Key Points
Question
Can a primary care intervention comprising electronic health record (EHR) reminders and patient outreach with or without patient navigation improve timely follow-up of overdue abnormal cancer screening test results?
Findings
Among 11 980 patients in 44 primary care practices, completion of follow-up for an abnormal breast, cervical, colorectal, or lung cancer screening test result within 120 days of study enrollment was higher among patients exposed to EHR reminders, outreach, and navigation (31.4%) or EHR reminders and outreach (31.0%) than those exposed to EHR reminders only (22.7%) or usual care (22.9%).
Meaning
Systems-based outreach in primary care settings can improve the timely follow-up of abnormal cancer screening results, but gaps in follow-up care need to be addressed if the full benefits of preventive cancer screening are to be realized.
Abstract
Importance
Realizing the benefits of cancer screening requires testing of eligible individuals and processes to ensure follow-up of abnormal results.
Objective
To test interventions to improve timely follow-up of overdue abnormal breast, cervical, colorectal, and lung cancer screening results.
Design, Setting, and Participants
Pragmatic, cluster randomized clinical trial conducted at 44 primary care practices within 3 health networks in the US enrolling patients with at least 1 abnormal cancer screening test result not yet followed up between August 24, 2020, and December 13, 2021.
Intervention
Automated algorithms developed using data from electronic health records (EHRs) recommended follow-up actions and times for abnormal screening results. Primary care practices were randomized in a 1:1:1:1 ratio to (1) usual care, (2) EHR reminders, (3) EHR reminders and outreach (a patient letter was sent at week 2 and a phone call at week 4), or (4) EHR reminders, outreach, and navigation (a patient letter was sent at week 2 and a navigator outreach phone call at week 4). Patients, physicians, and practices were unblinded to treatment assignment.
Main Outcomes and Measures
The primary outcome was completion of recommended follow-up within 120 days of study enrollment. The secondary outcomes included completion of recommended follow-up within 240 days of enrollment and completion of recommended follow-up within 120 days and 240 days for specific cancer types and levels of risk.
Results
Among 11 980 patients (median age, 60 years [IQR, 52-69 years]; 64.8% were women; 83.3% were White; and 15.4% were insured through Medicaid) with an abnormal cancer screening test result for colorectal cancer (8245 patients [69%]), cervical cancer (2596 patients [22%]), breast cancer (1005 patients [8%]), or lung cancer (134 patients [1%]) and abnormal test results categorized as low risk (6082 patients [51%]), medium risk (3712 patients [31%]), or high risk (2186 patients [18%]), the adjusted proportion who completed recommended follow-up within 120 days was 31.4% in the EHR reminders, outreach, and navigation group (n = 3455), 31.0% in the EHR reminders and outreach group (n = 2569), 22.7% in the EHR reminders group (n = 3254), and 22.9% in the usual care group (n = 2702) (adjusted absolute difference for comparison of EHR reminders, outreach, and navigation group vs usual care, 8.5% [95% CI, 4.8%-12.0%], P < .001). The secondary outcomes showed similar results for completion of recommended follow-up within 240 days and by subgroups for cancer type and level of risk for the abnormal screening result.
Conclusions and Relevance
A multilevel primary care intervention that included EHR reminders and patient outreach with or without patient navigation improved timely follow-up of overdue abnormal cancer screening test results for breast, cervical, colorectal, and lung cancer.
Trial Registration
ClinicalTrials.gov Identifier: NCT03979495
This cluster randomized trial compares the effects of electronic health record reminders and patient outreach with or without patient navigation on overdue follow-up of abnormal cancer screening test results for colorectal, cervical, breast, or lung cancer among primary care patients.
Introduction
Recommended screening for breast, cervical, colorectal, and lung cancer reduces cancer-specific mortality.1,2,3,4,5 Cancer screening starts with identifying and screening eligible individuals and has been a focus of health care reform legislation and quality improvement efforts.6,7,8 Realizing the maximal benefits of screening requires both population-based screening and timely follow-up of abnormal test results. However, less effort has been paid to systematically ensuring high rates of timely follow-up of abnormal cancer screening test results.9,10,11,12,13
Barriers to follow-up of abnormal cancer screening test results exist at multiple levels, including those involving the patient, primary care clinician, care team, specialist, and health system.9,14,15 Most cancer screening is initiated in primary care settings. Apart from legislated requirements for radiologists to follow-up on abnormal mammograms,16 the responsibility for managing abnormal test results is often less organized and variably falls to the performing or ordering clinician. Even when primary care clinicians are not the ordering clinician, they often coordinate care with various specialists depending on the cancer screening test result.17,18 Moreover, primary care clinicians commonly view themselves as responsible for managing the diagnostic evaluation of all abnormal test results.19 The large volume of abnormal cancer screening test results, along with increasingly complex recommendations for test completion, leads to considerable burden and potential medicolegal risk.20,21,22,23 Few clinicians and practice networks have met the challenge with integrated, population-management systems to comprehensively track all abnormal test results and manage follow-up.24
To improve the systematic follow-up of abnormal cancer screening test results, we developed a multilevel intervention and evaluated the intervention in a pragmatic, primary care practice–based, cluster randomized clinical trial. We hypothesized that improving the follow-up of patients with abnormal cancer screening test results requires multiple components, including a system-level comprehensive health informatics platform; team engagement of patients, primary care clinicians, and specialist clinicians through the use of population outreach and patient navigation to promote coordination; and components directed at individuals and their clinicians. The intervention was designed as a system to supplement rather than replace usual care for patients overdue for recommended follow-up based on the cancer type and risk of the abnormal test result.
Methods
Study Design and Participants
Details of the study have been published.25 The trial protocol appears in Supplement 1 and the eMethods appear in Supplement 2. Briefly, we designed a pragmatic, cluster randomized clinical trial involving 44 primary care practices within 3 primary care networks: Brigham and Women’s Hospital (15 practices), Dartmouth Health (12 practices), and Massachusetts General Hospital (17 practices). Individuals in the practices with an overdue, abnormal cancer screening test result for breast, cervical, colorectal, or lung cancer were eligible. The included patients were (1) women aged 40 to 80 years with an abnormal mammogram; (2) women aged 21 to 65 years with an abnormal Papanicolaou test with or without a human papillomavirus test; (3) adults aged 40 to 80 years with a positive fecal immunochemical test or an abnormal short-interval colonoscopy (1-5 years); and (4) adults aged 55 to 80 years with current or former smoking and an abnormal low-dose computed tomographic result.
Patients who were unable to speak English or Spanish or who had a history of cancer for the organ associated with the abnormal test result were excluded. For colorectal cancer, individuals with inflammatory bowel disease also were excluded, but those undergoing short-interval colonoscopy screening for a high-risk family history alone were included because they could not be reliably identified and excluded.
Relevant guideline recommendations and specialist input were used to create automated electronic health record (EHR) algorithms to identify patient eligibility and determine a recommended follow-up period and appropriate diagnostic follow-up (eTable 1 in Supplement 2).25,26 The exception was short-interval colonoscopy in which the follow-up time frame was determined by the gastrointestinal specialist performing the procedure. Designed to supplement usual care, additional time beyond the due date for the abnormal test result was added to allow for completion of the recommended follow-up before a patient became eligible (eFigure 1 in Supplement 2).
The study was approved by the Mass General Brigham institutional review board for all participating networks. The study was considered minimal risk and was granted a waiver of informed consent because all patient care remained under the direction of the patient’s clinical team and the intervention supplemented rather than replaced usual care. Data for all sociodemographic, clinical covariates, and outcomes were obtained from the EHR. The race and ethnicity data recorded in the EHR were self-reported by the patient using open-ended questions and were included and assessed in this study due to the association previously reported between race and ethnicity and the likelihood of completing cancer screening.15
Randomization
Primary care practices were the unit of randomization and individual patients were the unit of analysis. Practice-level cluster randomization was used to minimize crossover of intervention strategies within practices and was based on patient volume and the number of patients who were women or insured through Medicaid. Each of these 3 practice characteristics was dichotomized, forming 8 strata, and each practice fell into 1 of the 8 strata. Four practices were then chosen from within a stratum and randomized by the study statistician (E.J.O.) with each practice randomized in a 1:1:1:1 ratio to the following groups: (1) usual care, (2) EHR reminders, (3) EHR reminders and outreach, or (4) EHR reminders, outreach, and navigation (Figure 1; additional information appears in the eMethods in Supplement 2).
Figure 1. Selection of Primary Care Practices and Flow of Patients Through the Study.
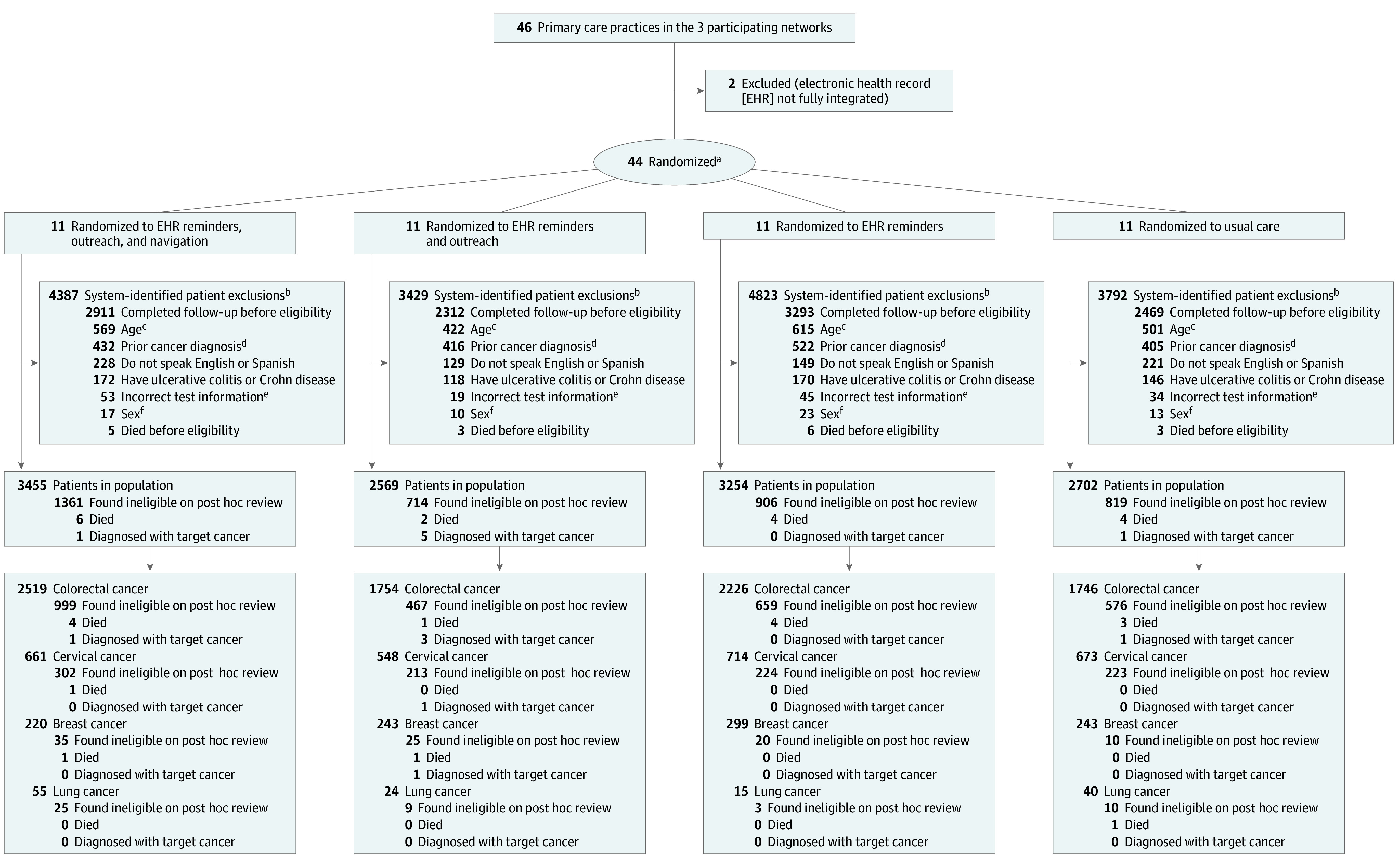
aStratified by practice volume and the number of patients who were female or had Medicaid insurance.
bPatients were excluded based on information derived from the EHR.
cDid not meet age-related inclusion criteria.
dPrior cancer diagnosis for target organ.
eIncorrect test date or had a normal test result.
fMen with abnormal mammogram results.
Given the nature of the interventions, neither the patients nor the practices were blinded to the allocation. All patients had access to usual care, which included outreach and follow-up (at the discretion of their clinician) and the ability to view test results in an online patient portal (eMethods and eTable 2 in Supplement 2).
Interventions
The trial interventions were developed with the support of network leadership, and presentations were given to educate the clinical staff at the practices randomized to the 3 intervention groups. Practice leaders received emails prior to initiating enrollment describing the study with additional information relevant to the study group for their practice. Patients from participating practices were enrolled after their first overdue abnormal cancer screening test result and remained in the assigned study group even if they changed practices during the study period. If a patient had more than 1 overdue abnormal cancer screening test result during the study period, only the first abnormal test result was analyzed for the study outcomes.
The intervention components were added sequentially. After enrollment, patients in the intervention groups (EHR reminders, outreach, and navigation; EHR reminders and outreach; and EHR reminders) had health maintenance topics added to the screening reminders in the EHR identifying the type of abnormal test result and the recommended follow-up (see sample EHR reminder in the eMethods in Supplement 2). Patients and primary care practices could view these EHR reminders any time the record was accessed, but most commonly they were seen around the time of an office encounter. Completing the recommended follow-up led to closure of the EHR reminder.
Patients in the EHR reminders and outreach group and in the EHR reminders, outreach, and navigation group remaining overdue after 2 weeks received a reminder letter through the EHR patient web portal or via postal mail (see sample letter in the eMethods in Supplement 2). Patients remaining overdue for follow-up after 4 weeks received a reminder phone call by study staff informing them of the test result and providing contact information to schedule a follow-up visit. Patients in the EHR reminders, outreach, and navigation group remaining overdue after an additional 4 weeks received a follow-up call by a patient navigator that assessed social barriers to care across 9 domains: housing insecurity, food insecurity, paying for basic utilities, family caregiving, legal, transportation, financial compensation for treatment, education, and employment. Navigators used an online network of verified social service programs to help connect patients with services available in their community.25,27
Outcomes Assessment
The primary outcome was completion of recommended follow-up within 120 days of study enrollment (a time frame that would permit scheduling and completion of follow-up in response to the study interventions.) The secondary outcomes included completion of recommended follow-up within 240 days of enrollment and completion of recommended follow-up within 120 days and 240 days for specific cancer types and levels of risk for the abnormal screening result. Completion of the follow-up outcome included a test or procedure defined by guidelines or specialist consensus based on the risk level of the cancer screening test result and organ type and identified using automated algorithms that ran daily.20,28,29,30
Sample Size and Power
We anticipated the ability to recruit approximately 3324 participants from 40 practices over a 2-year period. Based on this sample size, the study had 80% power to detect an 11% difference in the proportion who completed follow-up. The sample size calculation allowed for a within-primary care clinician correlation of 0.02 (assuming 550 clinicians), a within-practice correlation of 0.01 (assuming 40 practices), and a Bonferroni-adjusted significance level of 0.0167 to allow for comparison of each of the 3 active intervention groups vs usual care (control group).
Statistical Analysis
Continuous patient characteristics are presented as means with SDs and categorical characteristics as frequencies and proportions. The primary intention-to-treat population included all patients who met protocol eligibility criteria for at least 1 abnormal cancer screening test result (additional details appear in the statistical analysis plan in Supplement 3). A secondary, as-treated population excluded those patients in the primary analysis subsequently found to be ineligible (eg, those with a prior cancer diagnosis, those who were no longer receiving care at a participating practice, or those who received follow-up care prior to the eligibility assessment) based on manual chart review (eMethods in Supplement 2).
All analysis models and covariates were specified in the statistical analysis plan that was finalized prior to unblinding (Supplement 3). The analyses for the dichotomous outcomes (completed follow-up or did not complete follow-up) were performed using mixed logistic regression models (SAS Proc GLIMMIX, SAS Institute Inc) with the patient as the unit of analysis. Initial prespecified partially adjusted models included indicator variables for the 3 intervention groups as the primary exposure, random effects for practices (accounting for cluster randomization) and physicians, and fixed effects for cancer type, calendar month, and the month × study site interaction. The fully adjusted models also included covariates for the risk status of the abnormal cancer screening test result (eg, low, medium, or high), marital status, race and ethnicity, and having a primary care visit within the past year. These covariates were chosen from the patient characteristics believed to be potentially important or that differed among the groups at P < .05 and changed the coefficient of any of the 3 indicators for treatment group by greater than 20%. Adjusted completion proportions were calculated by marginal standardization.
For time to completion of follow-up analyses, we plotted Kaplan-Meier time-to-event curves. Censoring occurred at the first time point of missing outcome data for a patient, at study withdrawal, or at the end of the planned study follow-up. We calculated cumulative follow-up completion proportions at 120 and 240 days for the intention-to-treat population. The same mixed logistic regression model with 120- and 240-day follow-up completion proportions was used for the secondary as-treated population and for the exploratory subgroup analyses based on organ (breast, cervical, colorectal, or lung) and risk of the abnormal cancer screening test result (low, medium, or high) (eTable 3 in Supplement 2).
For the primary adjusted intention-to-treat analyses for completion of follow-up at 120 days, an overall global P value of .05 or less was considered statistically significant. If the global test was significant, 3 pairwise tests were done (each intervention group vs the usual care group) using a P value of .0167 or less for statistical significance. All other analyses and comparisons were based on 95% CIs. All analyses used SAS version 9.4 (SAS Institute Inc).
Results
Patients
Patient enrollment occurred sequentially over time by cancer organ type at the Brigham and Women’s Hospital and the Massachusetts General Hospital sites beginning in August 2020 and at the Dartmouth Health sites beginning in November 2020 (eMethods in Supplement 2). Patient enrollment ended at all sites in December 2021; 11 980 patients had at least 1 overdue abnormal cancer screening test result and met the intention-to-treat eligibility criteria. The flow of patients is depicted in Figure 1.
Each study group included 11 practices; patients were assigned to the study group of the practice in which they were being treated at the time of enrollment (Table 1).31 The median age of the patients was 60 years (IQR, 52-69 years), 64.8% were women, 83.3% were White, and 15.4% were insured through Medicaid; there was no clinically meaningful imbalance across the groups. The most common type of abnormal cancer screening test result was for colorectal cancer (8245 patients [69%]), followed by cervical cancer (2596 patients [22%]), breast cancer (1005 patients [8%]), and lung cancer (134 patients [1%]). The most common risk level of the abnormal cancer screening test result was low (6082 patients [51%]), followed by medium (3712 patients [31%]), and high (2186 patients [18%]). The type and risk level for the abnormal cancer screening test result were similar across study groups.
Table 1. Characteristics of the Practices and Patients.
| EHR reminders, outreach, and navigation | EHR reminders and outreach | EHR reminders | Usual care | |
|---|---|---|---|---|
| Practice characteristics | ||||
| No. of practices | 11 | 11 | 11 | 11 |
| Primary care clinicians, median/practice (IQR) | 13.5 (7.5-30.8) | 13 (11.3-14.8) | 11 (10.0-28.5) | 16 (11.5-17.5) |
| No. and type of clinician | ||||
| Physicians | 11 | 10.5 | 10 | 13 |
| Advance practice | 3 | 2 | 3 | 3 |
| No. of patients, median/practice (IQR) | 7843 (4279-14 121) | 7101 (5897-11 165) | 10 379 (6982-15 542) | 8967 (6613-13 585) |
| Patient characteristics | ||||
| No. of patients | 3455 | 2569 | 3254 | 2702 |
| Age, median (IQR), y | 61 (54-69) | 61 (52-69) | 60 (52-68) | 59 (50-68) |
| Sex, No. (%)a | ||||
| Female | 2172 (62.9) | 1680 (65.4) | 2139 (65.7) | 1772 (65.6) |
| Male | 1283 (37.1) | 889 (34.6) | 1115 (34.3) | 930 (34.4) |
| Race, No. (%)a | ||||
| American Indian or Alaska Native | 12 (0.4) | 6 (0.2) | 7 (0.2) | 4 (0.2) |
| Asian | 101 (3.0) | 77 (3.1) | 89 (2.9) | 90 (3.4) |
| Black | 201 (6.0) | 184 (7.4) | 265 (8.5) | 139 (5.3) |
| Native Hawaiian or Other Pacific Islander | 2 (0.1) | 1 (<1) | 1 (<1) | 2 (0.1) |
| White | 2790 (82.8) | 2059 (82.9) | 2556 (82.3) | 2241 (85.5) |
| Otherb | 264 (7.8) | 157 (6.3) | 186 (6.0) | 145 (5.5) |
| Hispanic or Latino ethnicity, No. (%)a | 344 (10.0) | 228 (8.9) | 316 (9.7) | 207 (7.7) |
| Primary language is Spanish, No. (%) | 218 (6.3) | 117 (4.6) | 165 (5.1) | 75 (2.8) |
| Married or have a life partner, No. (%)a | 2050 (59.3) | 1455 (56.6) | 1958 (60.2) | 1541 (57.0) |
| Primary health insurance, No. (%)c | ||||
| Commercial | 1816 (52.6) | 1425 (55.5) | 1865 (57.3) | 1553 (57.5) |
| Medicare | 1054 (30.5) | 735 (28.6) | 817 (25.1) | 678 (25.1) |
| Dual Medicaid and Medicare | 519 (15.0) | 381 (14.8) | 521 (16.0) | 421 (15.6) |
| Self-pay or without insurance | 66 (1.9) | 28 (1.1) | 51 (1.6) | 50 (1.9) |
| Charlson score, No. (%)d | ||||
| 0 | 1651 (47.8) | 1260 (49.0) | 1574 (48.4) | 1339 (49.6) |
| 1 | 744 (21.5) | 523 (20.4) | 708 (21.8) | 569 (21.1) |
| ≥2 | 1060 (30.7) | 786 (30.6) | 972 (29.9) | 794 (29.4) |
| Type of cancer screening, No. (%) | ||||
| Colorectal | 2519 (72.9) | 1754 (68.3) | 2226 (68.4) | 1746 (64.6) |
| Cervical | 661 (19.1) | 548 (21.3) | 714 (21.9) | 673 (24.9) |
| Breast | 220 (6.4) | 243 (9.5) | 299 (9.2) | 243 (9.0) |
| Lung | 55 (1.6) | 24 (0.9) | 15 (0.5) | 40 (1.5) |
| Risk of abnormal screening test result, No. (%)e | ||||
| Low | 1819 (52.6) | 1282 (49.9) | 1672 (51.4) | 1309 (48.4) |
| Medium | 1050 (30.4) | 807 (31.4) | 1025 (31.5) | 830 (30.7) |
| High | 586 (17.0) | 480 (18.7) | 557 (17.1) | 563 (20.8) |
| COVID-19 pandemic enrollment period, No. (%) | ||||
| Before the vaccine rollout (August-December 2020) | 421 (12.2) | 372 (14.5) | 459 (14.1) | 418 (15.5) |
| During the vaccine rollout (January-June 2021) | 2174 (62.9) | 1531 (59.6) | 1944 (59.7) | 1618 (59.9) |
| After the vaccine rollout (July-December 2021) | 860 (24.9) | 666 (25.9) | 851 (26.2) | 666 (24.6) |
| Primary care visit within same year as abnormal result, No. (%) | 2280 (66.0) | 1651 (64.3) | 2088 (64.2) | 1750 (64.8) |
Abbreviation: EHR, electronic health record.
Self-reported response to open-ended questions; collected during patient registration.
Self-reported option for race.
Collected during patient registration and updated at visits.
Score range 0-17. Higher scores indicate more comorbidity. Calculated using diagnosis codes including myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer, liver disease, diabetes, hemiplegia or paraplegia, malignancy, metastatic solid tumor, and HIV/AIDS.31
Based on guideline or specialist consensus as detailed in the eMethods and eTable 3 in Supplement 2.
Among patients randomized to the more intensive outreach groups, most were exposed to patient portal letters, mailed letters, or both (2969 patients [85.9%] in the EHR reminders, outreach, and navigation group; 2226 patients [86.6%] in the EHR reminders and outreach group) and coordinator phone calls (1830 patients [53%] in the EHR reminders, outreach, and navigation group; 1531 patients [59.6%] in the EHR reminders and outreach group), and fewer required or received navigator phone calls (1421 patients [41.1%] in the EHR reminders, outreach, and navigation group) or were screened for social determinants of health (315 patients [9.1%] in the EHR reminders, outreach, and navigation group) (eTable 4 in Supplement 2).
Patients often completed follow-up before the scheduled letter was sent (101 patients [2.9%] in the EHR reminders, outreach, and navigation group; 64 patients [2.5%] in the EHR reminders and outreach group), before the outreach phone call (533 patients [15.4%] in the EHR reminders, outreach, and navigation group; 374 patients [14.6%] in the EHR reminders and outreach group), or before the navigator phone call (820 patients [23.7%] in the EHR reminders, outreach, and navigation group).
Outcomes in the Intention-to-Treat Population
For the primary outcome of completion of recommended follow-up within 120 days of study enrollment, the adjusted proportion was 31.4% (n = 3455) in the EHR reminders, outreach, and navigation group, 31.0% (n = 2569) in the EHR reminders and outreach group, 22.7% (n = 3254) in the EHR reminders group, and 22.9% (n = 2702) in the usual care group (adjusted absolute difference for comparison of EHR reminders, outreach, and navigation group vs usual care, 8.5% [95% CI, 4.8%-12.0%], P < .001). There was no statistically significant interaction by health system (P = .35) or enrollment period (before the COVID-19 vaccine rollout [August-December 2020], during the COVID-19 vaccine rollout [January-June 2021], or after the COVID-19 vaccine rollout [July-December 2021], P = .73) (Table 2).
Table 2. Primary and Secondary Outcomes of 120-Day and 240-Day Follow-Up.
| Patient population (N = 11 980)a | Absolute difference among proportions (95% CI)a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EHR reminders, outreach, and navigation | EHR reminders and outreach | EHR reminders | Usual care | EHR reminders, outreach, and navigation vs usual care | EHR reminders and outreach vs usual care | EHR reminders vs usual care | EHR reminders, outreach, and navigation vs EHR reminders | EHR reminders and outreach vs EHR reminders | EHR reminders, outreach, and navigation vs EHR reminders and outreach | |
| Primary outcome | ||||||||||
| Intention-to-treat population, No. (%) | 3455 (28.8) | 2569 (21.4) | 3254 (27.2) | 2702 (22.6) | ||||||
| Completed follow-up within 120 d of study enrollment | ||||||||||
| Unadjusted, No. (%) | 1062 (30.7) | 831 (32.3) | 796 (24.5) | 672 (24.9) | 5.8 (3.6 to 8.1) | 7.4 (5.0 to 9.9) | −0.4 (−2.6 to 1.8) | 6.2 (4.1 to 8.4) | 7.8 (5.6 to 10.2) | −1.6 (−4.0 to 0.8) |
| Adjusted %b | 31.4 | 31.0 | 22.7 | 22.9 | 8.5 (4.8 to 12.0) | 8.1 (4.5 to 11.7) | −0.2 (−3.5 to 3.0) | 8.7 (5.1 to 12.2) | 8.3 (4.8 to 11.9) | 0.4 (−3.6 to 4.2) |
| Secondary outcomes | ||||||||||
| Completed follow-up within 240 d of study enrollmentc | ||||||||||
| Unadjusted, No. (%) | 1470 (42.5) | 1122 (43.7) | 1170 (36.0) | 955 (35.3) | 7.2 (4.8 to 9.6) | 8.4 (5.7 to 11.0) | 0.7 (−1.8 to 3.1) | 6.5 (4.3 to 8.9) | 7.7 (5.2 to 10.2) | −1.2 (−3.7 to 1.4) |
| Adjusted %b | 43.8 | 42.6 | 34.7 | 34.1 | 9.7 (5.4 to 14.2) | 8.5 (4.1 to 13.0) | 0.6 (−3.5 to 4.9) | 9.1 (4.7 to 13.5) | 7.9 (3.5 to 12.3) | 1.2 (−3.3 to 5.9) |
| As-treated populationd | 2094 (25.6) | 1855 (22.7) | 2348 (28.7) | 1883 (23.0) | ||||||
| Completed follow-up within 120 d of study enrollment | ||||||||||
| Unadjusted, No. (%) | 786 (37.5) | 649 (35.0) | 597 (25.4) | 453 (24.1) | 13.4 (10.6 to 16.3) | 10.9 (8.0 to 13.8) | 1.3 (−1.2 to 4.0) | 12.1 (9.4 to 14.8) | 9.6 (6.8 to 12.4) | 2.5 (−0.5 to 5.6) |
| Adjusted %b | 38.9 | 33.7 | 23.6 | 22.6 | 16.3 (12.3 to 20.4) | 11.1 (7.2 to 15.0) | 1.0 (−2.4 to 4.5) | 15.3 (11.3 to 19.3) | 10.1 (6.2 to 13.9) | 5.2 (0.8 to 9.6) |
| Completed follow-up within 240 d of study enrollment | ||||||||||
| Unadjusted, No. (%) | 1095 (52.3) | 895 (48.2) | 900 (38.3) | 691 (36.7) | 15.6 (12.5 to 18.6) | 11.5 (8.4 to 14.7) | 1.6 (−1.3 to 4.6) | 14.0 (11.1 to 16.9) | 9.9 (6.9 to 12.9) | 4.1 (0.9 to 7.2) |
| Adjusted %b | 52.2 | 46.9 | 37.3 | 35.9 | 18.3 (13.0 to 23.4) | 11.0 (5.8 to 16.2) | 1.4 (−3.6 to 6.3) | 16.9 (11.7 to 22.0) | 9.6 (4.5 to 14.8) | 7.3 (1.8 to 12.7) |
Abbreviation: EHR, electronic health record.
The comparisons overall yielded P<.001, which was derived from the adjusted model.
Mixed logistic regression models (SAS Proc GLIMMIX) were used with the patient as the unit of analysis and included indicator variables for the 3 intervention groups (EHR reminders, outreach, and navigation group, the EHR reminders and outreach group, and the EHR reminders group) as the primary exposure. Random effects were used for practices and physicians. Fixed effects were used for cancer type, study site, enrollment period, and the enrollment period × study site interaction. In addition, the primary adjusted models also included covariates for the risk level of the abnormal test results (eg, low, medium, or high), marital status, having a primary care visit within the past year, race, and ethnicity. The adjusted percentages were calculated using marginal standardization.
Data are for the intention-to-treat population.
Excluded those included in the intention-to-treat population (additional details appear in the eMethods in Supplement 2).
The absolute differences in the proportions were statistically significantly higher in comparisons of follow-up among patients in the EHR reminders and outreach group vs patients in the usual care group or patients in the EHR reminders group. The absolute differences in the proportions were statistically significantly higher in comparisons of follow-up among patients in the EHR reminders, outreach, and navigation group vs patients in the usual care group or patients in the EHR reminders group. The absolute differences in the proportions were not statistically significantly higher in comparisons of follow-up among patients in the EHR reminders, outreach, and navigation group vs patients in the EHR reminders and outreach group or in comparisons of follow-up among patients in the EHR reminders group vs patients in the usual care group (Table 2).
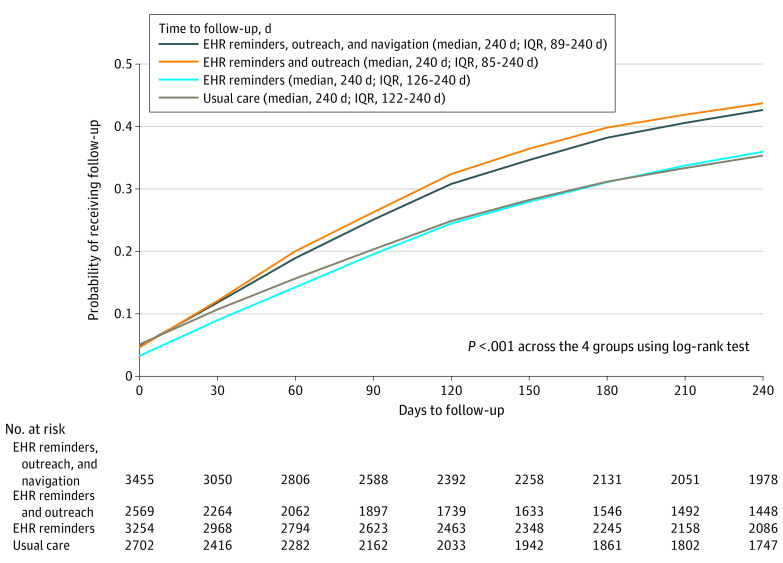
The follow-up completion proportions at 240 days showed similar results. The cumulative follow-up completion proportions through 240 days using Kaplan-Meier estimates for the primary population showed patients in the EHR reminders and outreach group and patients in the EHR reminders, outreach, and navigation group were statistically significantly more likely to complete follow-up during the study period (log-rank test, P < .001; Figure 2).
Figure 2. Cumulative Follow-Up for the Intention-to-Treat Population.
Outcome completion was defined by a follow-up test or procedure as recommended by guideline or specialist consensus and was specific to the organ type and risk of the abnormal screening test result. EHR indicates electronic health record.
Exploratory Analyses
Findings from prespecified exploratory partially adjusted models were similar to those from the full model (eTable 5 in Supplement 2).
In subgroup analyses, patients with abnormal cancer screening test results for cervical cancer in the EHR reminders, outreach, and navigation group (odds ratio [OR], 1.28 [95% CI, 0.93-1.77]) and in the EHR reminders and outreach group (OR, 1.51 [95% CI, 1.09-2.09]) had greater odds of completing follow-up compared with patients in the usual care group. Patients with abnormal cancer screening test results for colorectal cancer in the EHR reminders, outreach, and navigation group (OR, 1.68 [95% CI, 1.32-2.16]) and in the EHR reminders and outreach group (OR, 1.71 [95% CI, 1.33-2.19]) had greater odds of completing follow-up compared with patients in the usual care group (eTable 6 in Supplement 2).
Similarly, patients with abnormal cancer screening test results that were low or medium risk (eTable 3 in Supplement 2) in the EHR reminders, outreach, and navigation group (OR for low risk, 1.66 [95% CI, 1.36-2.02]; OR for medium risk, 1.57 [95% CI, 1.25-1.96]) and in the EHR reminders and outreach group (OR for low risk, 1.58 [95% CI, 1.28-1.95]; OR for medium risk, 1.55 [95% CI, 1.23-1.96]) had greater odds of completing follow-up compared with patients in the usual care group.
Outcomes in the As-Treated Population
Among the 11 980 patients in the study sample, 3800 were found to be ineligible based on manual chart review (eFigure 2 in Supplement 2). The secondary population thus comprised 8180 patients. The patient characteristics, cancer type, and risk of abnormal test result were similar across study groups in the as-treated population (eTable 7 in Supplement 2). The proportions completing follow-up at 120 days and 240 days in the secondary population were similar to those in the primary study population (Table 2), as were cumulative follow-up completion proportions through 240 days using Kaplan-Meier estimates (eFigure 3 in Supplement 2).
Discussion
Primary care practices within 3 large networks were randomized to a multilevel intervention to improve recommended follow-up of patients with overdue abnormal cancer screening test results. Designed to supplement rather than replace usual care, the intervention used automated EHR algorithms to classify abnormal cancer screening test results based on the test and the risk of the result, and to assign an appropriate follow-up action and time frame for each abnormal test result.
Almost 12 000 patients were enrolled during a study period encompassing the COVID-19 pandemic. Compared with patients treated at practices randomized to usual care or passive EHR reminders, completion of follow-up within 120 days of enrollment was higher among patients in practices randomized to EHR reminders and outreach (via letters and phone calls with or without patient navigation). Similar findings were noted for outcomes through 240 days and in subgroups based on cancer type and risk level of the abnormal test result.
The intervention was embedded in primary care practices because primary care clinicians take a whole-person approach to patients and have responsibility for integrating follow-up management for the 4 US Preventive Services Task Force recommended cancer screening tests evaluated in the trial.1,2,4,5 The trial’s design allowed for evaluation of the effectiveness at the information technology system level and the individual clinician level (EHR reminders), and at the individual patient and the team outreach levels (the EHR reminders and outreach group; the EHR reminders, outreach, and navigation group), and reinforce prior research showing incomplete follow-up varying across cancer organ types and health care settings.9,14,15,24,32,33
Even though active outreach by population health coordinators or patient navigators resulted in improved proportions of follow-up completion, many patients did not complete follow-up, which may in part reflect care disruptions resulting from the COVID-19 pandemic. However, studies conducted prior to the pandemic also show many patients do not complete recommended follow-up,23 highlighting the need to understand factors associated with not completing follow-up that go beyond reminder efforts. Patient factors may include need for education about the meaning of the test results, what follow-up procedures involve, and assessing patient preferences.34,35 Practitioner factors such as knowledge of guidelines and improved access to prior test results may also be important.19
The current study was designed to examine the additive effect of EHR reminders, direct patient outreach, and patient navigation. The EHR reminders alone did not improve follow-up proportions and highlight the limited ability of passive reminders among patients who are overdue for follow-up.36 The lack of additive benefit of patient navigation beyond that of reminder letters and phone calls may reflect the relatively modest remote navigation approach that was implemented; only 315 patients (9.1%) in the EHR reminders, outreach, and navigation group were screened for social determinants of health.37,38,39 It may also reflect that in this population, social determinants of health were less important impediments or that the resources to address barriers were insufficient, especially given that the trial took place during the COVID-19 pandemic.40
Exploratory subgroup analyses suggest that the primary benefit was seen in those with abnormal screening test results for cervical cancer or colorectal cancer that were of mild or moderate risk. The small number of patients enrolled with abnormal test results for lung cancer likely reflects low adoption of lung cancer screening in practice, perhaps with closer management of abnormal test results because of the relative novelty of recommendations for lung cancer screening.41 For both types of abnormal test results (breast cancer and those of other cancer types that are high risk), the intervention’s lack of benefit may reflect existing systems that promote follow-up.32 It is also uncertain if the intervention would have been more effective compared with usual care if implemented earlier (when a patient first becomes due for follow-up).
Limitations
The study has limitations. First, it may not be generalizable beyond the 3 large participating primary care networks with well-integrated EHRs and existing population management infrastructure. Second, even though each network used a common proprietary EHR, there were differences in local implementation due to the need for a coded data infrastructure to assess breast, cervical, and lung cancer screening test results.
Third, the 3800-patient difference between the intention-to-treat population and the as-treated population reflects limitations either in EHR data or in the accuracy of our algorithms to correctly identify history, abnormal test results, complete ascertainment of follow-up care, and documented alternate care plans that did not fulfill the recommended follow-up algorithms. Fourth, defining appropriate follow-up for abnormal cancer screening test results for cervical cancer is particularly complex,42 and our algorithms did not reflect the most recent changes in the consensus guidelines for cervical and colorectal cancer.20,29 Fifth, given these limitations, health care systems may be reluctant to adapt such data systems; our findings suggest the need for national organizations to develop and maintain computable algorithms that are broadly interoperable among EHRs.43
Sixth, as a pragmatic trial, randomized treatment assignment was not blinded. Seventh, based in primary care, the intervention did not actively engage specialists involved in managing abnormal test results, and tailoring outreach to the risk of the abnormal test result was not feasible. Eighth, the study did not evaluate the cost of implementing the intervention, but the marginal cost of the population outreach for the EHR reminders and outreach intervention is likely modest.44 Ninth, the entire study took place during the COVID-19 pandemic, which likely contributed both to more patients having overdue abnormal test results and not completing follow-up.
Conclusions
A multilevel primary care intervention that included EHR reminders and patient outreach with or without patient navigation improved timely follow-up of overdue abnormal cancer screening test results for breast, cervical, colorectal, and lung cancer.
Trial protocol
eMethods
eTable 1. Description of included test results, follow-up period, appropriate diagnostic follow-up, and estimated follow-up rates pre-trial
eTable 2. Overview of Usual Care Outreach by Organ Type and Risk Status
eTable 3. Assignment of Risk Status to Screening Test Results by Cancer Type
eTable 4. Outreach Activities for Intervention Arms 4 (EHR Reminders + Outreach + Navigation) and 3 (EHR Reminders + Outreach)
eTable 5. Primary 120-day follow-up and secondary 240-day follow-up completion outcomes from the partially adjusted model by study population and intervention
eTable 6. Adjusted odds ratios of 120-day follow-up completion for primary intention to treat subgroups among study arms
eTable 7. Patient characteristics for as-treated population by study arm
eFigure 1. Flow of patients in the study
eFigure 2. Consort diagram for as-treated population
eFigure 3. Cumulative follow-up completion rates (Kaplan-Meier estimates) and KM plot for as-treated population
Statistical analysis plan
Data sharing statement
References
- 1.Siu AL; US Preventive Services Task Force . Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(4):279-296. doi: 10.7326/M15-2886 [DOI] [PubMed] [Google Scholar]
- 2.Curry SJ, Krist AH, Owens DK, et al. ; US Preventive Services Task Force . Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320(7):674-686. doi: 10.1001/jama.2018.10897 [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society . American Cancer Society guidelines for the early detection of cancer. Published February 24, 2023. Accessed July 24, 2023. https://www.cancer.org/cancer/screening/american-cancer-society-guidelines-for-the-early-detection-of-cancer.html
- 4.Davidson KW, Barry MJ, Mangione CM, et al. ; US Preventive Services Task Force . Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi: 10.1001/jama.2021.6238 [DOI] [PubMed] [Google Scholar]
- 5.Krist AH, Davidson KW, Mangione CM, et al. ; US Preventive Services Task Force . Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(10):962-970. doi: 10.1001/jama.2021.1117 [DOI] [PubMed] [Google Scholar]
- 6.Yabroff KR, Gansler T, Wender RC, Cullen KJ, Brawley OW. Minimizing the burden of cancer in the United States: goals for a high-performing health care system. CA Cancer J Clin. 2019;69(3):166-183. doi: 10.3322/caac.21556 [DOI] [PubMed] [Google Scholar]
- 7.Koh HK, Sebelius KG. Promoting prevention through the Affordable Care Act. N Engl J Med. 2010;363(14):1296-1299. doi: 10.1056/NEJMp1008560 [DOI] [PubMed] [Google Scholar]
- 8.Sabatino SA, Lawrence B, Elder R, et al. ; Community Preventive Services Task Force . Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: nine updated systematic reviews for the guide to community preventive services. Am J Prev Med. 2012;43(1):97-118. doi: 10.1016/j.amepre.2012.04.009 [DOI] [PubMed] [Google Scholar]
- 9.Tosteson AN, Beaber EF, Tiro J, et al. ; PROSPR Consortium . Variation in screening abnormality rates and follow-up of breast, cervical and colorectal cancer screening within the PROSPR Consortium. J Gen Intern Med. 2016;31(4):372-379. doi: 10.1007/s11606-015-3552-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawaya GF, Kulasingam S, Denberg TD, Qaseem A; Clinical Guidelines Committee of American College of Physicians . Cervical cancer screening in average-risk women: best practice advice from the clinical guidelines committee of the American College of Physicians. Ann Intern Med. 2015;162(12):851-859. doi: 10.7326/M14-2426 [DOI] [PubMed] [Google Scholar]
- 11.Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370(14):1298-1306. doi: 10.1056/NEJMoa1309086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinsky PF, Gierada DS, Black W, et al. Performance of Lung-RADS in the National Lung Screening Trial: a retrospective assessment. Ann Intern Med. 2015;162(7):485-491. doi: 10.7326/M14-2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aberle DR, Adams AM, Berg CD, et al. ; National Lung Screening Trial Research Team . Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi: 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feldman S, Lykken JM, Haas JS, et al. Factors associated with timely colposcopy following an abnormal cervical cancer test result. Prev Med. 2022;164:107307. doi: 10.1016/j.ypmed.2022.107307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spencer JC, Kim JJ, Tiro JA, et al. Racial and ethnic disparities in cervical cancer screening from three US healthcare settings. Am J Prev Med. Published online May 2, 2023. doi: 10.1016/j.amepre.2023.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Destouet JM, Bassett LW, Yaffe MJ, Butler PF, Wilcox PA. The ACR’s Mammography Accreditation Program: ten years of experience since MQSA. J Am Coll Radiol. 2005;2(7):585-594. doi: 10.1016/j.jacr.2004.12.005 [DOI] [PubMed] [Google Scholar]
- 17.Zapka JG, Taplin SH, Solberg LI, Manos MM. A framework for improving the quality of cancer care: the case of breast and cervical cancer screening. Cancer Epidemiol Biomarkers Prev. 2003;12(1):4-13. [PubMed] [Google Scholar]
- 18.Beaber EF, Kim JJ, Schapira MM, et al. ; Population-based Research Optimizing Screening through Personalized Regimens Consortium . Unifying screening processes within the PROSPR consortium: a conceptual model for breast, cervical, and colorectal cancer screening. J Natl Cancer Inst. 2015;107(6):djv120. doi: 10.1093/jnci/djv120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atlas SJ, Tosteson ANA, Burdick TE, et al. Primary care practitioner perceptions on the follow-up of abnormal cancer screening test results. JAMA Netw Open. 2022;5(9):e2234194. doi: 10.1001/jamanetworkopen.2022.34194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perkins RB, Guido RS, Castle PE, et al. ; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee . 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24(2):102-131. doi: 10.1097/LGT.0000000000000525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poon EG, Gandhi TK, Sequist TD, Murff HJ, Karson AS, Bates DW. “I wish I had seen this test result earlier!”: dissatisfaction with test result management systems in primary care. Arch Intern Med. 2004;164(20):2223-2228. doi: 10.1001/archinte.164.20.2223 [DOI] [PubMed] [Google Scholar]
- 22.Yabroff KR, Washington KS, Leader A, Neilson E, Mandelblatt J. Is the promise of cancer-screening programs being compromised? quality of follow-up care after abnormal screening results. Med Care Res Rev. 2003;60(3):294-331. doi: 10.1177/1077558703254698 [DOI] [PubMed] [Google Scholar]
- 23.Zapka J, Taplin SH, Price RA, Cranos C, Yabroff R. Factors in quality care—the case of follow-up to abnormal cancer screening tests—problems in the steps and interfaces of care. J Natl Cancer Inst Monogr. 2010;2010(40):58-71. doi: 10.1093/jncimonographs/lgq009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuccotti G, Samal L, Maloney FL, Ai A, Wright A. The need for closed-loop systems for management of abnormal test results. Ann Intern Med. 2018;168(11):820-821. doi: 10.7326/M17-2425 [DOI] [PubMed] [Google Scholar]
- 25.Haas JS, Atlas SJ, Wright A, et al. Multilevel Follow-up of Cancer Screening (mFOCUS): protocol for a multilevel intervention to improve the follow-up of abnormal cancer screening test results. Contemp Clin Trials. 2021;109:106533. doi: 10.1016/j.cct.2021.106533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diamond CJ, Laurentiev J, Yang J, et al. Natural language processing to identify abnormal breast, lung, and cervical cancer screening test results from unstructured reports to support timely follow-up. Stud Health Technol Inform. 2022;290:433-437. doi: 10.3233/SHTI220112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeVetter N, Westfall JM, Carrozza M, Westfall E. Calling your aunt Bertha for social assets: family medicine and social determinants of health. J Prim Care Community Health. 2022;13:21501319221131405. doi: 10.1177/21501319221131405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehman CD, Arao RF, Sprague BL, et al. National performance benchmarks for modern screening digital mammography: update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283(1):49-58. doi: 10.1148/radiol.2016161174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta S, Lieberman D, Anderson JC, et al. Recommendations for follow-up after colonoscopy and polypectomy: a consensus update by the US Multi-Society Task Force on colorectal cancer. Gastrointest Endosc. 2020;91(3):463-485.e5, e5. doi: 10.1016/j.gie.2020.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wood DE, Kazerooni EA, Aberle D, et al. NCCN guidelines® insights: lung cancer screening, version 1.2022. J Natl Compr Canc Netw. 2022;20(7):754-764. doi: 10.6004/jnccn.2022.0036 [DOI] [PubMed] [Google Scholar]
- 31.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 32.Barlow WE, Beaber EF, Geller BM, et al. Evaluating screening participation, follow-up, and outcomes for breast, cervical, and colorectal cancer in the PROSPR Consortium. J Natl Cancer Inst. 2020;112(3):238-246. doi: 10.1093/jnci/djz137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rivera MP, Durham DD, Long JM, et al. Receipt of recommended follow-up care after a positive lung cancer screening examination. JAMA Netw Open. 2022;5(11):e2240403. doi: 10.1001/jamanetworkopen.2022.40403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bastani R, Yabroff KR, Myers RE, Glenn B. Interventions to improve follow-up of abnormal findings in cancer screening. Cancer. 2004;101(5)(suppl):1188-1200. doi: 10.1002/cncr.20506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yabroff KR, Zapka J, Klabunde CN, et al. Systems strategies to support cancer screening in US primary care practice. Cancer Epidemiol Biomarkers Prev. 2011;20(12):2471-2479. doi: 10.1158/1055-9965.EPI-11-0783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimolzak AJ, Shahid U, Giardina TD, et al. Why test results are still getting “lost” to follow-up: a qualitative study of implementation gaps. J Gen Intern Med. 2022;37(1):137-144. doi: 10.1007/s11606-021-06772-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Percac-Lima S, Aldrich LS, Gamba GB, Bearse AM, Atlas SJ. Barriers to follow-up of an abnormal Pap smear in Latina women referred for colposcopy. J Gen Intern Med. 2010;25(11):1198-1204. doi: 10.1007/s11606-010-1450-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paskett ED, Dudley D, Young GS, et al. ; PNRP Investigators . Impact of patient navigation interventions on timely diagnostic follow up for abnormal cervical screening. J Womens Health (Larchmt). 2016;25(1):15-21. doi: 10.1089/jwh.2014.5094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramachandran A, Freund KM, Bak SM, Heeren TC, Chen CA, Battaglia TA. Multiple barriers delay care among women with abnormal cancer screening despite patient navigation. J Womens Health (Larchmt). 2015;24(1):30-36. doi: 10.1089/jwh.2014.4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Percac-Lima S, Ashburner JM, Zai AH, et al. Patient navigation for comprehensive cancer screening in high-risk patients using a population-based health information technology system: a randomized clinical trial. JAMA Intern Med. 2016;176(7):930-937. doi: 10.1001/jamainternmed.2016.0841 [DOI] [PubMed] [Google Scholar]
- 41.Doan C, Li S, Goodwin JS. Breast and lung cancer screening among Medicare enrollees during the COVID-19 pandemic. JAMA Netw Open. 2023;6(2):e2255589. doi: 10.1001/jamanetworkopen.2022.55589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perkins RB, Guido RL, Saraiya M, et al. Summary of current guidelines for cervical cancer screening and management of abnormal test results: 2016-2020. J Womens Health (Larchmt). 2021;30(1):5-13. doi: 10.1089/jwh.2020.8918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saraiya M, Colbert J, Bhat GL, et al. Computable guidelines and clinical decision support for cervical cancer screening and management to improve outcomes and health equity. J Womens Health (Larchmt). 2022;31(4):462-468. doi: 10.1089/jwh.2022.0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levy DE, Munshi VN, Ashburner JM, Zai AH, Grant RW, Atlas SJ. Health IT-assisted population-based preventive cancer screening: a cost analysis. Am J Manag Care. 2015;21(12):885-891. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eMethods
eTable 1. Description of included test results, follow-up period, appropriate diagnostic follow-up, and estimated follow-up rates pre-trial
eTable 2. Overview of Usual Care Outreach by Organ Type and Risk Status
eTable 3. Assignment of Risk Status to Screening Test Results by Cancer Type
eTable 4. Outreach Activities for Intervention Arms 4 (EHR Reminders + Outreach + Navigation) and 3 (EHR Reminders + Outreach)
eTable 5. Primary 120-day follow-up and secondary 240-day follow-up completion outcomes from the partially adjusted model by study population and intervention
eTable 6. Adjusted odds ratios of 120-day follow-up completion for primary intention to treat subgroups among study arms
eTable 7. Patient characteristics for as-treated population by study arm
eFigure 1. Flow of patients in the study
eFigure 2. Consort diagram for as-treated population
eFigure 3. Cumulative follow-up completion rates (Kaplan-Meier estimates) and KM plot for as-treated population
Statistical analysis plan
Data sharing statement