Abstract
Background:
Bisphenol A (BPA) has been linked to changes in the dopamine system and development of an Attention-Deficit/Hyperactivity Disorder (ADHD) phenotype in animal models, with differing effects in males compared to females. We examined the association between urinary BPA concentrations and ADHD in a national sample of U.S. children, and whether this association differs by child sex.
Methods:
We used data from the 2003–2004 National Health and Nutrition Examination Survey, a cross-sectional, nationally representative sample of the U.S. population. Participants were 8–15 years of age (N=460). Using a diagnostic interview to ascertain the presence of ADHD in the past year, multivariable logistic regression examined the link between concurrent urinary BPA concentrations and ADHD status.
Results:
Of the 460 participants, 7.1% [95% CI: 4.4–11.3] met Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition (DSM-IV) criteria for ADHD. Children who had BPA concentrations at or above the median of the sample had higher prevalence of meeting criteria for ADHD (11.2% [95% CI: 6.8–17.8]) than those with BPA concentrations below the median (2.9% [95% CI: 1.1–7.2]). Higher urinary BPA concentrations were associated with ADHD (adjusted odds ratio [aOR]: 5.68 [95% CI: 1.6–19.8] for BPA concentrations above vs. below the median). In sex-stratified analyses, these associations were stronger in boys (aOR=10.9 [95% CI: 1.4–86.0]) than in girls (aOR=2.8 [95% CI: 0.4–21.3]), although the BPA by sex interaction term was not significant (p=0.25).
Conclusion:
We found evidence that higher urinary BPA concentrations were associated with ADHD in U. S. children; these associations were stronger in boys than in girls. Considering the widespread use of BPA and growing literature on neurobehavioral effects of BPA in children, further study is warranted to determine if reducing exposure to BPA may represent an important avenue for ADHD prevention.
Keywords: ADHD, Attention, Hyperactivity, Behavior, Bisphenol A, Environmental exposure, Endocrine disruptor
1. Introduction
Attention Deficit Hyperactivity Disorder (ADHD), the most common neurobehavioral disorder in children, is characterized by difficulty sustaining attention, controlling impulsivity and modulating activity levels (Froehlich et al., 2007; Merikangas et al., 2010). Both familial and environmental factors have been implicated in the development of ADHD (Braun et al., 2006; Nigg, 2006). Concerns have been raised regarding the possible association between Bisphenol A (BPA) exposure and childhood neurobehavioral disorders (Center for the Evaluation of Risks to Human Reproduction, 2008), such as ADHD (Harley et al., 2013; Hong et al., 2013). BPA is an industrial chemical widely used to manufacture polycarbonate plastics and epoxy resins, and can be found in a broad range of products, including metal food can linings, reusable water bottles, dental sealants, sports safety equipment, thermal receipts, and adhesives (Vandenberg et al., 2007). The primary source of human exposure to BPA is the ingestion of food and drink containing BPA (Kang et al., 2006; Miyamoto and Kotake, 2006; Vandenberg et al., 2010; Vandenberg et al., 2007; Wilson et al., 2007). BPA exposure is widespread in developed countries (Calafat et al., 2008; Vandenberg et al., 2010). For example, BPA was detected in the urine of 93% of the general U.S. population (Calafat et al., 2008).
Animal studies of BPA’s neuroendocrine effects offer potential mechanisms supporting a linkage to ADHD. BPA has been implicated in modulating dopaminergic neurotransmission (Jones and Miller, 2008). Animal studies have shown that perinatal exposure to BPA causes accelerated turnover of dopamine (Honma et al., 2006), reduction in functional dopamine D3 receptors in the forebrain (Mizuo et al., 2004b), and abnormalities in dopamine transporter (DAT) gene expression in the neuronal membrane (Alyea and Watson, 2009; Ishido et al., 2007; Masuo et al., 2004). Given that imbalance in dopamine neurotransmission is believed to underlie the pathophysiology of ADHD (Levy, 1991; Levy and Swanson, 2001; Swanson et al., 2007), it is plausible that exposure to BPA may contribute to the development of an ADHD phenotype. Indeed, animal studies have shown that changes in the dopaminergic system with BPA exposure are associated with hyperactivity (Ishido et al., 2004, 2005b, 2007, 2011; Masuo et al., 2004).
There is a growing body of literature examining the impact of BPA exposure on neurodevelopmental outcomes in children (Braun et al., 2009, 2011; Casas et al., 2015; Evans et al., 2014; Findlay and Kohen, 2015; Harley et al., 2013; Hong et al., 2013; Miodovnik et al., 2011; Perera et al., 2012; Roen et al., 2015; Yolton et al., 2011). For example, prenatal exposure to BPA has been associated with externalizing behaviors in early childhood (Braun et al., 2009, 2011; Casas et al., 2015; Perera et al., 2012). However, there have been few published studies examining the association between childhood BPA exposure and meeting diagnostic criteria for ADHD (Harley et al., 2013). Of note, the majority of prior studies of childhood BPA exposure and neurobehavior have been conducted in regional cohorts and have focused on children younger than 8 years of age (Braun et al., 2009, 2011; Casas et al., 2015; Harley et al., 2013; Maserejian et al., 2012; Perera et al., 2012), whereas ADHD and its impairments are often not fully manifest until children enter school. The findings of these prior studies have been mixed, with some reporting an association between childhood exposure to BPA and hyperactivity and inattention (Casas et al., 2015; Findlay and Kohen, 2015; Harley et al., 2013; Hong et al., 2013) while others did not (Braun et al., 2011; Maserejian et al., 2012; Perera et al., 2012).
Due to BPA’s endocrine disrupting properties and agonist effects on estrogen receptors, concerns have been raised regarding possible sex specific effects on neurobehavior (Adriani et al., 2003; Gioiosa et al., 2007; Patisaul and Polston, 2008; Tando et al., 2007). Postnatal exposure to BPA has been associated with hyperactivity in male rats in animal studies (Ishido et al., 2007; Nojima et al., 2013), but there is a paucity of data regarding effects in female animals. Most animal models used only males to study BPA effects on these outcomes (Ishido et al., 2004, 2005a, 2007; Masuo et al., 2004; Mizuo et al., 2004a; Zhou et al., 2011). In addition, findings regarding possible sex-specific effects of BPA on human neurodevelopment, specifically ADHD-related behaviors, have been mixed. Prenatal BPA exposure was associated with increased hyperactivity and inattention symptoms in preschool boys (Casas et al., 2015), but these associations did not persist at later ages. A study by Hong et al. showed childhood BPA exposure was associated with increased externalizing problems and aggressive behaviors in boys but not in girls (Hong et al., 2013), while Harley et al. (2013) reported an association between childhood BPA exposure and increased inattention and hyperactivity in children of both sexes at seven years of age. Two additional studies found that childhood BPA exposure was associated with hyperactivity and externalizing behaviors in girls but not boys (Findlay and Kohen, 2015; Roen et al., 2015). Given these mixed findings, the purpose of our study was to determine the association between childhood BPA exposure and ADHD in a national sample of U.S. children aged 8–15 years old, and whether sex modifies this association.
2. Materials and methods
2.1. Study participants
This study was reviewed by the Institutional Review Board (IRB) of Cincinnati Children’s Hospital Medical Center and was determined to be exempt from regulatory criteria for research involving human subjects (Study # 2011–1686) due to its use of de-identified data. Our study sample was derived from the 2003–2004 dataset of the National Health and Nutrition Examination Survey (NHANES). NHANES is a stratified multistage probability sample of the civilian non-institutionalized population of the U.S. with an oversampling of certain minority populations (Centers for Disease Control and Prevention, 2014). Urine samples were analyzed for BPA in one third of the random subset of participants in the NHANES 2003–2004. Among children 8–15 years old who participated in the NHANES 2003–2004 cycle (N=1,771), data for both urinary BPA and ADHD diagnostic interview was available for 460 participants (30% of total) for analyses. There were no significant differences observed between participants included versus excluded from the analyses with regards to demographic characteristics, ADHD status, and measured environmental exposures (See Table 1).
Table 1.
Comparison of Characteristics of Children 8–15 Years of Age Not Included and Included in Study Sample.
| Not Included | Included | p-value | |
|---|---|---|---|
|
| |||
| Age n (%) a | |||
| 8–11 years | 490 (49.2) (N = 1311) | 176 (48.9) (N = 460) | 0.83 |
| 12–15 years | 821 (50.8) (n = 1311) | 284 (51.1) (n = 460) | |
| Male, n (%) a | 639 (50.8) (n = 1311) | 237 (53.3) (n = 460) | 0.67 |
| Race/ethnicity, n (%) a | 0.94 | ||
| White, non-Hispanic | 339(60.9) (N = 1311) | 122 (62.2) (N = 460) | |
| African American | 479 (15.9) (n = 1311) | 162 (15.4) (n = 460) | |
| Mexican American | 397 (12.1) (n = 1311) | 143 (12.0) (n = 460) | |
| Other race/ethnicity | 96 (11.1) (N = 1311) | 33 (10.4) (n = 459) | |
| DSM-IV defined ADHD | 60 (7.9) (n =980) | 32 (7.1) (N = 454) | 0.61 |
| Caregiver report of ADHD, n (%) a | 109 (10.0) (N = 1308) | 49 (13.4) (n = 459) | 0.12 |
| ADHD by DSM-IV criteria and/or Caregiver report Poverty-Income Ratio, n (%)a | 147 (17.3) (N =1002) | 71 (17.3) (n = 460) | 0.97 |
| <1.00 | 421 (23.6) (N = 1249) | 126 (19.3) (N=444) | 0.08 |
| 1.00–1.85 | 287 (18.8) (n = 1249) | 120 (23.6) (n=444) | |
| > 1.85–3.00 | 236 (22.4) (n = 1249) | 91 (21.6) (n=444) | |
| >3.00 | 305 (35.2) (n = 1249) | 107 (35.5) (n=444) | |
| Reported health insurance, n (%)a | 1087 (87.6) (n = 1293) | 289 (90.4) (n = 456) | 0.14 |
| Maternal Smoking during pregnancy, n (%) a | 217 (21.0) (n = 1297) | 71 (19.2) (n = 455) | 0.42 |
| Birth weight < 2.5 kg, n (%) a | 119 (6.6) (N = 1291) | 46 (8.0) (n = 452) | 0.54 |
| Reported current household tobacco smoke exposure, n (%) a | 289 (23.5) (N = 1295) | 93 (23.8) (N =457) | 0.97 |
| Urine BPA, weighted geometric mean ± SE, pg/L | 4.27 + 1.17 (N = 90) | 3.67 + 1.09 (N = 460) | 0.35 |
| Urine BPA < median, n (%)a | 42 (37.9) (n = 90) | 227 (48.9) (n = 460) | 0.13 |
Abbreviations: ADHD, Attention-Deficit/Hyperactivity Disorder; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; SE, standard error; BPA, Bisphenol A.
n reflects actual sample size; % is weighted to reflect national prevalence estimates.
2.2. ADHD measurement
Our primary outcome was the presence of ADHD, defined as meeting DSM-IV criteria for ADHD using the National Institute of Mental Health Diagnostic Interview Schedule for Children–IV (DISC-IV), a structured diagnostic interview module. The DISC was administered to caregivers by a telephone interview within one to four weeks of urine collection. Caregivers were queried about the child’s ADHD symptoms, age of onset, symptom pervasiveness and related impairments at both home and school during the past 12 months. The ADHD DISC-IV caregiver module has evidence of substantial validity (Shaffer et al., 2000), and reliability in both English (Shaffer et al., 2000), and Spanish versions (Bravo et al., 2001; Canino et al., 2004).
Given that more than half of the children diagnosed with ADHD earlier in life continue to experience ADHD symptoms despite no longer meeting the DSM criteria for ADHD later during adolescence (Biederman et al., 2000; Oord et al., 2012), we also examined the secondary outcomes of 1) having a caregiver report of a previous diagnosis of ADHD and, 2) having DSM-defined ADHD and/or caregiver report of ADHD. To determine whether children had a prior diagnosis of ADHD, caregivers were asked, “has a doctor or a health professional ever told you that (child’s name) had attention deficit disorder?” (National Health and Nutrition Examination Survey, 2006).
2.3. Measurement of Bisphenol A
Our independent variable was childhood exposure to BPA. BPA concentrations were measured by a single, spot urine sample collected during the child’s Mobile Examination Center visit. The total urine concentration of BPA was measured using online solidphase extraction (SPE) coupled to high-performance liquid chromatography (HPLC)- isotope dilution tandem mass spectrometry (MS/MA) with peak focusing as described previously (Calafat et al., 2008; Ye et al., 2005). The limit of detection (LOD) was 0.36 μg/L; concentrations below the LOD were ascribed a value of LOD/√2 (Hornung and Reed, 1990).
2.4. Covariates
We examined the prior literature to identify factors linked to ADHD and included these covariates as potential confounders for the association between childhood BPA exposure and ADHD in all multivariable models. These covariates included child sex (Froehlich et al., 2007; Pastor and Reuben, 2008), age (Pastor and Reuben, 2008), income (Froehlich et al., 2007), race/ethnicity (Froehlich et al., 2007), health insurance status (Lingineni et al., 2012), prenatal tobacco exposure reported by caregivers (Braun et al., 2006; Froehlich et al., 2009), blood lead level (Froehlich et al., 2009), and urine organophosphate metabolite (3 dimethyl alkylphosphate [DMAP]) level (Bouchard et al., 2010). Information about demographics was collected concurrently during the mobile examination center visit. Income was measured using the Poverty Income Ratio (PIR, the ratio of self -reported household income to the poverty threshold appropriate for the size of the household), which was categorized into quintiles. Race/ethnicity was reported by the caregiver as non-Hispanic white, African American, Mexican-American, and “other.” The “other” race category included participants who identified themselves as non-Mexican Hispanics or other race combined into one category due to small numbers in each individual group. Blood samples for lead levels and urine samples for organophosphate metabolite levels, BPA concentrations and creatinine were collected concurrently at the time of mobile examination center visit. Blood lead level and urinary organophosphate metabolite concentrations were log10-transformed in the adjusted models to account for skewed distribution. In addition, urinary creatinine concentrations were included in models to account for urine dilution (Barr et al., 2004).
2.5. Statistical analyses
Descriptive statistics on the prevalence of ADHD using all three case definitions (DSM-IV defined ADHD, DSM-IV defined ADHD and/or caregiver report of ADHD, and caregiver report of previous diagnosis of ADHD) were reported for participants with urinary BPA concentrations at or above median vs. below median.
We used logistic regression models to analyze the association between a dichotomous indicator of urinary BPA concentrations (i.e., at or above vs. below the median) and ADHD while adjusting for covariates. We further examined the association between continuous BPA levels (log10 transformed to account for skewed distribution of data) and ADHD diagnosis using logistic regression analyses, again adjusting for potential confounders.
We used Poisson regression to evaluate the relationship between urinary BPA concentrations and symptom counts for inattention and hyperactivity-impulsivity reported as occurring ‘often’ at home and in an additional setting on the DISC-IV. Using Poisson regression to account for the skewed distribution of ADHD symptom counts, urinary BPA was analyzed as a dichotomous variable (levels below vs. at or above median) and separately as a continuous (log10 transformed) variable. Adjusted Symptom Count Ratios were calculated for each model to determine the mean percent increase in symptom counts beyond the reference group for each 10-fold increase in urinary BPA concentrations.
Sex-stratified analyses were conducted to examine the relationship between BPA levels and ADHD, based on suggested differences in the neurobehavioral effects of BPA in males compared to females in some prior studies. We statistically tested whether sex modified the association between BPA and ADHD diagnosis by including a sex by BPA interaction term in our statistical models.
Because of the complex differential probabilities of selection to achieve over sampling of selected groups in the NHANES cohort, sample weights were applied according to the National Center for Health Statistics guidelines to generate all estimates. Analyses were performed using SUDAAN (Version 9, Research Triangle Institute, Research Triangle Park, NC) procedures for analyses of complex surveys.
3. Results
3.1. Descriptive statistics
Descriptive statistics of the study sample are provided in Table 1. The mean age of the included participants (N=460) was 11.5 years (Standard Error ±0.1), and the sample was almost evenly divided between males and females based on weighted percentages. BPA was detected in 97.1% of the urine samples, with concentrations ranging from 0.4 μg/L to 149 μg/L. The median concentration for urinary BPA was 3.9 μg/L, and the geometric mean was 3.6 μg/L (Standard Error ±1.09) (See Table 1). Among all participants, 7.1% (95% Confidence Interval [CI]: 4.4–11.3) met DSM-IV criteria for ADHD. For the secondary outcomes, 13.4% (95% CI: 10.5–16.9) participants had caregiver reported ADHD and 17.3% (95% CI: 14.3–20.8) participants had DSM-IV defined ADHD/and or ADHD by caregiver report.
3.2. Association between BPA exposure and ADHD
In bivariate analyses, children with urinary BPA concentrations above the median had a higher prevalence of DSM-defined ADHD compared with children who had BPA concentrations below the median (11.2% vs. 2.9%, p=0.01). A similar pattern was observed using the secondary outcomes of DSM-defined ADHD and/or caregiver reported previous diagnosis of ADHD (25.1% in BPA above median vs. 9.1% in BPA below median, p=0.001) and caregiver reported ADHD alone (20.2% in BPA above median vs. 6.2% in BPA below median, p=0.02).
In multivariable analyses, after adjusting for potential confounders, children with BPA concentrations at or above the median were more than five times as likely to have a DSM-defined diagnosis of ADHD compared to children with BPA levels below the median (adjusted Odds Ratio [aOR] 5.68; 95% CI:1.63–19.83)(See Table 2). When urinary BPA was modeled as a log10 transformed variable, a 10-fold increase in BPA concentrations was associated with a more than two-fold increased odds of DSM-defined ADHD (aOR 2.73; 95% CI: 0.78–9.59), but the relationship was not statistically significant. However, the link between increasing log10-transformed BPA and ADHD was stronger and more precise for the secondary outcomes of 1) having DSM-defined and/or caregiver-reported ADHD (aOR: 3.04; 95% CI: 1.36–6.79), and 2) caregiver-reported ADHD (aOR: 3.72; 95% CI: 1.35–10.20) (Fig. 1).
Table 2.
Adjusteda Odds Ratio for association between Urinary BPA and ADHD.
| Urine BPA concentration | Adjusted Odds Ratio for ADHD (95% CI) |
||
|---|---|---|---|
| DSM-IV defined ADHD | ADHD by DSM-IV criteria and/or caregiver report | Caregiver reported ADHD alone | |
|
| |||
| < median | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) |
| ≥ median | 5.68 (1.63–19.83) | 3.82 (1.19–12.28) | 4.51 (0.83–24.55) |
Abbreviations: ADHD, Attention-Deficit/Hyperactivity Disorder; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; BPA, Bisphenol A.
Adjusted for child's age in years, income, sex, race/ethnicity, urine creatinine, prenatal tobacco exposure, blood lead level (log10-transformed), urine organophosphate metabolite level (log10-transformed), insurance status.
Fig. 1.
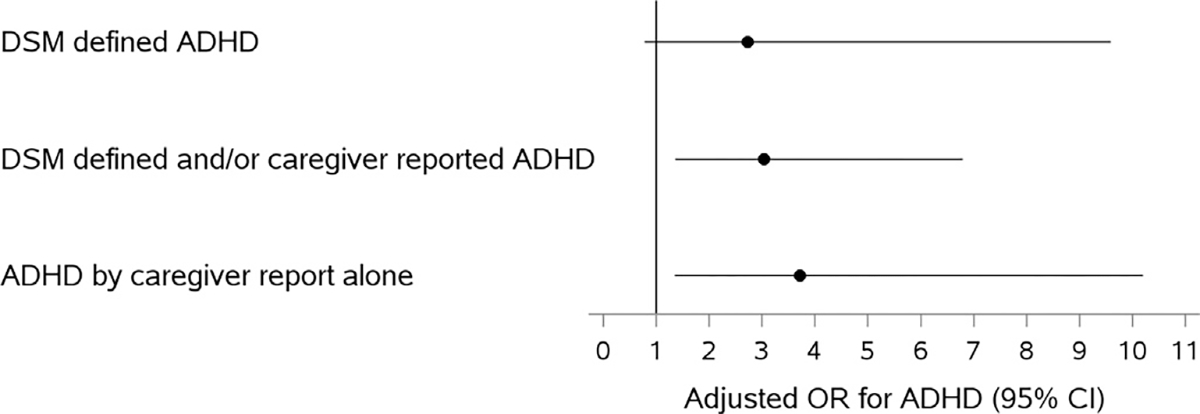
Adjusted1 (1Adjusted for child’s age in years, income, sex, race/ethnicity, urine creatinine, prenatal tobacco exposure, blood lead level (log10-transformed), urine organophosphate metabolite level (log10-transformed), insurance status.) Odds Ratio for association between Log10-transformed BPA and ADHD. Abbreviations: ADHD, Attention-Deficit/Hyperactivity Disorder; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; BPA, Bisphenol A; OR- Odds Ratio; CI, Confidence Interval.
3.3. Associations between BPA concentrations and ADHD symptom counts
In Poisson regression models for the overall sample, urinary BPA concentrations at or above median were associated with a 54% increase in inattentive symptom counts compared to urinary BPA concentrations below median (Adjusted Count Ratio [aCR]: 1.54; 95% CI: 1.01–2.35). The relationship was not statistically significant when BPA concentrations were modeled as a continuous log10 transformed variable. No significant associations were observed between urinary BPA concentrations and total or hyperactive-impulsive symptom counts in the overall sample (Table 3).
Table 3.
Adjusted Count Ratiosa for Urine Bisphenol A Concentrations and ADHD Symptom Counts.
| Total Symptom Count | Inattentive Symptom Count | Hyperactive-Impulsive Symptom Count | |
|---|---|---|---|
|
| |||
| ACR (95% CI) | ACR (95% CI) | ACR (95% CI) | |
|
| |||
| Urine BPA concentration ≥ Median | |||
| Overall | 1.34 (0.81–2.21) | 1.54 (1.01–2.35) | 1.15 (0.59–2.23) |
| Boys | 1.91 (1.01–3.60) | 1.87 (1.10–3.18) | 1.97 (0.78–4.97) |
| Girls | 0.85 (0.39–1.86) | 1.25 (0.59–2.67) | 0.52 (0.24–1.14) |
| Log10 transformed | |||
| Urine BPA | |||
| Overall | 1.22 (0.74–2.00) | 1.20 (0.82–1.76) | 1.25 (0.57–2.74) |
| Boys | 1.38 (0.69–2.76) | 1.21 (0.70–2.11) | 1.67 (0.54–5.20) |
| Girls | 0.71 (0.31–1.64) | 0.80 (0.23–2.80) | 0.63 (0.31–1.30) |
Abbreviations: ACR, Adjusted Count Ratio; ADHD, Attention-Deficit/Hyperactivity Disorder.
Adjusted for child's age, race/ethnicity, income, health insurance status, prenatal tobacco exposure, blood lead level (log10-transformed), urine organophosphate metabolite level (log10-transformed), and urine creatinine level. Overall models also adjust for sex.
3.4. Modification of effects of BPA on ADHD by child sex
We found stronger associations between urinary BPA concentrations and ADHD in boys compared to girls in sex-stratified analyses. In adjusted analyses, boys with urinary BPA concentrations at or above the median were almost eleven times more likely to have DSM-defined ADHD compared to boys with levels below median (aOR: 10.93; 95% CI: 1.39–86.0). In contrast, the odds of having DSM-defined ADHD were not statistically higher in girls (aOR: 2.8; 95% CI: 0.37–21.29) for above vs. below median urinary BPA concentrations. In adjusted sex-stratified analyses for the secondary outcomes of 1) DSM defined and/or caregiver reported ADHD, and 2) caregiver reported prior ADHD, the association between BPA concentrations and ADHD was robust in boys, but attenuated and non-significant in girls (see Supplemental Table S1 and S2).
When urinary BPA was modeled as a continuous log10-transformed variable, boys showed a 3-fold increase in odds of DSM-defined ADHD, although the finding was not statistically significant (aOR: 3.48; 95% CI: 0.6–19.9). However, in sex-stratified analyses for the secondary outcomes of 1) DSM defined and/or caregiver reported ADHD, and 2) caregiver reported prior ADHD, the association between log10-transformed BPA concentrations and ADHD was robust and significant in boys, but attenuated and non-significant in girls (Fig. 2). However, formal tests of BPA by sex interaction, when BPA was modeled as a log10-transformed variable, did not show significant joint BPA-sex effects (p>0.51).
Fig. 2.
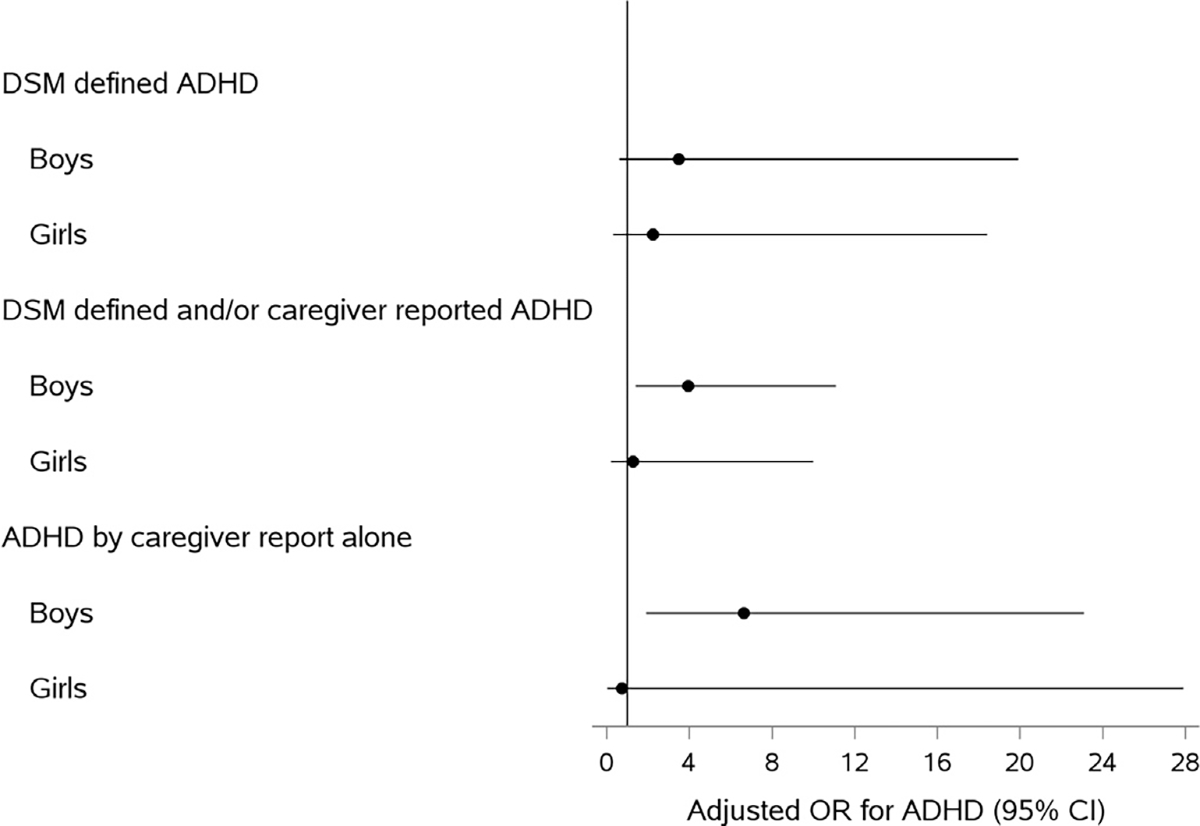
Adjusted1 (1Adjusted for child’s age in years, income, race/ethnicity, urine creatinine, prenatal tobacco exposure, blood lead level (log10-transformed), urine organophosphate metabolite level (log10-transformed), insurance status.) Odds Ratio for Association between Log10-transformed BPA and ADHD by sex. Abbreviations: ADHD, Attention-Deficit/Hyperactivity Disorder; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; BPA, Bisphenol A; OR, Odds Ratio; CI, Confidence Interval.
In Poisson regression models for sex-stratified analyses (Table 3), boys with urinary BPA at or above median had higher total ADHD symptom counts (aCR: 1.91; 95% CI: 1.01–3.60) and elevated inattentive symptom counts (aCR: 1.87; 95% CI: 1.1–3.28) compared to boys with BPA concentrations below median. However, when BPA was modeled as a log10 transformed continuous variable, these associations did not reach statistical significance. No association was observed between BPA concentrations and ADHD symptom counts in girls, whether BPA was modeled as a dichotomous or continuous variable.
4. Discussion
We observed that higher urinary BPA concentrations were associated with increased odds of ADHD in this nationally representative sample of U.S. children aged 8–15 years. This is the first study to evaluate the association between childhood BPA exposure and ADHD that incorporates a DSM-based ADHD measure in a nationally representative sample of U.S. children. Of note, our findings showed a consistent pattern across multiple ADHD outcomes and different analytic techniques, and are consistent with several prior studies reporting associations with childhood BPA exposure and parent- and/or teacher-reported inattention or hyperactivity symptoms in school age children (Findlay and Kohen, 2015; Harley et al., 2013; Hong et al., 2013). Other studies (Braun et al., 2011; Perera et al., 2012; Perez-Lobato et al., 2015; Roen et al., 2015) found no associations between childhood exposure to BPA and ADHD-specific behaviors. These contrasting results may be due to differences in study methodology, including characteristics of the cohort, child age and primary outcome definition. Of note, we found a stronger association between BPA exposure and inattentive compared to hyperactive-impulsive symptoms in our sample. It is plausible that the prior studies in preschool samples (Braun et al., 2011; Perera et al., 2012) did not observe an association between BPA exposure and ADHD-related symptoms because it is difficult to accurately evaluate inattention in younger children (McGee et al., 1992).
Our results suggest sex-specific associations between BPA exposure and ADHD. Higher BPA concentrations were associated with increased odds of ADHD and elevated ADHD symptom counts in boys, but findings were weaker and non-significant in girls. In prior studies, Harley et al. (2013) found that childhood BPA exposure was associated with increased ADHD behaviors in both boys and girls (although higher BPA exposure was linked to more inattention in boys and more externalizing behaviors in girls), and Findlay and Kohen (2015) reported a link between BPA exposure and increased hyperactivity in girls but not boys. However, compared to the present study, Findlay and Kohen used less specific measures for hyperactivity and inattention and their Canadian sample had lower BPA exposures (geometric mean of 1.3 μg/L vs. 3.6 μg/L). Of note, our sample’s mean BPA levels were comparable to those of other U.S.-based cohorts (Braun et al., 2011; Harley et al., 2013; Roen et al., 2015; Teitelbaum et al., 2008). Our findings should be interpreted cautiously because of the smaller samples sizes limiting our statistical power to detect modification of the association between BPA and ADHD by sex. Still, despite our wide confidence intervals, these findings show a trend towards sex-specific effects of BPA, with an increased vulnerability in BPA-exposed boys. Future studies should explore if BPA impacts girls and boys differently at various stages of development, as has been suggested by several researchers (Mustieles et al., 2015; Roen et al., 2015; Rosenfeld, 2015).
Strengths of this study included our use of a nationally representative population-based sample, which increases the generalizability of our findings. Compared to previous studies (Braun et al., 2009, 2011; Harley et al., 2013; Maserejian et al., 2012; Perera et al., 2012; Perez-Lobato et al., 2015; Roen et al., 2015), which evaluated preschoolers and children younger than 11 years of age, our sample consisted of a broader age range of school age children (8–15 years old). As it is difficult to diagnose inattentive type ADHD in children younger than seven years of age (McGee et al., 1992), our study may have better accounted for children with inattentive type ADHD or those who present later in childhood, compared to studies which focused on younger children. In addition, our study employed a DSM based measure to assess for ADHD, whereas only one other prior study has done so (Harley et al., 2013). Finally, our study used a variety of outcomes and analyses, including adjusting for environmental exposure to lead and organophosphate insecticides, to verify a consistent pattern of findings across these models.
Our study is limited in that we used a single spot urine measurement to estimate BPA exposure. BPA has an estimated half-life of approximately 6 h (Center for the Evaluation of Risks to Human Reproduction, 2008). Given that BPA has a short half-life and is eliminated quickly, obtaining serial measurements for accurate estimation of exposure is recommended in future studies. Despite the short half-life, BPA was detected in 97% of our study sample, suggesting widespread continuous low-level exposure. Future studies should aim to determine a threshold for problematic effects of BPA exposure on ADHD-related behaviors, as this study design does not enable us to do so.
Additional study limitations include the cross sectional study design and lack of BPA measurements during the prenatal period or early childhood. For this reason, we are not able to investigate neurobehavioral effects of prenatal exposure to BPA. In addition, we cannot verify a causative relationship between BPA exposure and ADHD. For example, the association between higher BPA levels and ADHD in our sample could be due to reverse causality, as children with ADHD may have different food intake patterns compared to children without ADHD (Blunden et al., 2011; Howard et al., 2011; Park et al., 2012; Woo et al., 2014), and therefore may have increased BPA exposure through their diet. Whether the link between ADHD and BPA exposure precedes or is subsequent to increased dietary BPA intake should be investigated in future studies, although animal studies conducted in carefully controlled environment support the findings of hyperactivity after exposure to BPA in diet (Ishido et al., 2007). Other animal studies reported that postnatal BPA exposure led to ADHD-like behaviors, as well as alterations in dopaminergic systems (Ishido et al., 2004) and dopamine transporter gene expression in the brain (Ishido et al., 2007; Masuo et al., 2004), suggesting biologic mechanisms for development of an ADHD phenotype secondary to BPA exposure.
We adjusted for a number of possible confounders and covariates, but we did not control for genetic influences and other unmeasured factors (e.g., other environmental chemical exposures, prematurity, pubertal development) which may have affected our outcomes. These factors would have confounded our association if they were associated with both ADHD symptoms and urinary BPA concentrations. Outcome misclassification might also have played a role in our analyses, as we assessed for ADHD using the DISC-IV caregiver interview. However, the DISC is well-validated (Bravo et al., 2001; Canino et al., 2004; Shaffer et al., 2000) and our measured prevalence of ADHD (7.1%) is consistent with those of prior U.S. community-based studies (Akinbami et al., 2011; Wolraich et al., 1998) and a recent meta-analysis reporting a pooled prevalence estimate of 7.2% worldwide (Thomas et al., 2015). In addition, children effectively treated with ADHD medications may fall below the threshold for meeting ADHD DSM criteria, and hence would not be captured by our primary outcome. In that case, our secondary outcomes (which included caregiver report of prior ADHD diagnosis) would account for these children.
5. Conclusions
We found evidence that higher urinary BPA concentrations were associated with ADHD in U.S. children; these associations were stronger in boys than girls. Given the robust animal literature documenting adverse effects of BPA exposure on neuroendocrine systems and behaviors, and mounting evidence of adverse associations in children, further study is warranted to determine if reducing exposure to BPA represents an important avenue for ADHD prevention.
Supplementary Material
Acknowledgements
Independent Statistical Analysis: Data analyzed for this investigation was collected by the National Center for Health Statistics. All analyses, interpretations, and conclusions expressed in this manuscript are those of the authors and not the National Center for Health Statistics, which is responsible only for the initial data.
Sources of funding
This study was supported by the National Institutes of Health, United States Grants K23 MH083881 (TF), K24 MH064478 (JE), R00 ES020346 (JB) and R01ES015517–01A1 (KY). Manuscript preparation and submission was supported by the Division of Developmental and Behavioral Pediatrics, Cincinnati Children’s Hospital Medical Center.
Abbreviations:
- ADHD
Attention-Deficit/Hyperactivity Disorder
- ACR
Adjusted Count Ratio
- aOR
Adjusted Odds Ratio
- BPA
Bisphenol A
- CI
Confidence Interval
- DAT
Dopamine Transporter
- DMAP
3-dimethyl alkylphosphate
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition
- DISC
Diagnostic Interview Schedule for Children
- LOD
Limit of Detection
- NHANES
National Health and Nutrition Examination Survey
- PIR
Income to Poverty line Ratio
Footnotes
Financial disclosures
The authors have no financial conflicts of interest (relationship with or receipt of funds from any organization that may gain or lose financially from the publication of this manuscript) to disclose.
Non-financial disclosures
Dr. Lanphear served as an expert witness in California for the plaintiffs in a public nuisance case of childhood lead poisoning, a Proposition 65 case on behalf of the California Attorney General’s Office, a case involving lead-contaminated water in a new housing development in Maryland, and Canadian tribunal on trade dispute about using lead-free galvanized wire in stucco lathing but he received no personal compensation for these services. He is currently representing the government of Peru as an expert witness in a suit involving Doe Run vs Peru, but he is receiving no personal compensation for these cases. Dr. Lanphear has served as a paid consultant on a US Environmental Protection Agency research study, NIH research awards and the California Department of Toxic Substance Control. Dr. Lanphear has received federal research awards from the National Institute of Environmental Health, the US Environmental Protection Agency, the Centers for Disease Control and the US Department of Housing and Urban Development. He is also the recipient of federal research awards from the Canada Institutes of Health Research and Health Canada.
Dr. Braun was financially compensated for conducting a reanalysis of a study of child lead exposure for the plaintiffs in a public nuisance case related to childhood lead poisoning. These activities are not directly related to the present study. The other authors have no potential non-financial conflicts of interest.
Institutional review board
This study was reviewed by the Institutional Review Board (IRB) of Cincinnati Children’s Hospital Medical Center and was determined to be exempt from regulatory criteria for research involving human subjects (Study # 2011–1686).
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.envres.2016.05.040.
References
- Adriani W, et al. , 2003. Altered profiles of spontaneous novelty seeking, impulsive behavior, and response to D-amphetamine in rats perinatally exposed to bisphenol A. Environ. Health Perspect. 111, 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinbami LJ, et al. , 2011. Attention deficit hyperactivity disorder among children aged 5–17 years in the United States, 1998–2009. NCHS Data Brief. National Center for Health Statistics, Hyattsville, MD. [PubMed] [Google Scholar]
- Alyea RA, Watson CS, 2009. Differential regulation of dopamine transporter function and location by low concentrations of environmental estrogens and 17beta-estradiol. Env. Health Perspect. 117, 778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, et al. , 2004. Concentrations of dialkyl phosphate metabolites of organophosphorus pesticides in the U.S. population. Env. Health Perspect. 112, 186–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, et al. , 2000. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am. J. psychiatry 157, 816–818. [DOI] [PubMed] [Google Scholar]
- Blunden SL, et al. , 2011. Diet and sleep in children with attention deficit hyperactivity disorder: preliminary data in Australian children. J. Child. Health Care 15, 14–24. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, et al. , 2010. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics 125, e1270–e1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, et al. , 2006. Exposures to environmental toxicants and attention deficit hyperactivity disorder in U.S. children. Env. Health Perspect. 114, 1904–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, et al. , 2011. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics 128, 873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, et al. , 2009. Prenatal bisphenol A exposure and early childhood behavior. Env. Health Perspect. 117, 1945–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo M, et al. , 2001. Test-retest reliability of the Spanish version of the diagnostic interview schedule for children (DISC-IV). J. Abnorm. Child Psychol. 29, 433–444. [DOI] [PubMed] [Google Scholar]
- Calafat AM, et al. , 2008. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Env. Health Perspect. 116, 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canino G, et al. , 2004. The DSM-IV rates of child and adolescent disorders in Puerto Rico: prevalence, correlates, service use, and the effects of impairment. Arch. General Psychiatry 61, 85–93. [DOI] [PubMed] [Google Scholar]
- Casas M, et al. , 2015. Exposure to bisphenol A during pregnancy and child neuropsychological development in the INMA-Sabadell cohort. Env. Res. 142, 671–679. [DOI] [PubMed] [Google Scholar]
- Center for the Evaluation of Risks to Human Reproduction, 2008. NTP_CERHR Expert Panel Report on the Reproductive and Developmental Toxicity of Bisphenol A. In: U. S. D. o. H. a. H. S. National Toxicological Program, (Ed.), Research Triangle Park, NC. [Google Scholar]
- Centers for Disease Control and Prevention, 2014. About the National Health and Nutrition Examination Survey. Vol. 2014. [Google Scholar]
- Evans SF, et al. , 2014. Prenatal Bisphenol A Exposure and maternally reported behavior in boys and girls. Neurotoxicology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay LC, Kohen DE, 2015. Bisphenol A and child and youth behaviour: Canadian health measures survey 2007 to 2011. Health Reports. 26, pp. 3–9. [PubMed] [Google Scholar]
- Froehlich TE, et al. , 2009. Association of tobacco and lead exposures with attention-deficit/hyperactivity disorder. Pediatrics 124, e1054–e1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich TE, et al. , 2007. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch. Pediatr. Adolesc. Med. 161, 857–864. [DOI] [PubMed] [Google Scholar]
- Gioiosa L, et al. , 2007. Developmental exposure to low-dose estrogenic endocrine disruptors alters sex differences in exploration and emotional responses in mice. Horm. Behav. 52, 307–316. [DOI] [PubMed] [Google Scholar]
- Harley KG, et al. , 2013. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environ. Res. 126, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SB, et al. , 2013. Bisphenol A in relation to behavior and learning of school-age children. J. Child Psychol. Psychiatry 54, 890–899. [DOI] [PubMed] [Google Scholar]
- Honma T, et al. , 2006. Effects of perinatal exposure to bisphenol A on brain neurotransmitters in female rat offspring. Ind. Health 44, 510–524. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD, 1990. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 5, 46–51. [Google Scholar]
- Howard AL, et al. , 2011. ADHD is associated with a “Western” dietary pattern in adolescents. J. Atten. Disord. 15, 403–411. [DOI] [PubMed] [Google Scholar]
- Ishido M, et al. , 2004. Bisphenol A causes hyperactivity in the rat concomitantly with impairment of tyrosine hydroxylase immunoreactivity. J. Neurosci. Res. 76, 423–433. [DOI] [PubMed] [Google Scholar]
- Ishido M, et al. , 2011. Rat hyperactivity by bisphenol A, but not by its derivatives, 3-hydroxybisphenol A or bisphenol A 3,4-quinone. Toxicol. Lett. 206, 300–305. [DOI] [PubMed] [Google Scholar]
- Ishido M, et al. , 2005a. Alteration of gene expression of G protein-coupled receptors in endocrine disruptors-caused hyperactive rats. Regul. Pept. 126, 145–153. [DOI] [PubMed] [Google Scholar]
- Ishido M, et al. , 2005b. Alteration of gene expression of G protein-coupled receptors in endocrine disruptors-caused hyperactive rats. Regul. Pept. 126, 145–153. [DOI] [PubMed] [Google Scholar]
- Ishido M, et al. , 2007. Mesencephalic neurodegeneration in the orally administered bisphenol A-caused hyperactive rats. Toxicol. Lett. 173, 66–72. [DOI] [PubMed] [Google Scholar]
- Jones DC, Miller GW, 2008. The effects of environmental neurotoxicants on the dopaminergic system: a possible role in drug addiction. Biochem. Pharmacol. 76, 569–581. [DOI] [PubMed] [Google Scholar]
- Kang JH, et al. , 2006. Human exposure to bisphenol A. Toxicology 226, 79–89. [DOI] [PubMed] [Google Scholar]
- Levy F, 1991. The dopamine theory of attention deficit hyperactivity disorder (ADHD). Aust. N. Z. J. Psychiatry 25, 277–283. [DOI] [PubMed] [Google Scholar]
- Levy F, Swanson JM, 2001. Timing, space and ADHD: the dopamine theory revisited. Aust N. Z. J. Psychiatry 35, 504–511. [DOI] [PubMed] [Google Scholar]
- Lingineni RK, et al. , 2012. Factors associated with attention deficit/hyperactivity disorder among US children: results from a national survey. BMC Pediatr. 12, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maserejian NN, et al. , 2012. Dental composite restorations and psychosocial function in children. Pediatrics 130, e328–e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuo Y, et al. , 2004. Motor hyperactivity caused by a deficit in dopaminergic neurons and the effects of endocrine disruptors: a study inspired by the physiological roles of PACAP in the brain. Regul. Pept. 123, 225–234. [DOI] [PubMed] [Google Scholar]
- McGee R, et al. , 1992. Attention deficit disorder and age of onset of problem behaviors. J. Abnorm. Child Psychol. 20, 487–502. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, et al. , 2010. Prevalence and treatment of mental disorders among US children in the 2001–2004 NHANES. Pediatrics 125, 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miodovnik A, et al. , 2011. Endocrine disruptors and childhood social impairment. Neurotoxicology 32, 261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, Kotake M, 2006. Estimation of daily bisphenol a intake of Japanese individuals with emphasis on uncertainty and variability. Env. Sci. 13, 15–29. [PubMed] [Google Scholar]
- Mizuo K, et al. , 2004a. Functional changes in dopamine D3 receptors by prenatal and neonatal exposure to an endocrine disruptor bisphenol-A in mice. Addict. Biol. 9, 19–25. [DOI] [PubMed] [Google Scholar]
- Mizuo K, et al. , 2004b. Functional changes in dopamine D3 receptors by prenatal and neonatal exposure to an endocrine disruptor bisphenol-A in mice. Addict. Biol. 9, 19–25. [DOI] [PubMed] [Google Scholar]
- Mustieles V, et al. , 2015. Bisphenol A: human exposure and neurobehavior. Neurotoxicology 49, 174–184. [DOI] [PubMed] [Google Scholar]
- National Health and Nutrition Examination Survey, 2006. 2003–2004 data documentation, codebook, and frequencies. Medical Conditions (MCQ_C). Question MCQ 060., vol. 2014. US Department of Health and Human Services, Centers for Disease Control and Prevention, Hyattsville, MD. [Google Scholar]
- Nigg JT, 2006. What causes ADHD?: understanding what goes wrong and why. Guilford Press, New York, NY. [Google Scholar]
- Nojima K, et al. , 2013. Prolonged exposure to a low-dose of bisphenol A increases spontaneous motor activity in adult male rats. J. Physiol. Sciences: JPS 63, 311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oord S, et al. , 2012. The adolescent outcome of children with attention deficit hyperactivity disorder treated with methylphenidate or methylphenidate combined with multimodal behaviour therapy: results of a naturalistic follow-up study. Clin. Psychol. Psychother. 19, 270–278. [DOI] [PubMed] [Google Scholar]
- Park S, et al. , 2012. Association between dietary behaviors and attention-deficit/hyperactivity disorder and learning disabilities in school-aged children. Psychiatry Res. 198, 468–476. [DOI] [PubMed] [Google Scholar]
- Pastor PN, Reuben CA, 2008. Diagnosed attention deficit hyperactivity disorder and learning disability: United States, 2004–2006. Vital Health Stat. 10, 1–14. [PubMed] [Google Scholar]
- Patisaul HB, Polston EK, 2008. Influence of endocrine active compounds on the developing rodent brain. Brain Res. Rev. 57, 352–362. [DOI] [PubMed] [Google Scholar]
- Perera F, et al. , 2012. Prenatal bisphenol a exposure and child behavior in an innercity cohort. Env. Health Perspect. 120, 1190–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Lobato R, et al. , 2015. Exposure to bisphenol A and behavior in school-age children. Neurotoxicology 53, 12–19. [DOI] [PubMed] [Google Scholar]
- Roen EL, et al. , 2015. Bisphenol A exposure and behavioral problems among inner city children at 7–9 years of age. Env. Res. 142, 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS, 2015. Bisphenol A and phthalate endocrine disruption of parental and social behaviors. Front. Neurosci. 9, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, et al. , 2000. NIMH diagnostic interview schedule for children version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J. Am. Acad. Child. Adolesc. Psychiatry 39, 28–38. [DOI] [PubMed] [Google Scholar]
- Swanson J, et al. , 2007. Etiologic subtypes of attention-deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychol. Rev. 17, 39–59. [DOI] [PubMed] [Google Scholar]
- Tando S, et al. , 2007. Effects of pre- and neonatal exposure to bisphenol A on murine brain development. Brain Dev. 29, 352–356. [DOI] [PubMed] [Google Scholar]
- Teitelbaum S, et al. , 2008. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ. Res. 106, 257–269. [DOI] [PubMed] [Google Scholar]
- Thomas R, et al. , 2015. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics 135, e994–1001. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, et al. , 2010. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Env. Health Perspect. 118, 1055–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, et al. , 2007. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 24, 139–177. [DOI] [PubMed] [Google Scholar]
- Wilson NK, et al. , 2007. An observational study of the potential exposures of preschool children to pentachlorophenol, bisphenol-A, and nonylphenol at home and daycare. Environ. Res. 103, 9–20. [DOI] [PubMed] [Google Scholar]
- Wolraich ML, et al. , 1998. Examination of DSM-IV criteria for attention deficit/hyperactivity disorder in a county-wide sample. J. Dev. Behav. Pediatr. 19, 162–168. [DOI] [PubMed] [Google Scholar]
- Woo HD, et al. , 2014. Dietary patterns in children with attention deficit/hyperactivity disorder (ADHD). Nutrients 6, 1539–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, et al. , 2005. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal. Chem. 77, 5407–5413. [DOI] [PubMed] [Google Scholar]
- Yolton K, et al. , 2011. Prenatal exposure to bisphenol A and phthalates and infant neurobehavior. Neurotoxicol. Teratol. 33, 558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, et al. , 2011. Abnormal synaptic plasticity in basolateral amygdala may account for hyperactivity and attention-deficit in male rat exposed perinatally to low-dose bisphenol-A. Neuropharmacology 60, 789–798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


