe AUD, even after accounting for total criterion count.
Meaning
Emphasis on more severe criteria as indicators of vulnerability for severe AUD in current diagnostic approaches may increase detection of individuals with greater likelihood for disorder progression.
Abstract
Importance
Current Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) (DSM-5) diagnoses of substance use disorders rely on criterion count–based approaches, disregarding severity grading indexed by individual criteria.
Objective
To examine correlates of alcohol use disorder (AUD) across count-based severity groups (ie, mild, moderate, mild-to-moderate, severe), identify specific diagnostic criteria indicative of greater severity, and evaluate whether specific criteria within mild-to-moderate AUD differentiate across relevant correlates and manifest in greater hazards of severe AUD development.
Design, Setting, and Participants
This cohort study involved 2 cohorts from the family-based Collaborative Study on the Genetics of Alcoholism (COGA) with 7 sites across the United States: cross-sectional (assessed 1991-2005) and longitudinal (assessed 2004-2019). Statistical analyses were conducted from December 2022 to June 2023.
Main Outcomes and Measures
Sociodemographic, alcohol-related, psychiatric comorbidity, brain electroencephalography (EEG), and AUD polygenic score measures as correlates of DSM-5 AUD levels (ie, mild, moderate, severe) and criterion severity–defined mild-to-moderate AUD diagnostic groups (ie, low-risk vs high-risk mild-to-moderate).
Results
A total of 13 110 individuals from the cross-sectional COGA cohort (mean [SD] age, 37.8 [14.2] years) and 2818 individuals from the longitudinal COGA cohort (mean baseline [SD] age, 16.1 [3.2] years) were included. Associations with alcohol-related, psychiatric, EEG, and AUD polygenic score measures reinforced the role of increasing criterion counts as indexing severity. Yet within mild-to-moderate AUD (2-5 criteria), the presence of specific high-risk criteria (eg, withdrawal) identified a group reporting heavier drinking and greater psychiatric comorbidity even after accounting for criterion count differences. In longitudinal analyses, prior mild-to-moderate AUD characterized by endorsement of at least 1 high-risk criterion was associated with more accelerated progression to severe AUD (adjusted hazard ratio [aHR], 11.62; 95% CI, 7.54-17.92) compared with prior mild-to-moderate AUD without endorsement of high-risk criteria (aHR, 5.64; 95% CI, 3.28-9.70), independent of criterion count.
Conclusions and Relevance
In this cohort study of a combined 15 928 individuals, findings suggested that simple count-based AUD diagnostic approaches to estimating severe AUD vulnerability, which ignore heterogeneity among criteria, may be improved by emphasizing specific high-risk criteria. Such emphasis may allow better focus on individuals at the greatest risk and improve understanding of the development of AUD.
Introduction
Alcohol use disorder (AUD), as defined by the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition)1 (DSM-5), is conceptualized as a syndrome of sustained problematic alcohol use and clinically significant impairment. A diagnosis is based on endorsement of 2 or more of 11 criteria assessing behavioral and physical manifestations of heavy alcohol use that occur in a 12-month period.1 Recent US estimates indicate that 11% and 30% of adults meet criteria for past-year and lifetime AUD, respectively.2,3 This level of disordered alcohol use results in significant social, economic, and public health costs.4,5,6
DSM-5 AUD is diagnosed on a continuum of severity based on the number of criteria endorsed (2-3 = mild, 4-5 = moderate, ≥6 = severe),1 and identifying individuals at high risk for severe AUD is a priority. Several studies document increased comorbid burden and reduced likelihood of recovery as a function of increasing criterion count.7,8,9 However, count-based indices are limited by their equal weighting of criteria, suggesting all diagnostic criteria are interchangeable.10 Extensive cross-national psychometric evidence shows that certain AUD criteria (eg, withdrawal) are more likely to be endorsed by those with more severe AUD and may represent superior indicators of risk.11,12,13,14 Thus, criteria heterogeneity is an important factor to consider in the advancement of personalized treatment approaches.15 Whether “high-risk” AUD criteria endorsement results in differing associations with a wide range of psychiatric, genetic, and neurobiological correlates could further guide delineation of risk severity.
In light of the above evidence,12 here we investigate the impact of criteria heterogeneity within criterion count–based AUD diagnoses. As other research also suggests that severe AUD may be substantively different from mild or moderate AUD in terms of treatment needs,16 functional impairment,17 and other health sequelae,18 we sought to compare mild-to-moderate AUD (ie, endorsing 2-5 criteria, meeting criteria for either mild or moderate AUD) vs severe AUD while accounting for individual differences in criterion count among those with mild-to-moderate AUD. The current study aimed to (1) validate criterion count–based severity using DSM-5 categorizations of AUD, including comparing mild-to-moderate vs severe AUD, in a sample of 13 110 individuals; (2) use item response theory (IRT) modeling to identify criteria indicative of greater severity; and (3) evaluate whether the presence of certain high-risk criteria, identified through IRT modeling, indexes greater hazards of developing severe AUD in a related cohort of 2818 adolescents and young adults.
Methods
Participants
The Collaborative Study on the Genetics of Alcoholism (COGA) is a family-based study with deep and repeated phenotyping of substance use disorders (SUDs), comorbid psychiatric disorders, and related traits (including polygenic liability and electrophysiological markers) designed to examine the genetic substrates of AUD and its development across the life span.19 AUD probands were recruited primarily from treatment facilities across 7 US collection sites. Families of probands that included 3 or more individuals with alcohol dependence were also recruited and included members with and without alcohol dependence. Comparison families (ie, without ascertainment for alcohol dependence or exclusion for it) were also selected from a variety of community sources.20 The institutional review boards at all 7 sites approved this study, and written consent was obtained from all participants. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines for cohort studies. Data were restricted to alcohol-exposed individuals (ie, endorsing lifetime use of any alcohol) from 2 COGA subsamples: (1) a cross-sectional cohort of 13 110 individuals from 2234 families assessed from 1991 to 2005, and (2) a longitudinal cohort comprising 2818 offspring of individuals from the cross-sectional cohort, born after 1981 and assessed from 2004 to 2019.20
Measures
Cross-Sectional Cohort
The cross-sectional cohort was used to (1) examine correlates of mild, moderate, mild-to-moderate, and severe AUD; (2) identify AUD criteria indicative of heightened risk using IRT analysis; and (3) evaluate whether individuals with mild-to-moderate AUD who endorsed high-risk criteria differed from those who did not, and from those with severe AUD, across relevant factors, including alcohol-related, comorbid psychiatric, and electroencephalography (EEG)-derived traits and polygenic indices. Given extant literature demonstrating substantive differences between severe AUD and mild or moderate AUD,16,17,18 and statistical considerations for examining effects of criteria heterogeneity separately within mild and moderate AUD (ie, reduced power, large number of statistical tests17), we elected to combine mild and moderate AUD into a single referent group for analyses in both cohorts.
AUD criteria, diagnoses, and several correlates were derived from the Semi-Structured Interview for the Genetics of Alcoholism (SSAGA).21,22 Sociodemographic variables included sex, race and ethnicity, current income, educational attainment, and relationship status. Psychiatric lifetime Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV)23 diagnoses included major depressive disorder (MDD), antisocial personality disorder (ASPD), and other SUD diagnoses (endorsing ≥2 DSM-5 criteria for cannabis, cocaine, opiate, stimulant, sedative, or other use disorder or DSM-IV nicotine dependence). In addition, several alcohol-related measures were included as correlates in cross-sectional analyses: (1) lifetime endorsement of drinking every day for a week or more, (2) largest number of drinks consumed each day during this period, (3) lifetime endorsement of experiencing blackouts, (4) age at first intoxication, (5) age at regular drinking onset (ie, drinking once per month for 6 months or more), (6) lifetime maximum number of drinks ever consumed in a single 24-hour period, and (7) lifetime endorsement of seeking professional help or engaging in treatment for drinking problems.
COGA includes EEG-derived event-related oscillation (ERO) response measures.24 Prior studies have found the P300 component during the standard visual oddball paradigm to be associated with family history of AUD.24 Subgroup differences for parietal delta (1-3 Hz) and frontal theta (3-7 Hz) band EROs (300-700 millisecond window) and parietal P300 amplitude were also included as correlates of AUD severity in cross-sectional analyses. Polygenic scores (PGS) for AUD diagnostic status (ie, case/control), derived from a meta-analysis of large-scale genome-wide association study summary statistics,25,26,27,28 were calculated for genotyped individuals of European (n = 5396) and African American (n = 1774) ancestry separately using PRS-CS-auto29 and PRS-CSx,30 respectively (eMethods in Supplement 1).
Longitudinal Cohort
The longitudinal cohort included adolescent and young adult participants who were followed up approximately every 2 years (mean [SD] number of time points, 3.2 [1.8]). The SSAGA was administered at each biennial assessment. Given the longitudinal design, this sample was used to examine whether prior mild-to-moderate AUD diagnoses, with and without endorsement of high-risk criteria, were associated with increased hazards of progression to severe AUD. Other well-studied correlates assessed via the SSAGA, including alcohol involvement milestones (ie, age at first drink, regular drinking, and intoxication), and MDD, ASPD, and other SUD diagnoses were also examined.
Statistical Analyses
Cross-Sectional Cohort
First, descriptive statistics were estimated for sociodemographic, alcohol-related, and comorbid psychiatric measures. Means and proportions were calculated for continuous and categorical variables, respectively, and compared across AUD severity categories. Statistical comparisons of mild vs moderate AUD and moderate vs severe AUD were conducted across correlate measures using Wilcoxon rank sum and Fisher exact tests to evaluate the validity of combining mild and moderate as mild-to-moderate AUD. Second, a 1-parameter logistic IRT model assuming unidimensional structure for the 11 AUD criteria was applied to estimate criteria severity using the mirt package (version 1.37.1)31 in R32 (eMethods in Supplement 1). Criteria were rank ordered by severity, and those with severity parameter values greater than 2 (ie, 50% endorsement probability by individuals ≥2 SD above mean AUD latent severity) were considered high risk (Figure 1 and eTable 1 in Supplement 1). Third, individuals with mild-to-moderate AUD either endorsing no high-risk criteria or at least 1 high-risk criterion were classified into low-risk (n = 2486) and high-risk (n = 993) groups, respectively. Low-risk mild-to-moderate, high-risk mild-to-moderate, and severe AUD groups were then compared across alcohol-related, psychiatric, and EEG correlates using mixed-effects logistic and linear regression models fitted using the lme4 package (version 1.1-30)33 in R controlling for sex, age, self-declared race and ethnicity, and criterion count. Criterion count was included as a covariate given our combined grouping of mild and moderate AUD and our goal to examine associations independent of or not simply due to individual differences in the number of criteria endorsed. Mixed-effects models adjusted for familial clustering. Similar analyses, conducted separately by genetic ancestry, were used to examine associations between AUD PGS and diagnostic groups (eMethods in Supplement 1).
Figure 1. Cross-Sectional Collaborative Study on the Genetics of Alcoholism (COGA) Cohort (N = 13 110) Endorsement Rates and Item Response Theory (IRT) Response Curves for 11 Lifetime Alcohol Use Disorder (AUD) Criteria.
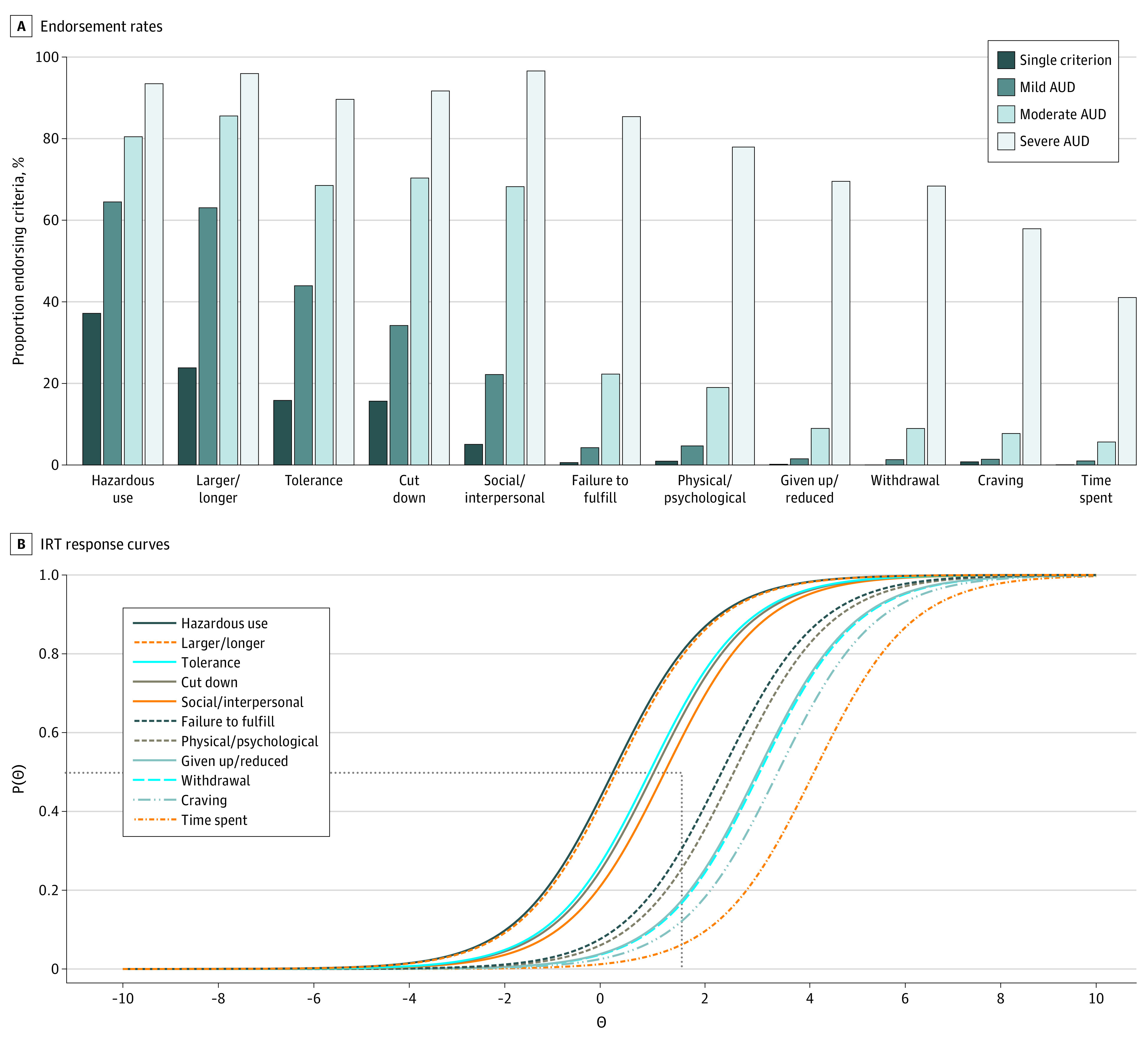
B, The probability of endorsement of each AUD criteria, P(θ) (y-axis), is plotted as a function of increasing severity of the underlying AUD latent trait, θ (x-axis). The horizontal dotted line represents a 50% probability of endorsing criteria; the vertical dotted line represents 2 SD above the mean of AUD latent severity. Criteria with difficulty parameters of 2 or above (ie, to the right of intersection of vertical and horizontal lines: Failure to fulfill, Physical/psychological, Craving, Withdrawal, Given up/reduced, and Time spent) were identified as high-risk diagnostic criteria. Hazardous use refers to recurrent alcohol use (≥3 times) in situations in which it is physically hazardous; Larger/longer = drinking in larger amounts or over longer periods than intended; Tolerance = need for markedly increased amounts of alcohol to achieve intoxication or desired effect or a markedly diminished effect with continued use of the same amount of alcohol; Cut down = persistent desire or 3 or more unsuccessful efforts to stop, cut down, or control drinking; Social/interpersonal = continued alcohol use despite having persistent or recurrent (≥3 times) social or interpersonal problems caused or exacerbated by the effects of alcohol; Failure to fulfill = recurrent use of alcohol resulting in a failure to fulfill major role obligations at work, school, or home; Physical/psychological = continued drinking despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to be caused or exacerbated by drinking; Craving = craving or a strong desire or urge to use alcohol; Given up/reduced = important social, occupational, or recreational activities given up or reduced because of drinking; Withdrawal = the characteristic withdrawal syndrome for alcohol or drinking (or using a closely related substance) to relieve or avoid withdrawal symptoms; Time spent = a great deal of time spent in activities necessary to obtain, use, or recover from the effects of drinking.
Longitudinal Cohort
Hazards of progression to severe AUD (≥6 criteria) were estimated for individuals who, at any previous time point, had endorsed a single criterion or met criteria for mild, moderate, or mild-to-moderate AUD via Cox proportional hazards survival analyses conducted using the survival (version 3.4-0)34 and adjustedCurves (version 0.9.1)35 packages in R. Low-risk (n = 699) and high-risk (n = 317) mild-to-moderate AUD subgroups were defined by a prior mild-to-moderate AUD diagnosis and differentiated by endorsement of 1 or more high-risk criteria identified in the cross-sectional IRT analysis. Additional survival analyses were conducted examining hazards of progression to severe AUD based on alcohol involvement milestones, MDD, ASPD, and other SUD diagnoses. Covariates for all models included sex, race and ethnicity, and criterion count to examine associations independent of individual differences in the number of prior criteria endorsed. A family grouping variable was used to estimate robust standard errors to account for familial clustering.36 Violations of the proportional hazards assumption for predictor variables were tested using Schoenfeld residuals and resolved by including an interaction term with age at onset of severe AUD. Statistical analyses were conducted from December 2022 to June 2023.
Results
Participants included 13 110 individuals from the cross-sectional COGA cohort (mean [SD] age, 37.8 [14.2] years, 52.8% female) and 2818 individuals from the longitudinal COGA cohort (mean baseline [SD] age, 16.1 [3.2] years; range, 11-26 years; 52.5% female).20
Differences Across Criterion Count–Based AUD Groups
As expected, increasing criterion count (ie, single criterion, mild, moderate, and severe AUD) was associated with increasing levels of heavy alcohol use and greater psychiatric comorbidity (Table 1). For instance, 48.9% and 82.4% of individuals with mild-to-moderate and severe AUD, respectively, reported experiencing blackouts. Likewise, 80% of those with severe AUD met criteria for a comorbid SUD vs half of those with mild-to-moderate AUD. Count-based severity was also reflected in reduced P300 amplitude and theta and delta EROs in individuals with severe vs mild-to-moderate AUD. AUD PGS also differentiated between severe and no AUD in the European ancestry subsample (odds ratio [OR], 1.23; 95% CI, 1.12-1.35) and between severe and mild AUD in the African American ancestry subsample (OR, 1.27; 95% CI, 1.07-1.51) (eTable 3 in Supplement 1). Individuals with mild AUD differed from those with moderate AUD on alcohol-related and psychiatric variables, but overall differences between these 2 severity groups were less pronounced than those between moderate and severe AUD or between mild-to-moderate and severe AUD. The extent of observed differences between mild and moderate AUD, as opposed to severe AUD, and evidence for this pattern in the literature17 supported our combined mild-to-moderate group as a comparison with severe AUD.
Table 1. Cross-Sectional COGA Cohort (n = 13 110) Descriptive Statistics for Sociodemographic, Alcohol-Related, Psychiatric Comorbidity, Electroencephalography, and AUD Polygenic Score Associations Organized by Diagnostic Groupa.
| Variables | % (95% CI) | |||||
|---|---|---|---|---|---|---|
| No criteria (n = 4684) | Single criterion (n = 1649) | Mild AUD (2-3 criteria) (n = 2184) | Moderate AUD (4-5 criteria) (n = 1295) | Mild-to-moderate AUD (n = 3479) | Severe AUD (≥6 criteria) (n = 3298) | |
| Sex | ||||||
| Male | 28.0 (26.7-29.3) | 43.7 (41.3-46.1) | 52.7 (50.6-54.8) | 60.3 (57.6-62.9) | 55.5 (53.9-57.2) | 67.3 (65.6-68.8) |
| Female | 72.0 (70.7-73.3) | 56.3 (53.9-58.7) | 47.3 (45.2-49.4) | 39.7 (37.1-42.4) | 44.5 (42.8-46.1) | 32.7 (31.2-34.4) |
| Race and ethnicity | ||||||
| African American or Black | 26.8 (25.6-28.1) | 18.2 (16.4-20.1) | 17.2 (15.7-18.9) | 20.5 (18.4-22.8) | 18.5 (17.2-19.8) | 24.0 (22.6-25.5) |
| Asian | 1.1 (0.8-1.5) | 0.7 (0.4-1.3) | 0.5 (0.3-0.9) | 0.6 (0.3-1.2) | 0.5 (0.3-0.9) | 0.6 (0.4-0.9) |
| Hispanic | 7.2 (6.5-8.0) | 6.5 (5.4-7.8) | 5.7 (4.8-6.7) | 5.3 (4.2-6.7) | 5.5 (4.8-6.4) | 6.1 (5.3-7.0) |
| Otherb | 2.4 (2.0-2.9) | 2.0 (1.4-2.8) | 1.6 (1.2-2.3) | 2.9 (2.1-3.9) | 2.1 (1.7-2.6) | 2.6 (2.1-3.2) |
| Unknown | 0.0 (0.0-0.2) | 0 | 0 | 0 | 0 | 0.1 (0.0-0.2) |
| White | 69.6 (68.3-70.9) | 79.1 (77.0-81.0) | 80.6 (78.9-82.2) | 76.0 (73.6-78.2) | 78.9 (77.5-80.2) | 72.7 (71.2-74.2) |
| Income (median range), $ | 30 000-39 999 | 30 000-39 999 | 30 000-39 999 | 30 000-39 999 | 30 000-39 999 | 20 000-29 999 |
| Education, mean (SD), y | 13.0 (2.3) | 13.2 (2.2) | 13.1 (2.3) | 12.8 (2.2) | 13.0 (2.3) | 12.2 (2.2) |
| Relationship | ||||||
| Married or living as married | 52.1 (50.6-53.5) | 52.9 (50.5-55.3) | 49.1 (47.0-51.2) | 43.5 (40.8-46.2) | 47.0 (45.3-48.6) | 33.3 (31.7-34.9) |
| Never married | 31.2 (29.9-32.6) | 33.4 (31.1-35.7) | 36.7 (34.6-38.7) | 37.4 (34.8-40.1) | 36.9 (35.3-38.6) | 33.8 (32.2-35.4) |
| Separated or divorced | 12.7 (11.8-13.7) | 12.5 (11.0-14.2) | 13.0 (11.6-14.5) | 17.2 (15.2-19.4) | 14.6 (13.4-15.8) | 31.4 (29.8-33.0) |
| Widowed | 4.0 (3.5-4.6) | 1.2 (0.8-1.9) | 1.3 (0.9-1.9) | 1.9 (1.3-2.9) | 1.5 (1.2-2.0) | 1.6 (1.2-2.1) |
| Alcohol-related | ||||||
| Drinking every day for ≥1 wk | 16.8 (15.4-18.2) | 32.4 (30.2-34.7) | 53.1 (51.0-55.2) | 74.1 (71.6-76.4) | 60.9 (59.3-62.5) | 93.6 (92.7-94.4) |
| No. of drinks every day for ≥1 wk, mean (SD)c | 3.1 (3.0) | 4.4 (4.0) | 6.4 (6.1) | 8.8 (8.9) | 7.5 (7.6) | 16.1 (13.5) |
| Experienced blackouts | 11.2 (10.1-12.5) | 25.0 (23-27.2) | 41.3 (39.3-43.4) | 61.7 (59.0-64.3) | 48.9 (47.3-50.6) | 82.4 (81.1-83.7) |
| Age at first intoxication, mean (SD), y | 20.1 (6.6) | 17.8 (4.6) | 17.0 (4.3) | 16.4 (4.1) | 16.7 (4.3) | 15.3 (4.4) |
| Age at regular drinking, mean (SD), y | 22.1 (7.7) | 19.5 (5.0) | 18.7 (5.0) | 18.2 (5.0) | 18.5 (5.0) | 17.1 (4.9) |
| Maximum No. of drinks, mean (SD)d | 6.6 (6.6) | 12.5 (9.5) | 16.6 (11.5) | 22.0 (15.0) | 18.6 (13.2) | 34.1 (20.0) |
| Sought help/treatment | 0.7 (0.4-1.0) | 2.8 (2.1-3.7) | 8.8 (7.7-10.1) | 28.1 (25.7-30.6) | 16.0 (14.8-17.2) | 79.4 (78.0-80.8) |
| Psychiatric comorbidity | ||||||
| MDD | 14.0 (12.8-15.3) | 14.0 (12.0-16.2) | 15.4 (13.7-17.4) | 13.2 (11.1-15.8) | 14.7 (13.2-16.2) | 23.3 (21.4-25.4) |
| ASPDe | 2.5 (2.1-3.0) | 5.5 (4.4-6.7) | 8.1 (7.0-9.3) | 13.5 (11.7-15.5) | 10.1 (9.1-11.1) | 24.4 (22.9-25.9) |
| SUDf | 16.7 (15.6-17.7) | 31.8 (29.6-34.1) | 43.5 (41.4-45.6) | 59.2 (56.5-61.9) | 49.4 (47.7-51.0) | 80.0 (78.6-81.4) |
| Theta ERO, mean (SD) | 26.7 (18.1) | 25.9 (17.1) | 27.1 (17.6) | 23.8 (14.1) | 25.8 (16.5) | 20.5 (14.0) |
| Delta ERO, mean (SD) | 49.3 (29.5) | 49.4 (28.8) | 49.6 (36.5) | 42.8 (21.3) | 47 (31.8) | 38.1 (22.4) |
| P300 amplitude, mean (SD) | 17.5 (9.4) | 18 (8.9) | 18.2 (9.5) | 16.8 (8.6) | 17.6 (9.2) | 13.9 (8.3) |
| AUD PGS, % in top quintile (95% CI)g | 16.8 (15.3-18.3) | 17.5 (15.1-20.2) | 20.4 (18.2-22.7) | 23.4 (20.5-26.5) | 21.5 (19.8-23.4) | 23.4 (21.6-25.3) |
Abbreviations: ASPD, antisocial personality disorder; AUD, alcohol use disorder; COGA, Collaborative Study on the Genetics of Alcoholism; DSM-IV and DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition and Fifth Edition; ERO, event-related oscillation; MDD, major depressive disorder; PGS, polygenic score; SUD, comorbid substance use disorder.
Comparison sample sizes varied across correlates according to patterns of missing data (eTable 2 in Supplement 1).
Other race and ethnicity comprises Native American, Pacific Islander, and self-declared “other” race.
Sample sizes for maximum number of drinks consumed every day during period of drinking every day for ≥1 week are restricted based on endorsement of ever drinking every day for ≥1 week.
Maximized over available interviews.
Nonsignificant difference between odds ratio for mild vs moderate AUD and odds ratio for moderate vs severe AUD. For all alcohol-related and psychiatric comorbidity variables, rank-biserial correlations from Wilcoxon rank sum tests for continuous measures were converted to odds ratios and compared with odds ratios calculated from Fisher exact tests for dichotomous measures. Significant differences in odds ratios were calculated using z statistics computed from log odds differences divided by pooled standard error estimates. All other comparisons were significant at 2-tailed P < .05 with differences between moderate and severe AUD being larger than differences between mild and moderate AUD.
DSM-5 cannabis use disorder, cocaine use disorder, opiate use disorder, simulant use disorder, sedative use disorder, and other drug use disorder and DSM-IV nicotine dependence.
Based on AUD PGS quintiles calculated separately by ancestral subsample prior to combined presentation.
High- and Low-Risk Criteria
IRT analysis of the 11 DSM-5 criteria in the cross-sectional cohort revealed that failure to fulfill major role obligations because of drinking, drinking despite physical/psychological problems, giving up/reducing important activities, withdrawal, craving, and spending a great deal of time drinking represented more severe criteria, indexing greater risk (Figure 1 and eTable 1 in Supplement 1). Endorsement of these 6 high-severity criteria differed considerably across criterion count–based severity groups (single criterion, mild, moderate, and severe AUD). For instance, withdrawal was endorsed by 4.2% of individuals with mild-to-moderate AUD and, in contrast, by 68.3% of those with severe AUD. As expected, individuals with mild AUD were less likely to endorse high-risk criteria (eg, 1.3% endorsing withdrawal) compared with those with moderate AUD (eg, 9.0% endorsing withdrawal).
High- and Low-Risk Mild-to-Moderate AUD vs Severe AUD
Individuals with high-risk mild-to-moderate AUD were more likely to endorse a greater number of criteria than those with low-risk mild-to-moderate AUD (eg, 42.5% vs 6.8% endorsed 5 criteria). Even after accounting for the number of criteria endorsed, individuals with mild-to-moderate AUD who endorsed high-risk criteria were more likely to consume a greater number of drinks during heavy drinking episodes and periods of frequent drinking; endorse seeking help or treatment; meet criteria for other SUDs, MDD, and ASPD; and have lower theta EROs when compared with individuals with low-risk mild-to-moderate AUD (Table 2 and eTable 5 in Supplement 1). AUD PGS also distinguished between low-risk mild-to-moderate and severe AUD in the African American ancestry subsample (OR, 1.30; 95% CI, 1.09-1.56) (eTable 3 in Supplement 1). Notably, the high-risk mild-to-moderate group did not statistically differ from the severe AUD group on theta EROs or P300 amplitude after accounting for criterion count differences.
Table 2. Cross-Sectional COGA Cohort Comparisons of Alcohol-Related, Psychiatric Comorbidity, Electroencephalography, and AUD Polygenic Score Associations by Low- and High-Risk Mild-to-Moderate and Severe AUDa.
| Variables | Mean (SD) | ||
|---|---|---|---|
| Mild-to-moderate AUD | Severe AUD (n = 3298) | ||
| Endorsed only low-risk criteria (n = 2486) | Endorsed ≥1 high-risk criteria (n = 993) | ||
| Alcohol-related | |||
| Drinking every day for ≥1 wk, % (95% CI)b | 57.2 (55.2-59.1) | 70.2 (67.3-73.0) | 93.6 (92.7-94.4) |
| No. of drinks every day for ≥1 wkb,c,d | 6.6 (5.9) | 9.4 (10.1) | 16.1 (13.5) |
| Experienced blackouts, % (95% CI)b | 46.1 (44.1-48.1) | 56 (52.9-59.1) | 82.4 (81.1-83.7) |
| Age at first intoxication, yb | 16.8 (3.9) | 16.7 (5.1) | 15.3 (4.4) |
| Age at regular drinking, yb | 18.5 (4.7) | 18.5 (5.8) | 17.1 (4.9) |
| Maximum No. of drinksb,c,e | 17.5 (11.4) | 21.5 (16.4) | 34.1 (20.0) |
| Sought help/treatment, % (95% CI)b,c | 11.3 (10.2-12.7) | 27.6 (24.9-30.5) | 79.4 (78.0-80.8) |
| Psychiatric comorbidity, % (95% CI) | |||
| MDDb,c | 13.9 (12.3-15.7) | 16.7 (13.9-19.9) | 23.3 (21.4-25.4) |
| ASPDb,c | 8.2 (7.1-9.3) | 15.0 (12.9-17.5) | 24.4 (22.9-25.9) |
| SUDb,c,f | 44.4 (42.5-46.4) | 61.7 (58.7-64.7) | 80.0 (78.6-81.4) |
| Theta EROc | 27.0 (17.3) | 22.9 (13.9) | 20.5 (14.0) |
| Delta ERO | 48.3 (33.8) | 43.7 (25.9) | 38.1 (22.4) |
| P300 amplitude | 18.3 (9.3) | 15.9 (8.6) | 13.9 (8.3) |
| AUD PGS, % in top quintile (95% CI) | 21.0 (19.0-23.2) | 22.8 (19.5-26.4) | 23.4 (21.6-25.3) |
| African American ancestry subsample | 18.6 (13.8-24.6) | 19.5 (14.4-25.8) | 22.3 (18.9-26.1) |
| European ancestry subsample | 21.4 (19.2-23.8) | 24.2 (20.4-29.0) | 23.8 (21.7-26.1) |
Abbreviations: ASPD, antisocial personality disorder; AUD, alcohol use disorder; COGA, Collaborative Study on the Genetics of Alcoholism; DSM-IV and DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition and Fifth Edition; ERO, event-related oscillation; MDD, major depressive disorder; PGS, polygenic score; SUD, comorbid substance use disorder.
Comparison sample sizes varied across correlates according to patterns of missing data (eTable 4 in Supplement 1).
Denotes variables exhibiting significant differences (P < .05) between high-risk mild-to-moderate and severe AUD in mixed models comparing low-risk and high-risk mild-to-moderate AUD with severe AUD (reference group) controlling for age, sex, race and ethnicity, and criterion count and nested within family (for alcohol-related, psychiatric, and electroencephalography correlates) or age, age2, sex, 10 ancestral principal components, genotyping array (for European ancestry subsample), cohort, and nested within family (for PGS correlates).
Denotes variables exhibiting significant differences (P < .05) between low-risk and high-risk mild-to-moderate AUD in mixed models comparing low-risk mild-to-moderate and severe AUD with high-risk mild-to-moderate AUD (reference group) controlling for age, sex, race and ethnicity, and criterion count and nested within family (for alcohol-related, psychiatric, and electroencephalography correlates) or age, age2, sex, 10 ancestral principal components, genotyping array (for European ancestry subsample), cohort, and nested within family (for PGS correlates).
Sample sizes for maximum number of drinks consumed every day during period of drinking every day for ≥1 week are restricted based on endorsement of ever drinking every day for ≥1 week.
Maximized over available interviews.
DSM-5 cannabis use disorder, cocaine use disorder, opiate use disorder, simulant use disorder, sedative use disorder, and other drug use disorder and DSM-IV nicotine dependence.
Hazards of Progression to Severe AUD
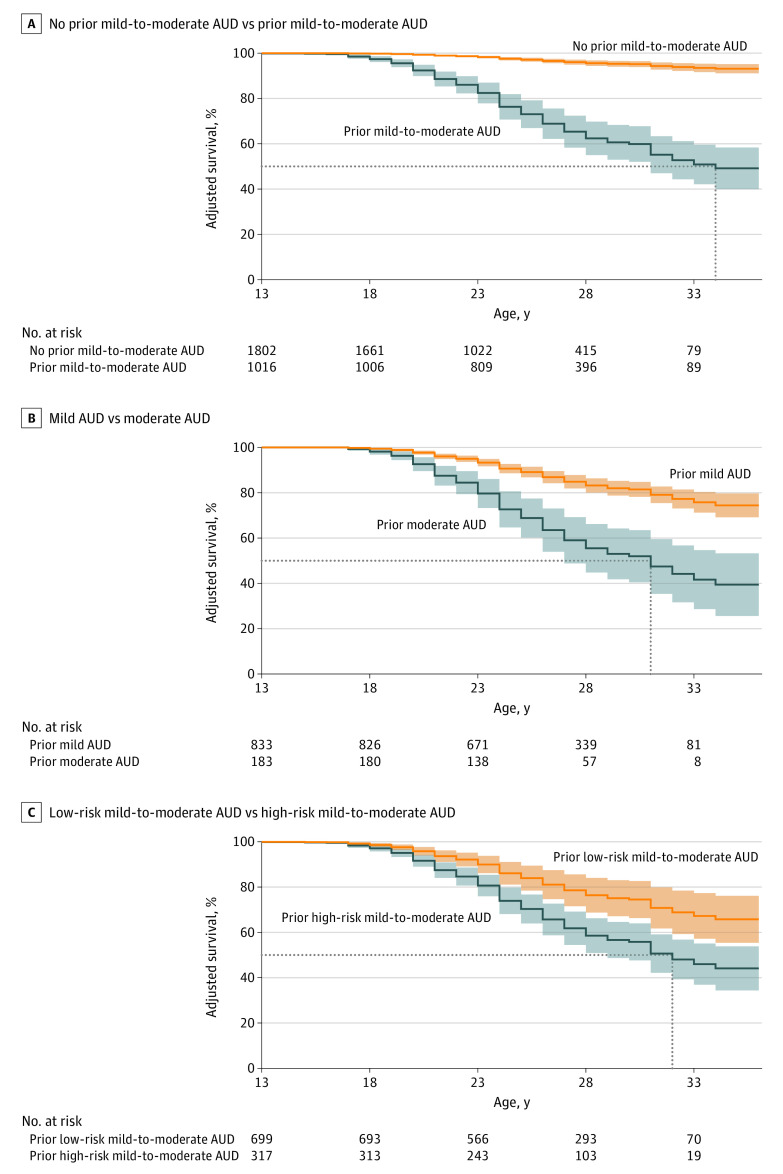
A majority of individuals who met criteria for severe AUD (77.8%) had a history of mild-to-moderate AUD (9.5% endorsed a single prior criterion, 12.7% endorsed no prior criteria). Consistent with cross-sectional findings, individuals with high-risk mild-to-moderate AUD were more likely (33.4%) to transition to severe AUD than those with low-risk mild-to-moderate AUD (12.9% transitioned to severe AUD) (Table 3 and eResults in Supplement 1). The hazard of transitioning from high-risk mild-to-moderate AUD to severe AUD (adjusted hazard ratio [aHR], 11.62; 95% CI, 7.54-17.92) was more than double that of transitioning to severe AUD from low-risk mild-to moderate AUD after accounting for criterion count (aHR, 5.64; 95% CI, 3.28-9.70; between-group aHR, 2.06; 95% CI, 1.47-2.88) (Figure 2). Earlier ages at first drink, regular drinking, and first intoxication and comorbid ASPD, MDD, and SUDs were significantly associated with progression to severe AUD; however, hazards for these characteristics were considerably lower than hazards for belonging to the high-risk mild-to-moderate AUD group. In multivariate models, high-risk mild-to-moderate was the strongest predictor of progression to severe AUD (aHR, 4.25; 95% CI, 2.57-7.04).
Table 3. Longitudinal COGA Cohort (n = 2818) Results From Cox Proportional Hazards Models for Progression to Severe AUDa.
| No criteria | Prior single criterion | Prior mild AUD (2-3 criteria) | Prior moderate AUD (4-5 criteria) | Prior mild-to-moderate | |||
|---|---|---|---|---|---|---|---|
| Endorsed only low-risk criteria | Endorsed ≥1 high-risk criterion | Total | |||||
| Sample size, No. | 1236 | 566 | 833 | 183 | 699 | 317 | 1016 |
| Proportion progressing to severe AUD (≥6 criteria), % (95% CI) | 2.6 (1.8-3.6) | 4.2 (2.9-6.3) | 15.4 (13.1-18.0) | 37.2 (30.4-44.4) | 12.9 (10.6-15.6) | 33.4 (28.5-38.8) | 19.3 (17.0-21.8) |
| Adjusted Cox proportional hazards ratio (95% CI) | NA | 1.13 (0.66-1.93) | 3.43 (2.33-5.05) | 11.10 (7.17-17.20) | 5.64 (3.28-9.70) | 11.62 (7.53-17.92) | 11.30 (7.33-17.43) |
Abbreviations: AUD, alcohol use disorder; COGA, Collaborative Study on the Genetics of Alcoholism; NA, not applicable.
All Cox proportional hazards models included sex and race and ethnicity as covariates clustered within family. Mild and moderate AUD models additionally included dummy-coded variables for criterion count (ie, 0/1 for 2 vs 3 criteria for mild; 0/1 for 4 vs 5 criteria for moderate). Mild-to-moderate AUD models additionally included dummy-coded variables for criterion count for 2 to 5 criteria.
Figure 2. Longitudinal Collaborative Study on the Genetics of Alcoholism (COGA) Cohort (N = 2818) Survival Curves and 95% Confidence Intervals for Progression to Severe Alcohol Use Disorder (AUD).
All survival curves include no prior mild-to-moderate AUD as comparison and are adjusted for sex, race and ethnicity, and mild-to-moderate AUD criterion count. A, Survival curves additionally adjusted for endorsement of prior mild-to-moderate AUD. B, Survival curves additionally adjusted for mild vs moderate AUD. C, Survival curves additionally adjusted for low-risk vs high-risk mild-to-moderate AUD. Dotted lines represent point estimates of median survival ages (not accounting for 95% CIs): A, prior mild-to-moderate AUD = 34 years; B, mild AUD = undefined, moderate AUD = 31 years; C, low-risk mild-to-moderate AUD = undefined, high-risk mild-to-moderate AUD = 32 years.
Discussion
We sought to examine whether endorsement of certain diagnostic criteria was associated with higher risk for severe AUD within the DSM-5 diagnostic scheme. IRT analyses revealed 6 high-risk criteria that reflect greater severity, and even after accounting for increasing criterion counts, there were significant differences among individuals who endorsed high- vs low-risk criteria. Moreover, individuals with mild-to-moderate AUD who endorsed 1 of these high-risk criteria were statistically indistinguishable from those with severe AUD with respect to theta EROs and P300 amplitude (after accounting for differences in criterion count). Individuals with moderate or high-risk mild-to-moderate AUD had the greatest hazards of progression to severe AUD across late adolescence and early adulthood. Overall, these analyses suggest that the presence of specific criteria are a superior indicator of risk for progression to severe AUD compared with criterion count alone. This difference is especially pronounced for the mild-to-moderate AUD group.
Many of the 6 empirically identified high-risk criteria have previously demonstrated stronger associations with comorbid psychopathology and greater severity of and discriminatory capabilities for AUD than other criteria.13,37,38,39 However, these high-risk criteria do not currently comprise a coherent DSM-5 AUD subtype.40 For example, although DSM-5 characterizes both withdrawal and tolerance as physiological components of AUD, withdrawal but not tolerance was identified as high risk. Interestingly, several of the high-risk criteria were markers of preoccupation (eg, craving, time spent, giving up/reducing important activities) and impairment in several domains (eg, role obligations, recurrent physical/psychological problems, withdrawal). These criteria were endorsed far more frequently by individuals with severe AUD and map onto 2 key stages (preoccupation/anticipation and withdrawal/negative affect) of one of the neurobiological models developed to characterize severe forms of SUDs.41
Unlike heavy drinking or psychiatric comorbidity, which may arise as a consequence of problematic drinking patterns, PGS and EEG measures provide a glimpse into potential and preexisting neurobiological vulnerabilities. EEG and AUD PGS results generally echoed other findings. Specifically, theta EROs distinguished between individuals with low-risk and high-risk mild-to-moderate AUD and demonstrated similarly blunted neurophysiological responses in both high-risk mild-to-moderate and severe AUD groups in the cross-sectional cohort. AUD PGS primarily exhibited associations with criterion count and overall diagnostic status (AUD vs no AUD) across both ancestry subsamples. As these PGS are calculated using genome-wide association studies of broadly conceptualized AUD status often obtained from electronic health record–derived diagnostic codes,25,26,27,28 findings here highlight the need for criterion-focused genome-wide association studies to resolve key genetic mechanisms that might underlie specific clinical presentations.38,39,42,43,44
In a recent conceptual proposal, McLellan et al45 note that while individuals with mild-to-moderate SUDs are currently not a high-priority population for treatment efforts, they represent “one reasonable starting point” for defining a state similar to prediabetes, indexing accumulating risk for progression to severe SUD diagnosis, and thus merit heightened vigilance and intervention. While the proposed concept has drawn criticism regarding potentially stigmatizing terminology,46,47,48 the importance of improving treatment efforts for all presentations of AUD cannot be overstated.6 In the current study, despite heightened risk, only 27.6% of the high-risk mild-to-moderate AUD group endorsed seeking professional help or engaging in treatment, which, although significantly more than rates endorsed by individuals in the low-risk mild-to-moderate AUD group, is far from ideal.
Our study provides mixed support for combining across mild and moderate AUD. In the cross-sectional cohort, differences between severe and moderate AUD were more pronounced than between moderate and mild AUD. However, endorsement of high-risk criteria was notably lower in those with mild vs moderate AUD, and in the longitudinal cohort, hazards of progressing to severe AUD from mild or low-risk mild-to-moderate AUD were much lower than hazards for moderate or high-risk mild-to-moderate AUD, which were largely equivalent. These findings are generally consistent with research suggesting that mild AUD may more accurately reflect temporally limited drinking problems consistent with endorsement of less severe criteria.49,50,51,52
Limitations
The current study should be interpreted in light of its limitations. First, identification of high-risk criteria may be influenced by the AUD-enriched family-based study design, though comparisons with prior IRT estimates suggest consistent results.13,14 Notwithstanding, findings here may not generalize to samples more representative of the general population. Second, although the cross-sectional cohort has a wide age distribution, longitudinal data are delimited to younger ages. Third, many DSM-5 criteria assessed using the SSAGA are compound criteria comprising multiple items of varying severity (eg, social/interpersonal problems: objections from others vs loss of friends). Disaggregation of compound criteria into individual items, though not always practical in clinical settings, may help further elucidate symptoms signaling increased risk for development of severe AUD.53
Conclusions
In this cohort study involving a combined 15 928 individuals, findings suggested that certain high-risk criteria may improve the identification of individuals at higher risk of progression to severe AUD. Defining severe AUD vulnerability using these criteria is consistent with extant etiological and clinical insight and may improve treatment penetrance.
eMethods. Supplemental methods
eTable 1. Cross-sectional COGA cohort (N = 13,110) item response theory severity parameters and endorsement rates for DSM-5 AUD criteria organized by diagnostic groups
eTable 2. All correlate sample sizes for COGA cross-sectional cohort organized by diagnostic groups
eTable 3. Results from mixed effect logistic AUD PGS regression models in COGA cross-sectional sample
eTable 4. Alcohol-related, psychiatric comorbidity, electroencephalography, and AUD polygenic score correlate sample sizes for COGA cross-sectional cohort organized by low- and high-risk mild-to-moderate and severe AUD
eTable 5. Results from mixed linear and logistic regression models comparing alcohol-related, psychiatric, electroencephalography, and polygenic score correlates across low-risk mild-to-moderate, high-risk mild-to-moderate, and severe AUD in COGA cross-sectional cohort
eResults. Supplemental survival analysis results
eReferences
Nonauthor Collaborators
Data Sharing Statement
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013. [Google Scholar]
- 2.Substance Abuse and Mental Health Services Administration . Key substance use and mental health indicators in the United States: results from the 2021 National Survey on Drug Use and Health [HHS Publ No PEP22-07-005, NSDUH Ser H-57]. Accessed September 7, 2023. https://www.samhsa.gov/data/report/2021-nsduh-annual-national-report
- 3.Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766. doi: 10.1001/jamapsychiatry.2015.0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rehm J, Gmel GE Sr, Gmel G, et al. The relationship between different dimensions of alcohol use and the burden of disease-an update. Addiction. 2017;112(6):968-1001. doi: 10.1111/add.13757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD. 2010 national and state costs of excessive alcohol consumption. Am J Prev Med. 2015;49(5):e73-e79. doi: 10.1016/j.amepre.2015.05.031 [DOI] [PubMed] [Google Scholar]
- 6.Carvalho AF, Heilig M, Perez A, Probst C, Rehm J. Alcohol use disorders. Lancet. 2019;394(10200):781-792. doi: 10.1016/S0140-6736(19)31775-1 [DOI] [PubMed] [Google Scholar]
- 7.Helle AC, Trull TJ, Watts AL, McDowell Y, Sher KJ. Psychiatric comorbidity as a function of severity: DSM-5 alcohol use disorder and HiTOP classification of mental disorders. Alcohol Clin Exp Res. 2020;44(3):632-644. doi: 10.1111/acer.14284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan AZ, Chou SP, Zhang H, Jung J, Grant BF. Prevalence and correlates of past-year recovery from DSM-5 alcohol use disorder: results from National Epidemiologic Survey on Alcohol and Related Conditions-III. Alcohol Clin Exp Res. 2019;43(11):2406-2420. doi: 10.1111/acer.14192 [DOI] [PubMed] [Google Scholar]
- 9.Lee MR, Boness CL, McDowell YE, Vergés A, Steinley DL, Sher KJ. Desistance and severity of alcohol use disorder: a lifespan-developmental investigation. Clin Psychol Sci. 2018;6(1):90-105. doi: 10.1177/2167702617736852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lane SP, Sher KJ. Limits of current approaches to diagnosis severity based on criterion counts: an example with DSM-5 alcohol use disorder. Clin Psychol Sci. 2015;3(6):819-835. doi: 10.1177/2167702614553026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mewton L, Slade T, McBride O, Grove R, Teesson M. An evaluation of the proposed DSM-5 alcohol use disorder criteria using Australian national data. Addiction. 2011;106(5):941-950. doi: 10.1111/j.1360-0443.2010.03340.x [DOI] [PubMed] [Google Scholar]
- 12.Preuss UW, Watzke S, Wurst FM; WHO/ISBRA Study on State; Trait Markers of Alcohol Use and Dependence Investigators . Dimensionality and stages of severity of DSM-5 criteria in an international sample of alcohol-consuming individuals. Psychol Med. 2014;44(15):3303-3314. doi: 10.1017/S0033291714000889 [DOI] [PubMed] [Google Scholar]
- 13.Lane SP, Steinley D, Sher KJ. Meta-analysis of DSM alcohol use disorder criteria severities: structural consistency is only ‘skin deep’. Psychol Med. 2016;46(8):1769-1784. doi: 10.1017/S0033291716000404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCutcheon VV, Agrawal A, Heath AC, et al. Functioning of alcohol use disorder criteria among men and women with arrests for driving under the influence of alcohol. Alcohol Clin Exp Res. 2011;35(11):1985-1993. doi: 10.1111/j.1530-0277.2011.01550.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, Koob GF. Heterogeneity of alcohol use disorder: understanding mechanisms to advance personalized treatment. Alcohol Clin Exp Res. 2015;39(4):579-584. doi: 10.1111/acer.12669 [DOI] [PubMed] [Google Scholar]
- 16.Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA. 2018;320(8):815-824. doi: 10.1001/jama.2018.11406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mannes ZL, Shmulewitz D, Livne O, Stohl M, Hasin DS. Correlates of mild, moderate, and severe alcohol use disorder among adults with problem substance use: validity implications for DSM-5. Alcohol Clin Exp Res. 2021;45(10):2118-2129. doi: 10.1111/acer.14701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rehm J, Shield KD. Global burden of alcohol use disorders and alcohol liver disease. Biomedicines. 2019;7(4):99. doi: 10.3390/biomedicines7040099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrawal A, Brislin SJ, Bucholz KK, et al. The Collaborative Study on the Genetics of Alcoholism: overview. Genes Brain Behav. Published online September 22, 2023. doi: 10.1111/gbb.12864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dick DM, Balcke E, McCutcheon V, et al. ; COGA Collaborators . The Collaborative Study on the Genetics of Alcoholism: sample and clinical data. Genes Brain Behav. 2023;e12860:e12860. doi: 10.1111/gbb.12860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA: a comparison with the SCAN. Addiction. 1999;94(9):1361-1370. doi: 10.1046/j.1360-0443.1999.94913618.x [DOI] [PubMed] [Google Scholar]
- 22.Bucholz KK, Cadoret R, Cloninger CR, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55(2):149-158. doi: 10.15288/jsa.1994.55.149 [DOI] [PubMed] [Google Scholar]
- 23.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; 1994. [Google Scholar]
- 24.Meyers JL, Brislin SJ, Kamarajan C, et al. ; COGA Collaborators . The Collaborative Study on the Genetics of Alcoholism: brain function. Genes Brain Behav. 2023;e12862:e12862. doi: 10.1111/gbb.12862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou H, Sealock JM, Sanchez-Roige S, et al. Genome-wide meta-analysis of problematic alcohol use in 435,563 individuals yields insights into biology and relationships with other traits. Nat Neurosci. 2020;23(7):809-818. doi: 10.1038/s41593-020-0643-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walters RK, Polimanti R, Johnson EC, et al. ; 23andMe Research Team . Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat Neurosci. 2018;21(12):1656-1669. doi: 10.1038/s41593-018-0275-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurki MI, Karjalainen J, Palta P, et al. ; FinnGen . FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613(7944):508-518. doi: 10.1038/s41586-022-05473-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kranzler HR, Zhou H, Kember RL, et al. Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nat Commun. 2019;10(1):1499. doi: 10.1038/s41467-019-09480-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ge T, Chen CY, Ni Y, Feng YA, Smoller JW. Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nat Commun. 2019;10(1):1776. doi: 10.1038/s41467-019-09718-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruan Y, Lin YF, Feng YA, et al. ; Stanley Global Asia Initiatives . Improving polygenic prediction in ancestrally diverse populations. Nat Genet. 2022;54(5):573-580. doi: 10.1038/s41588-022-01054-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chalmers RP. mirt: a multidimensional item response theory package for the R environment. J Stat Softw. 2012;48:1-29. doi: 10.18637/jss.v048.i06 [DOI] [Google Scholar]
- 32.R Foundation for Statistical Computing . The R project for statistical computing. Accessed May 15, 2023. https://www.r-project.org/
- 33.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1-48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 34.Therneau TM. A package for survival analysis in R. R package version 3.5-3. February 12, 2023. Accessed May 15, 2023. https://cran.r-project.org/web/packages/survival/index.html
- 35.Denz R, Klaaßen-Mielke R, Timmesfeld N. A comparison of different methods to adjust survival curves for confounders. Stat Med. 2023;42(10):1461-1479. doi: 10.1002/sim.9681 [DOI] [PubMed] [Google Scholar]
- 36.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. Springer; 2000. [Google Scholar]
- 37.Vize CE, Lane SP. Reliability of differential item functioning in alcohol use disorder: bayesian meta-analysis of criteria discrimination estimates. Assessment. 2022;29(5):925-939. doi: 10.1177/1073191120986613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gauld C, Baillet E, Micoulaud-Franchi JA, Kervran C, Serre F, Auriacombe M. The centrality of craving in network analysis of five substance use disorders. Drug Alcohol Depend. 2023;245:109828. doi: 10.1016/j.drugalcdep.2023.109828 [DOI] [PubMed] [Google Scholar]
- 39.Watts AL, Watson D, Heath AC, Sher KJ. Alcohol use disorder criteria exhibit different comorbidity patterns. Addiction. 2023;118(8):1457-1468. doi: 10.1111/add.16121 [DOI] [PubMed] [Google Scholar]
- 40.Watts AL, Boness CL, Loeffelman JE, Steinley D, Sher KJ. Does crude measurement contribute to observed unidimensionality of psychological constructs? a demonstration with DSM-5 alcohol use disorder. J Abnorm Psychol. 2021;130(5):512-524. doi: 10.1037/abn0000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760-773. doi: 10.1016/S2215-0366(16)00104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mallard TT, Savage JE, Johnson EC, et al. Item-level genome-wide association study of the Alcohol Use Disorders Identification Test in three population-based cohorts. Am J Psychiatry. 2022;179(1):58-70. doi: 10.1176/appi.ajp.2020.20091390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thorp JG, Campos AI, Grotzinger AD, et al. ; 23andMe Research Team . Symptom-level modelling unravels the shared genetic architecture of anxiety and depression. Nat Hum Behav. 2021;5(10):1432-1442. doi: 10.1038/s41562-021-01094-9 [DOI] [PubMed] [Google Scholar]
- 44.Cai N, Revez JA, Adams MJ, et al. ; MDD Working Group of the Psychiatric Genomics Consortium . Minimal phenotyping yields genome-wide association signals of low specificity for major depression. Nat Genet. 2020;52(4):437-447. doi: 10.1038/s41588-020-0594-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McLellan AT, Koob GF, Volkow ND. Preaddiction—a missing concept for treating substance use disorders. JAMA Psychiatry. 2022;79(8):749-751. doi: 10.1001/jamapsychiatry.2022.1652 [DOI] [PubMed] [Google Scholar]
- 46.Boness CL. Adopting the term ‘preaddiction’ would be a serious mistake. STAT. Published May 25, 2023. Accessed August 10, 2023. https://www.statnews.com/2023/05/25/preaddiction-substance-use-disorder-addiction-label/
- 47.Gallagher K. Preaddiction: a helpful term or reactionary misstep in addiction treatment? Mad In America. Published June 14, 2023. Accessed August 10, 2023. https://www.madinamerica.com/2023/06/preaddiction-a-helpful-shift-or-reactionary-misstep-in-addiction-treatment/
- 48.American Psychiatric Association . Re: Inviting input on use of a term like “preaddiction” for identifying and intervening in substance misuse and mild/early-stage substance use disorder [NOT-DA-23-019]. Published online April 27, 2023. Accessed August 10, 2023. https://www.psychiatry.org/getattachment/8044e50c-0a5d-4b6a-b12a-5bda60acfb23/APA-Letter-NIDA-NIAAA-RFI-Proposed-Preaddiction-Diagnosis-04272023.pdf
- 49.Slade T, Mewton L, O’Dean S, et al. DSM-5 and ICD-11 alcohol use disorder criteria in young adult regular drinkers: lifetime prevalence and age of onset. Drug Alcohol Depend. 2021;229(Pt B):109184. doi: 10.1016/j.drugalcdep.2021.109184 [DOI] [PubMed] [Google Scholar]
- 50.Martin CS, Steinley DL, Vergés A, Sher KJ. The proposed 2/11 symptom algorithm for DSM-5 substance-use disorders is too lenient [letter]. Psychol Med. 2011;41(9):2008-2010. doi: 10.1017/S0033291711000717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lynskey MT, Nelson EC, Neuman RJ, et al. Limitations of DSM-IV operationalizations of alcohol abuse and dependence in a sample of Australian twins. Twin Res Hum Genet. 2005;8(6):574-584. doi: 10.1375/twin.8.6.574 [DOI] [PubMed] [Google Scholar]
- 52.Bailey AJ, Ingram PF, Howe LK, Finn PR. Is lower severity alcohol use disorder qualitatively different than more severe manifestations? an evaluation of multivariate symptom clusters. Addiction. 2022;117(6):1598-1608. doi: 10.1111/add.15785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boness CL, Lane SP, Sher KJ. Not all alcohol use disorder criteria are equally severe: toward severity grading of individual criteria in college drinkers. Psychol Addict Behav. 2019;33(1):35-49. doi: 10.1037/adb0000443 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplemental methods
eTable 1. Cross-sectional COGA cohort (N = 13,110) item response theory severity parameters and endorsement rates for DSM-5 AUD criteria organized by diagnostic groups
eTable 2. All correlate sample sizes for COGA cross-sectional cohort organized by diagnostic groups
eTable 3. Results from mixed effect logistic AUD PGS regression models in COGA cross-sectional sample
eTable 4. Alcohol-related, psychiatric comorbidity, electroencephalography, and AUD polygenic score correlate sample sizes for COGA cross-sectional cohort organized by low- and high-risk mild-to-moderate and severe AUD
eTable 5. Results from mixed linear and logistic regression models comparing alcohol-related, psychiatric, electroencephalography, and polygenic score correlates across low-risk mild-to-moderate, high-risk mild-to-moderate, and severe AUD in COGA cross-sectional cohort
eResults. Supplemental survival analysis results
eReferences
Nonauthor Collaborators
Data Sharing Statement