Abstract
The present study reports the activity of BILD 1633 SE against acyclovir (ACV)-resistant herpes simplex virus (HSV) infections in athymic nude (nu/nu) mice. BILD 1633 SE is a novel peptidomimetic inhibitor of HSV ribonucleotide reductase (RR). In vitro, it is more potent than ACV against several strains of wild-type as well as ACV-resistant HSV mutants. Its in vivo activity was tested against cutaneous viral infections in athymic nude mice infected with the ACV-resistant isolates HSV type 1 (HSV-1) dlsptk and PAAr5, which contain mutations in the viral thymidine kinase gene and the polymerase gene, respectively. Following cutaneous infection of athymic nude mice, both HSV-1 dlsptk and PAAr5 induced significant, reproducible, and persistent cutaneous lesions that lasted for more than 2 weeks. A 10-day treatment regimen with ACV given topically four times a day as a 5% cream or orally at up to 5 mg/ml in drinking water was partially effective against HSV-1 PAAr5 infection with a reduction of the area under the concentration-time curve (AUC) of 34 to 48%. The effects of ACV against HSV-1 dlsptk infection were not significant when it was administered topically and were only marginal when it was given in drinking water. Treatment under identical conditions with 5% topical BILD 1633 SE significantly reduced the cutaneous lesions caused by both HSV-1 dlsptk and PAAr5 infections. The effect of BILD 1633 SE against HSV-1 PAAr5 infections was more prominent and was inoculum and dose dependent, with AUC reductions of 96 and 67% against infections with 106 and 107 PFU per inoculation site, respectively. BILD 1633 SE also significantly decreased the lesions caused by HSV-1 dlsptk infection (28 to 51% AUC reduction). Combination therapy with topical BILD 1633 SE (5%) and ACV in drinking water (5 mg/ml) produced an antiviral effect against HSV-1 dlsptk and PAAr5 infections that was more than the sum of the effects of both drugs. This is the first report that a selective HSV RR subunit association inhibitor can be effective against ACV-resistant HSV infections in vivo.
Herpes simplex virus (HSV) encodes a ribonucleotide reductase (RR) that catalyzes the synthesis of the deoxyribonucleotide precursors required for DNA synthesis. This enzyme shares many similarities with the host cellular RR but differs markedly from the latter in terms of primary amino acid sequences and allosteric regulation (24, 26, 35). Both RRs consist of two subunits (26, 35). The larger subunit contains redox-active thiols that provide the hydrogen for nucleotide reduction (24, 29, 31, 35). The smaller subunit contains a binuclear μ-oxo bridged iron center associated with a tyrosyl free radical (1, 17, 26, 32, 35). The carboxy terminus of the RR small subunit is critical for subunit association and for the catalytic activity of the enzyme (3, 13, 26). Although HSV RR is not required for virus replication in exponentially growing cells, it is necessary in nondividing cells (18) and is required for the full expression of pathogenicity of HSV in animal models of primary infection (3, 20, 21, 42) and for reactivation from latency (20, 21). Therefore, HSV RR represents an interesting target for antiherpesvirus therapy (3, 13, 21, 26). It has been reported that nonselective RR inhibitors, such as 2′-fluoromethylene-2′-deoxycytidine and A1110U, potentiate the activity of acyclovir (ACV) both in vitro and in vivo against wild-type and ACV-resistant HSV infections (6, 14, 28, 39). However, these RR inhibitors have not been shown to be useful as monotherapy, and therefore, the combination therapy has not been clearly validated due in part to the lack of specificity and the toxicity of these agents (14, 26, 28, 39). Our group has developed a class of selective HSV RR subunit association inhibitors that act as mimics of the carboxy terminus of the small subunit (4, 27, 30). We have recently reported the in vivo activity of one of the earliest lead compounds, BILD 1263, as a monotherapy against HSV type 1 (HSV-1)-induced ocular disease in mice (4, 27). In the present study, we examined the effects of BILD 1633 SE, a more potent HSV RR inhibitor, against cutaneous ACV-resistant HSV-1 infections in the athymic nude mouse model when BILD 1633 SE was used either alone or in combination with ACV. The athymic nude mouse was chosen because ACV-resistant HSV-1 fails to induce significant disease in normal mice (3, 14). A lack of functional T cells renders athymic nude mice more susceptible to cutaneous infections by ACV-resistant HSV-1 and more suitable for drug evaluation (14, 15). In addition, since ACV-resistant HSV infections cause significant disease mainly in immunocompromised patients (7, 8, 23), information obtained from these immunodeficient animals may be clinically relevant (14, 15). Our results indicate that BILD 1633 SE is effective in the treatment of ACV-resistant HSV infections in this model when it is used either alone or in combination with ACV. Therefore, selective inhibition of HSV RR by peptidomimetic subunit association inhibitors may offer a potential alternative topical therapy for the treatment of ACV-resistant HSV infections in humans.
MATERIALS AND METHODS
Viruses.
Four different laboratory strains of HSV-1 were used in the present study. These include two wild-type strains, HSV-1 F and KOS, and two ACV-resistant strains, dlsptk (11) and PAAr5 (9, 16). Both wild-type HSV-1 strains have been described elsewhere (27). The ACV-resistant, thymidine kinase (TK)-deficient HSV-1 strain dlsptk and the DNA polymerase mutant PAAr5 were kindly provided by D. Coen (9, 11). Virus stocks were routinely grown in Vero (African green monkey kidney) cells, and virus titers were determined by a standard plaque assay with confluent Vero cells.
Compounds.
The preparation of BILD 1633 SE was based on our previously published procedure (30). The [α-(R)-methyl-3-cyclohexyl]-propionyl functionality of the N-terminal part of BILD 1633 SE is introduced in the last stage of the synthesis by condensation of the corresponding acid chloride.
In vitro antiviral assay.
Antiviral assays were performed essentially as described previously (27). Baby hamster kidney (BHK)-21/C13 (ATCC CCL 10) cells were seeded in 96-well culture plates at a density of 5,000 cells per well in α-minimal essential medium containing 8% (vol/vol) fetal bovine serum (Gibco), and the plates were incubated at 37°C with 5% CO2. After 6 h, the concentration of fetal bovine serum was reduced to 0.5% (vol/vol) and the cells were serum starved for 3 days. Serum-starved cell monolayers were infected with HSV-1 F, KOS, PAAr5, and dlsptk at a multiplicity of infection of 0.05 in defined medium (5). The defined medium (5) consisted of F-12 medium, Dulbecco’s modified Eagle medium, and BGjb medium (6:3:1; vol/vol) with 2 g of bovine serum albumin per liter, 2.38 g of HEPES per liter, 50 mg of garamycin per liter, 100 μg of cortisol per liter, 1 μg of insulin per liter, 0.4 μg of triiodothyromine per liter, 0.2 μg of parathyroid hormone per liter, 10 μg of glucagon per liter, 0.1 μg of epidermal growth factor per liter, 0.2 μg of fibroblast growth factor per liter, and 10 mg of transferrin per liter. After 1 h, the HSV-infected cells were rinsed with defined medium and were then incubated with test compounds for 24 h for strains F and KOS and 44 h for strains PAAr5 and dlsptk. The extent of replication was assessed by a novel enzyme-linked immunosorbent assay (ELISA). The cells were fixed with 0.063% glutaraldehyde in phosphate-buffered saline (PBS) for 30 min and were blocked with 0.5% casein in PBS for 1 h. Thereafter, mouse monoclonal antibody C11 that recognizes the HSV-1 late glycoprotein C (41) was added to each well for 2 h. After the cells were washed three times with PBS containing 0.05% Tween 20, the bound monoclonal antibody was detected with sheep anti-mouse immunoglobulin G horseradish peroxidase for 1 h in the dark. The plates were washed three times with PBS and once with 0.1 M sodium citrate (pH 4.5). ortho-Phenylenediamine dihydrochloride (Gibco) was used as a substrate for 30 min in the dark, and color development in individual wells was monitored at 450 nm with a Titertek microplate spectrophotometer. For inhibition studies, compounds were tested in threefold serial dilutions. Stock solutions of compounds were prepared by dissolving each compound in dimethyl sulfoxide (DMSO) and diluting the solution with defined medium to a 1% DMSO final concentration. Stock solutions were routinely sonicated for 20 min in ice-cold water and were filtered through 0.22-μm-pore-size Millex (Millipore) filters. The 50% effective concentrations (EC50s) were determined from plots of inhibition of virus replication as a function of compound concentration. The cytotoxicity of BILD 1633 SE for serum-starved cells was determined by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (12).
In vitro drug combination studies.
The antiviral activity of BILD 1633 SE in combination with ACV against wild-type and ACV-resistant HSV-1 was assessed by the 96-well ELISA described above. Evaluation of drug interactions was performed by the isobole method (40). In this method, selected concentrations of BILD 1633 SE were tested in combination with various doses of ACV, and average EC50s of ACV were determined from duplicate dose-response curves. These values were used to calculate FEC50 (ACV), which represents the ratio of the concentration of ACV required to inhibit HSV-1 replication by 50% in the presence of a fixed concentration of BILD 1633 SE to the concentration of ACV required to inhibit HSV-1 replication by 50% in the absence of BILD 1633 SE. The isobologram representation is obtained by plotting FEC50 (ACV) as a function of the ratio of the fixed concentration of BILD 1633 SE to the EC50 of BILD 1633 SE in the absence of ACV. In this representation when experimental datum points for the drugs used in combination fall on the hypotenuse, the effects of the two drugs are additive. If experimental datum points fall below the theoretical line for noninteracting drugs, the effects of the two drugs are considered synergistic.
Animals.
Athymic nude mice (female nu/nu CD1 mice from Charles River Canada, St. Constant, Quebec, Canada) at 5 to 6 weeks of age were used for all experiments. Animals were housed in microisolator cages inside semirigid isolators with sterile food, water, and bedding. All manipulations were carried out within class II-type safety cabinets (Nuaire, Plymouth, Minn.), according to the protocols approved by the Canadian Council on Animal Care (Ottawa, Ontario, Canada).
Cutaneous inoculation.
Animals were inoculated with ACV-resistant HSV-1 dlsptk or PAAr5 mutants under halothane anesthesia by scarification and rubbing 10 μl of the desired virus stock on an area of about 1 cm2 on each side of the dorsal skin for 10 s.
Treatments and drug evaluation.
Both ACV and BILD 1633 SE were topically administered in a cream vehicle containing 16% DMSO, 5% linoleic acid, 16% Cremophor EL, and 63% polyvinyl alcohol (25% solution in 100 mM HEPES buffer [pH 8.5]). All topical treatments were started at 3 h postinoculation and continued for 10 days (four times per day between 8:30 a.m. and 5:30 p.m.). In some experiments, ACV was administered orally in drinking water immediately following inoculation. Topical lesions and systemic diseases were observed daily. The following criteria were applied to score topical lesions: 0, no lesions; 1, discrete vesicles; 2, two or more open lesions; 3, separate ulcerations; and 4, zoster band formations. The onset and time course of topical lesions are dependent on the titer of the inoculum. Under current experimental conditions, topical lesions became visible within 2 to 3 days and peaked within about 10 to 13 days; this was dependent on the titer of the inoculum. Although some degree of spontaneous regression occurred in animals infected with PAAr5 mutants, topical lesions persisted over the whole duration of experiment. Only a few (fewer than 10%) of the PAAr5-infected mice developed systemic disease (disseminated infections, neurological and physical abnormalities, and mortality) following the appearance of severe topical lesions. Therefore, systemic disease was not used for drug evaluation. Topical lesion data are presented in terms of the mean and the standard error of the mean (SEM). Daily lesion scores and the areas under the curve (AUC) of the lesion scores are compared for statistical significance by the analysis of variance followed by Student-Newman-Keuls multiple comparisons with SAS software (SAS Institute, Cary, N.C.). A P value of <0.05 was considered statistically significant.
RESULTS
Comparative in vitro activities of BILD 1633 SE and ACV against several strains of HSV-1.
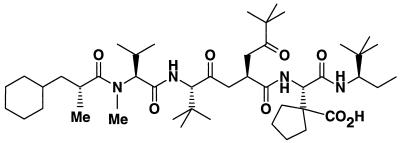
Figure 1 shows the structure of the HSV RR subunit association inhibitor BILD 1633 SE. This compound inhibits HSV RR with a 50% inhibitory concentration of 3 nM, as determined by a competitive binding assay (24). Like previously published inhibitors in this class, it does not affect the activity of the human RR at a concentration up to 250 μM, on the basis of the enzyme assay. Therefore, this compound represents a highly selective HSV RR inhibitor. As indicated in Table 1, BILD 1633 SE is about 10 times more potent than ACV in inhibiting the replication of the wild-type strains HSV-1 F and KOS (EC50 = 0.4 μM) and is about 100 times more potent then ACV against both ACV-resistant strains. In addition, this compound is about three times more active against the ACV-resistant mutant PAAr5 than against both wild-type strains and the dlsptk HSV-1 strains. The 50 percent cytotoxic concentration of BILD 1633 SE is 14 μM, as measured by the in vitro MTT assay, yielding a selectivity index of 35. Compared with the potency of BILD 1263 published previously (3, 27), BILD 1633 SE is about 5 to 10 times more potent in vitro against all the viruses studied (Table 1) and in vivo against HSV-1 infection in the mouse ocular model (unpublished data).
FIG. 1.
Molecular structure of BILD 1633 SE. Me, methyl.
TABLE 1.
Comparative in vitro antiviral activities of BILD 1633 SE and ACV against several strains of HSV-1
| HSV-1 strain | EC50 (μM)
|
|
|---|---|---|
| BILD 1633 SE | ACV | |
| F | 0.35 ± 0.10 | 2.7 ± 1.0 |
| KOS | 0.43 ± 0.06 | 5.2 ± 1.0 |
| dlsptk | 0.46 ± 0.09 | 60.2 ± 12.9 |
| PAAr5 | 0.14 ± 0.02 | 17.2 ± 2.9 |
Values represent the means ± standard deviations from at least 10 determinations.
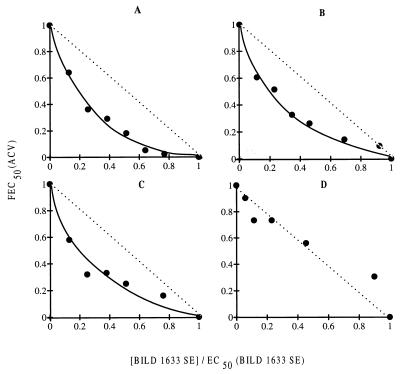
When BILD 1633 SE was tested in vitro in combination with ACV, the antiviral activity of ACV against HSV-1 F, KOS, and dlsptk was potentiated without producing any toxicity to the confluent BHK cells. The synergistic effect of BILD 1633 SE is clearly evident in Fig. 2A, B, and C, which shows the isobologram representation derived from an ELISA-based antiviral assay. At a concentration of BILD 1633 SE that produced 30% inhibition of viral replication, the EC50 of ACV for each virus decreased by about 10- to 20-fold. No synergy of ACV with BILD 1633 SE was observed against ACV-resistant virus strain PAAr5. As illustrated by the isobologram representation (Fig. 2D), both drugs acted in an additive manner to inhibit the replication of this ACV-resistant strain of HSV.
FIG. 2.
Effect of ACV in combination with BILD 1633 SE against wild-type and ACV-resistant HSV in vitro. Selected concentrations of BILD 1633 SE were tested in combination with various doses of ACV, and the EC50s for HSV KOS, F, PAAr5, and dlsptk were determined by the ELISA-based antiviral assay as described in Materials and Methods. These values were used to calculate FEC50 (ACV), and FEC50 (ACV) was plotted as a function of the ratio of the fixed concentration of BILD 1633 SE to the EC50 of BILD 1633 SE for the respective viruses to generate the isobologram. (A) HSV-1 F; (B) HSV-1 KOS; (C) HSV-1 dlsptk; (D) HSV-1 PAAr5.
Monotherapy with BILD 1633 SE or ACV against HSV-1 PAAr5 infection.
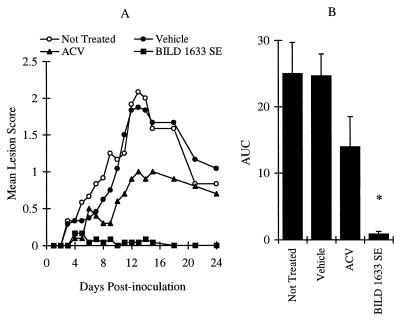
As shown in Fig. 3, inoculation of mice with 106 PFU of HSV-1 PAAr5 per site induced moderate lesions, with a maximum lesion score of 2.1 ± 0.4 on day 13 postinoculation (Fig. 3A). Vehicle treatment did not affect the maximum lesion score or the AUC value (Fig. 3A and B). Topical treatment with 5% ACV reduced the maximum lesion score (P < 0.05), but the effect on the AUC value did not reach statistical significance (P > 0.05). In contrast, treatment with 5% BILD 1633 SE almost completely abolished topical lesions (Fig. 3A and B).
FIG. 3.
Comparative effects of ACV and BILD 1633 SE against HSV-1 PAAr5 infection. Animals were cutaneously inoculated with 106 PFU/site, as described in Materials and Methods. ACV (5%) and BILD 1633 SE (5%) were applied topically four times per day. (A) Mean lesion scores. The mean lesion score was significantly reduced by ACV treatment on days 12-14 (n = 10; P < 0.05) and was further reduced by BILD 1633 SE on days 10 to 24 (n = 24; the result was significantly different [P < 0.05] from those for all other groups). (B) AUCs of the lesion scores. The AUCs of lesion scores are presented as means ± SEMs. ∗, P < 0.05 compared with the results for all other groups.
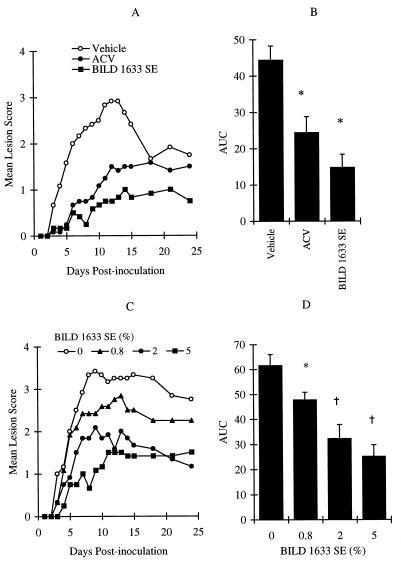
Increasing the virus inoculum to 107 PFU per inoculation site induced more prominent topical lesions that reached a maximum lesion score of 2.9 ± 0.3 on day 13 postinoculation (Fig. 4A and B). Treatment with 5% topical ACV for 10 days reduced both the maximum lesion score to 1.4 ± 0.3 and the AUC value by 45% (P < 0.05). Topical treatment of infected mice with 5% BILD 1633 SE reduced the maximum lesion score to 1 ± 0.3 and the AUC value by 66% (P < 0.05). This in vivo antiviral effect of BILD 1633 SE was highly reproducible, as verified by three additional independent experiments that showed reductions of the AUC values of the lesion scores of 60, 81, and 61%, respectively (n = 12 for both the vehicle- and the drug-treated groups; P was <0.05 for all experiments). The dose-dependent effect of topical BILD 1633 SE against HSV-1 PAAr5-induced topical lesions in athymic mice is shown in Fig. 4C and D.
FIG. 4.
Effects of BILD 1633 SE and ACV against HSV-1 PAAr5 infection. Animals were cutaneously inoculated with 107 PFU/site, as described in Materials and Methods. (A and B) BILD 1633 SE and ACV were applied in 5% topical formulation. (C and D) BILD 1633 SE was applied four times a day at concentrations of 0, 0.8, 2, and 5%. The AUCs of the lesion scores are presented as means ± SEMs (n = 12). ∗, P < 0.05 compared with the results for the vehicle group; †, P < 0.05 compared with the results for the vehicle and 0.8% BILD 1633 SE groups.
Combination therapy with oral ACV and topical BILD 1633 SE against HSV-1 PAAr5 infection.
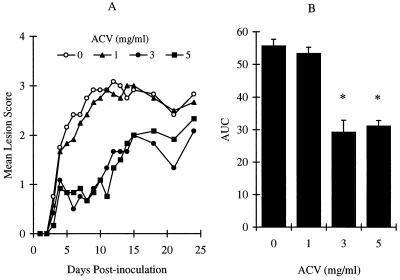
Since concomitant administration of two compounds by the same route may lead to chemical and/or physical interactions of the compounds, we administered ACV and BILD 1633 SE by two different routes. Figure 5 shows the effect of oral ACV supplied continuously in drinking water. When ACV was administered for 10 days in drinking water at a concentration of 1 mg/ml, no protection from HSV disease was seen (Fig. 5A and B). However, maximum protection was achieved with a concentration of 3 mg/ml (daily dose, 871 ± 49 mg/kg of body weight), resulting in a reduction of the AUC of the lesion score by 48%. This protection was similar to that achieved with topical ACV treatment, as described above. Increasing the ACV concentration to 5 mg/ml in drinking water (daily dose, 1,391 ± 29 mg/kg) did not improve the observed protection, perhaps because the maximum efficacy has been achieved with the dose of 3 mg/ml under current experimental conditions. At all doses tested, ACV did not change the behaviors or the body weights of the treated mice.
FIG. 5.
Antiviral effects of ACV in drinking water against HSV-1 PAAr5 infection. Animals were cutaneously inoculated with 107 PFU/site, as described in Materials and Methods. ACV was given in the drinking water at 0, 1, 3, and 5 mg/ml. (A) Mean lesion scores. (B) AUC of the lesion scores. The AUCs of the lesion scores are presented as means ± SEMs (n = 12). ∗, P < 0.05 compared with the results for the vehicle group.
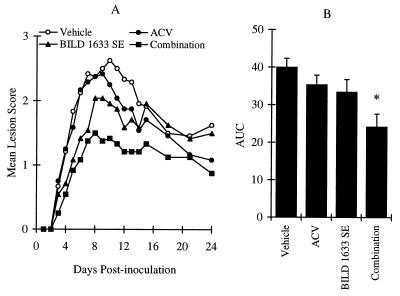
To determine the effectiveness of the combination therapy in the treatment of HSV-1 PAAr5-induced topical lesions, ACV was administered orally in drinking water at a concentration of 1.5 mg/ml in combination with 0.8% BILD 1633 SE in a topical cream. As illustrated in Fig. 6, treatment with ACV alone did not significantly affect the infection, while treatment with BILD 1633 SE alone reduced the mean disease score significantly for 7 days starting on day 4. Combination therapy was more effective than therapy with each agent alone. As shown in Fig. 6, the AUC values and the mean disease score for the combination of ACV and BILD 1633 SE were reduced significantly on days 8 to 11 compared to those for either treatment alone.
FIG. 6.
Antiviral effects of oral ACV and topical BILD 1633 SE against HSV-1 PAAr5 infection. Animals were cutaneously inoculated with 107 PFU/site, as described in Materials and Methods. ACV was given in the drinking water at a dose of 1.5 mg/ml. BILD 1633 SE (0.8%) was applied topically four times per day. (A) Mean lesion scores. (B) AUC of the lesion scores. The AUCs of the lesion scores are presented as means ± SEMs (n = 24). ∗, P < 0.05 compared with the results for the vehicle groups.
Monotherapy and combination therapy with ACV and BILD 1633 SE against HSV-1 dlsptk infection.
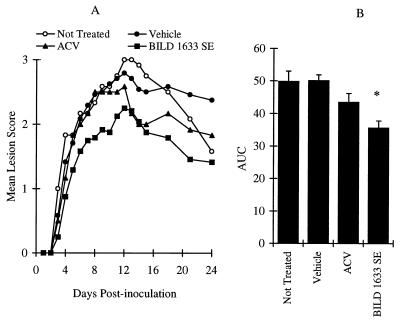
Reproducible and persistent topical lesions were induced by inoculating athymic nude mice with 107 PFU of HSV-1 dlsptk per inoculation site (Fig. 7). Infected animals that did not receive any treatment had a mean maximum lesion score of 3 ± 0.1 on day 12 postinoculation, with a mean AUC over 24 days of 50 ± 3 (n = 12). Vehicle-treated animals had a maximum lesion score of 2.8 ± 0.1, with an AUC of 50 ± 2 (n = 24; P > 0.05). Treatment with 5% topical ACV did not significantly affect the maximum lesion score or the AUC value (Fig. 7). However, topical treatment with 5% BILD 1633 SE for 10 days resulted in statistically significant reductions in the maximum disease score and the AUC value (Fig. 7).
FIG. 7.
Comparative effects of oral ACV and topical BILD 1633 SE against HSV-1 dlsptk infection. Animals were cutaneously inoculated with 107 PFU/site, as described in Materials and Methods. ACV (5%) and BILD 1633 SE (5%) were applied topically four times per day. (A) Mean lesion scores. (B) AUCs of the lesion scores. ACV only transiently (n = 12; P < 0.05) reduced the mean lesion score on days 13 to 15 compared with the scores for the no-treatment group (n = 12) and the vehicle group (n = 24). BILD 1633 SE more effectively reduced the mean lesion score (n = 24; P < 0.05 on days 8 to 24). The AUCs of the lesion scores are presented as means ± SEMs. ∗, P < 0.05 compared with the results for the vehicle groups.
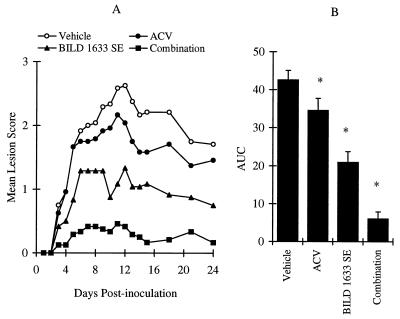
Figure 8 shows the effect of the combination therapy of oral ACV with topical BILD 1633 SE compared to the effect of treatment with either agent alone. Mice belonging to the vehicle-treated group had a mean maximum lesion score of 2.6 ± 0.1 and a mean AUC of 43 ± 3 (n = 24). Treatment with oral ACV at a concentration of 5 mg/ml in drinking water reduced the AUC by only 19%. In comparison, topical treatment with 5% BILD 1633 SE for 10 days reduced the AUC by 51% (P < 0.05) (Fig. 8). The combination therapy of oral ACV (5 mg/ml) and topical BILD 1633 SE (5% cream) was more effective than treatment with either agent alone (Fig. 8). Only a few animals in this group developed observable vesicles.
FIG. 8.
Antiviral effects of oral ACV and topical BILD 1633 SE against HSV-1 dlsptk infection. Animals were cutaneously inoculated with 107 PFU/site, as described in Materials and Methods. ACV was given in drinking water at a dose of 5 mg/ml. BILD 1633 SE (5%) was applied topically four times per day. (A) Mean lesion scores. (B) AUCs of the lesion scores. ACV transiently (P < 0.05) reduced the mean lesion score on days 12 to 15 compared with the scores for the vehicle control group. BILD 1633 SE more effectively reduced the mean lesion score (P < 0.05 on days 4 to 24 versus both the vehicle and the ACV groups). Combination therapy further reduced the mean lesion scores significantly from those for all other groups on days 3 to 24. The AUCs of lesion scores are presented as means ± SEMs (n = 24). ∗, P < 0.05 compared with the results for all other groups.
DISCUSSION
The present study reports for the first time an effective therapy against ACV-resistant HSV infections in athymic nude mice with a selective HSV RR subunit association inhibitor, BILD 1633 SE. Consistent with its in vitro antiviral properties, BILD 1633 SE was more effective against HSV-1 PAAr5-induced lesions than against HSV-1 dlsptk-induced lesions, with inoculum-dependent AUC reductions ranging from 67 to 96% and 28 to 51%, respectively. Although BILD 1633 SE was less effective against HSV-1 dlsptk-induced infections, it should be pointed out that the scoring system for topical lesions is based upon subjective criteria and that a 50% reduction of the disease lesion score from 3 to 1.5 is associated with significant abolition of HSV-induced ulceration.
Inhibition of HSV RR has previously been suggested as a potential strategy for therapy against both ACV-sensitive and ACV-resistant HSV infections (3, 13, 21, 26). However, a significant in vivo efficacy of specific RR inhibitors against ACV-resistant HSV infections has, to our knowledge, not been reported. Spector et al. (36, 39) have demonstrated the in vitro and limited in vivo antiherpesvirus effects of thiosemicarbazone derivatives, a series of compounds that disrupt the integrity of the free radical and/or iron center of the small subunit of the enzyme. Since the human RR also contains similar chemical features, those compounds do not appear to be very selective against HSV RR (26, 36). In addition, the in vivo efficacy of this class of RR inhibitors seems to be limited to potentiating the effects of ACV rather than acting as effective monotherapeutic antiherpesvirus agents (14, 28, 39). In a different study, Bridges et al. (6) have investigated the possibility of treating cutaneous HSV infections with an inhibitor of mammalian RR, MDL 101731. Although those investigators demonstrated the potent in vitro activity of this compound against both ACV-sensitive and ACV-resistant HSV, they failed to observe in vivo activity against ACV-resistant HSV infections (6). The lack of efficacy of these agents as monotherapy has in part been attributed to poor tissue penetration and to the fact that the viral RR may be dispensable in these infections because of elevated deoxynucleoside triphosphate (dNTP) pools. Our results clearly demonstrate that specific HSV RR inhibitors are effective as monotherapy in the athymic nude mouse model, implying that the viral RR is important for pathogenesis in this model.
Studies of ACV in combination with RR inhibitors have been designed previously to increase the efficacy of ACV. A number of studies have demonstrated that decreasing the dGTP pool levels with RR inhibitors increases the apparent potency of ACV by increasing the ACV triphosphate/dGTP ratio (36–38). Mechanism-based synergy between an RR inhibitor and ACV is also supported by the demonstration that HSV RR null mutants are hypersensitive to ACV (10). As expected, the combination of ACV and BILD 1633 SE also synergistically inhibited the replication of wild-type HSV strains and the ACV-resistant strain dlsptk. However, both drugs acted only in an additive manner against the polymerase mutant, ACV-resistant strain PAAr5, consistent with previous observation with nonspecific RR inhibitors (28). The precise reason for the lack of synergy of BILD 1633 SE and ACV in vitro against the HSV-1 polymerase mutant PAAr5 is unclear. It has been suggested that ACV-resistant polymerase mutants display lower affinities for normal dNTPs in their HSV DNA polymerase (19) than that in the wild-type virus. Therefore, a decrease in the dNTP concentration below the Km should occur at a lower concentration of an RR inhibitor and should enhance its antiviral effect. Because of this increased efficacy of BILD 1633 SE alone against this mutant virus, the synergistic effect of the combination may be less pronounced and is therefore difficult to observe experimentally in vitro. Nevertheless, the in vivo data indicate that combination therapy with ACV with BILD 1633 SE is more than additive against both ACV-resistant mutants. Thus, combination therapy may not have to be synergistic in vitro to achieve more than additive in vivo effects.
Although ACV has generally been successful in treating HSV infections, chronic therapy with this drug in patients who are immunocompromised due to AIDS, organ transplantation, or cancer chemotherapy leads to an increase in the viral burden and the possible emergence of resistance. It has recently been reported that ACV-resistant mutants are responsible for HSV pneumonia, progressive whitlow, meningoencephalitis, and mucocutaneous dissemination in AIDS patients (7, 8, 22–24, 34). The unfulfilled clinical needs call for continued investigation to identify novel therapeutic antiherpesvirus agents. In the present study, BILD 1633 SE reduced both dlsptk- and PAAr5-induced lesions more effectively than ACV did. In addition, the combination of BILD 1633 SE with ACV achieved better effects against dlsptk infection that did not respond to topical treatment with ACV. Although most ACV-resistant clinical isolates exhibit TK deficiency, some of them have been identified as DNA polymerase mutants or heterogeneous mixtures containing polymerase mutants (8, 24). Pathogenicity studies with mice and clinical observations indicate that ACV-resistant mutants, especially the TK-deficient mutants, usually have reduced virulence (8, 25, 33). In comparison, DNA polymerase mutants, especially heterogeneous mixtures containing polymerase mutants, may have great potential for causing dangerous ACV-resistant HSV infections (8, 25, 33, 34). Indeed, it has been shown that when a cell is coinfected with a TK-deficient mutant and a wild-type virus, ACV treatment will be effective. On the contrary, ACV has little effect on cells coinfected with the wild type and an ACV-resistant polymerase mutant (33). Considering the antiviral efficacy of BILD 1633 SE against both TK and polymerase mutants tested in the present study, alternative and/or combination therapy with ACV and RR inhibitors such as BILD 1633 SE may provide a good approach for the increasing challenge of ACV-resistant HSV infections. Due to the poor systemic absorption of peptidomimetic compounds like BILD 1633 SE, the therapeutic potential with this class of inhibitors may be limited to topical treatment only. Although HSV-1 isolates with resistance to peptidomimetic RR inhibitors have been isolated in vitro, these resistant isolates are more sensitive to ACV, possibly as a consequence of the compromised activity of their RRs (2). Therefore, combination therapy with ACV and RR inhibitors may be more beneficial.
In summary, the present study demonstrated the activity of BILD 1633 SE as monotherapy against both polymerase-altered and TK-deficient ACV-resistant HSV-1 infections. The combination of BILD 1633 SE and ACV almost abolished dlsptk-induced lesions that were not affected by topical ACV. These results further support the hypothesis that specific inhibition of the HSV RR subunit association is a valid strategy for the development of new antiherpesvirus drugs.
ACKNOWLEDGMENTS
We acknowledge valuable discussions with P. Anderson and G. Bolger. We thank N. Lapeyre-Paquette, N. Dansereau, and G. Di Lella for technical assistance. We also acknowledge D. Coen for providing ACV-resistant mutants and S. Larouche for secretarial assistance.
REFERENCES
- 1.Bollinger J M, Jr, Edmondson D E, Huynh B H, Filley J, Norton J R, Stubbe J. Mechanism of assembly of the tyrosyl radical-dinuclear iron cluster cofactor of ribonucleotide reductase. Science. 1991;253:292–298. doi: 10.1126/science.1650033. [DOI] [PubMed] [Google Scholar]
- 2.Bonneau A-M, Kibler P, White P, Bousquet C, Dansereau N, Cordingley M G. Resistance of herpes simplex virus type 1 to peptidomimetic ribonucleotide reductase inhibitors: selection and characterization of mutant isolates. J Virol. 1996;70:787–793. doi: 10.1128/jvi.70.2.787-793.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt C R, Kintner R L, Pumfery A M, Visalli R J, Grau D R. The herpes simplex virus ribonucleotide reductase is required for ocular virulence. J Gen Virol. 1991;72:2043–2049. doi: 10.1099/0022-1317-72-9-2043. [DOI] [PubMed] [Google Scholar]
- 4.Brandt C R, Spencer B, Imesch P, Garneau M, Deziel R. Evaluation of a peptidomimetic ribonucleotide reductase inhibitor with a murine model of herpes simplex virus type 1 ocular disease. Antimicrob Agents Chemother. 1996;40:1078–1084. doi: 10.1128/aac.40.5.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brazeau P, Ling N, Bohlen P, Esch F, Ying S-Y, Guillemin R. Growth hormone releasing factor, somatocrinin, releases pituitary growth hormone in vitro. Proc Natl Acad Sci USA. 1982;79:7909–7913. doi: 10.1073/pnas.79.24.7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bridges C G, Ahmed S P, Sunkara P S, McCarthy J R, Tyms A S. The ribonucleotide reductase inhibitor (E)-2′-fluoromethylene-2′-deoxycytidine (MDL 101,731): a potential topical therapy for herpes simplex virus infection. Antivir Res. 1995;27:325–334. doi: 10.1016/0166-3542(95)00015-e. [DOI] [PubMed] [Google Scholar]
- 7.Chatis P A, Crumpacker C S. Resistance of herpesviruses to antiviral drugs. Antimicrob Agents Chemother. 1992;36:1589–1595. doi: 10.1128/aac.36.8.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coen D M. The implications of resistance to antiviral agents for herpesvirus drug targets and drug therapy. Antivir Res. 1991;15:287–300. doi: 10.1016/0166-3542(91)90010-o. [DOI] [PubMed] [Google Scholar]
- 9.Coen D M, Aschman D P, Gelep P T, Retondo M J, Weller S K, Schaffer P A. Fine mapping and molecular cloning of mutations in the herpes simplex virus DNA polymerase locus. J Virol. 1984;49:236–247. doi: 10.1128/jvi.49.1.236-247.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coen D M, Goldstein D J, Weller S K. Herpes simplex virus ribonucleotide reductase mutants are hypersensitive to acyclovir. Antimicrob Agents Chemother. 1989;33:1395–1399. doi: 10.1128/aac.33.8.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coen D M, Kosz-Vnenchak M, Jacobson J G, Leib D A, Bogard C L, Schaffer P A, Tyler K L, Knipe D M. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci USA. 1989;86:4736–4740. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 13.Dutia B M, Frame M C, Subak-Sharpe J H, Clark W N, Marsden H S. Specific inhibition of herpesvirus ribonucleotide reductase by synthetic peptides. Nature. 1986;321:439–443. doi: 10.1038/321439a0. [DOI] [PubMed] [Google Scholar]
- 14.Ellis M N, Lobe D C, Spector T. Synergistic therapy by acyclovir and A1110U for mice orofacially infected with herpes simplex viruses. Antimicrob Agents Chemother. 1989;33:1691–1696. doi: 10.1128/aac.33.10.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellis M N, Waters R, Hill E L, Lobe D C, Selleseth D W, Barry D W. Orofacial infection of athymic mice with defined mixtures of acyclovir-susceptible and acyclovir-resistant herpes simplex virus type 1. Antimicrob Agents Chemother. 1989;33:304–310. doi: 10.1128/aac.33.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furman P A, Coen D M, St. Clair M H, Schaffer P A. Acyclovir-resistant mutants of herpes simplex virus type 1 express altered DNA polymerase or reduced acyclovir phosphorylating activities. J Virol. 1981;40:936–941. doi: 10.1128/jvi.40.3.936-941.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaudreau P, Paradis H, Langelier Y, Brazeau P. Synthesis and inhibitory potency of peptides corresponding to the subunit 2 C-terminal region of herpes virus ribonucleotide reductases. J Med Chem. 1990;33:723–730. doi: 10.1021/jm00164a040. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein D J, Weller S K. Factor(s) present in herpes simplex virus type 1-infected cells can compensate for the loss of the large subunit of the viral ribonucleotide reductase: characterization of an ICP6 deletion mutant. Virology. 1988;166:41–51. doi: 10.1016/0042-6822(88)90144-4. [DOI] [PubMed] [Google Scholar]
- 19.Hall J D, Furman P A, St. Clair M H, Knopf C W. Reduced in vivo mutagenesis by herpes simplex DNA polymerase involves improved nucleotide selection. Proc Natl Acad Sci USA. 1985;82:3889–3893. doi: 10.1073/pnas.82.11.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Idowu A D, Fraser-Smith E B, Poffenberger K L, Herman R C. Deletion of the herpes simplex virus type 1 ribonucleotide reductase gene alters virulence and latency in vivo. Antivir Res. 1992;17:145–156. doi: 10.1016/0166-3542(92)90048-a. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson J G, Leib D A, Goldstein D J, Bogard C L, Chaffer P A S, Weller S K, Coen D M. A herpes simplex virus ribonucleotide reductase deletion mutant is defective for productive acute and reactivatable latent infections of mice and for replication in mouse cells. Virology. 1989;173:276–283. doi: 10.1016/0042-6822(89)90244-4. [DOI] [PubMed] [Google Scholar]
- 22.Kimberlin D W, Coen D M, Biron K K, Cohen J I, Lamb R A, McKinlay M, Emini E A, Whitley R J. Molecular mechanisms of antiviral resistance. Antivir Res. 1995;26:369–401. doi: 10.1016/0166-3542(95)00027-j. [DOI] [PubMed] [Google Scholar]
- 23.Kimberlin D W, Crumpacker C S, Straus S E, Biron K K, Drew W L, Hayden F G, McKinlay M, Richman D D, Whitley R J. Antiviral resistance in clinical practice. Antivir Res. 1995;26:423–438. doi: 10.1016/0166-3542(95)00031-g. [DOI] [PubMed] [Google Scholar]
- 24.Krogsrud R L, Welchner E, Scouten E, Liuzzi M. A solid-phase assay for the binding of peptidic subunit association inhibitors to the herpes simplex virus ribonucleotide reductase large subunit. Anal Biochem. 1993;213:386–394. doi: 10.1006/abio.1993.1436. [DOI] [PubMed] [Google Scholar]
- 25.Laufer D S, Starr S E. Resistance to antivirals. Pediatric Clin N Am. 1995;42:583–599. doi: 10.1016/s0031-3955(16)38980-5. [DOI] [PubMed] [Google Scholar]
- 26.Liuzzi M, Déziel R. Inactivation of herpes simplex virus ribonucleotide reductase by subunit association inhibitors: a potential antiviral strategy. In: Adams J, Merluzzi V J, editors. The search for antiviral drugs. Boston, Mass: Birkhauser; 1993. pp. 225–238. [Google Scholar]
- 27.Liuzzi M, Déziel R, Moss N, Beaulieu P, Bonneau A-M, Bousquet C, Chafouleas J G, Garneau M, Jaramillo J, Krogsrud R L, Lagacé L, McCollum R S, Nawoot S, Guindon Y. A potent peptidomimetic inhibitor of HSV ribonucleotide reductase with antiviral activity in vivo. Nature. 1994;372:695–698. doi: 10.1038/372695a0. [DOI] [PubMed] [Google Scholar]
- 28.Lobe D C, Spector T, Ellis M N. Synergistic topical therapy by acyclovir and A1110U for herpes simplex virus induced zosteriform rash in mice. Antivir Res. 1991;15:87–100. doi: 10.1016/0166-3542(91)90027-o. [DOI] [PubMed] [Google Scholar]
- 29.McClements W, Yamanaka G, Garsky V, Perry H, Bacchetti S, Colonno R, Stein R B. Oligopeptides inhibit the ribonucleotide reductase of herpes simplex virus by causing subunit separation. Virology. 1988;162:270–273. doi: 10.1016/0042-6822(88)90421-7. [DOI] [PubMed] [Google Scholar]
- 30.Moss N, Beaulieu P, Duceppe J-S, Ferland J-M, Garneau M, Gauthier J, Ghiro E, Goulet S, Guse I, Jaramillo J, Llinas-Brunet M, Malenfant E, Plante R, Poirier M, Soucy F, Wernic D, Yoakim C, Déziel R. Peptidomimetic inhibitors of herpes simplex virus ribonucleotide reductase with improved in vivo antiviral activity. J Med Chem. 1996;39:4173–4180. doi: 10.1021/jm960324r. [DOI] [PubMed] [Google Scholar]
- 31.Nordlund P, Sjoberg B-M, Eklund H. Three-dimensional structure of the free radical protein of ribonucleotide reductase. Nature. 1990;345:593–598. doi: 10.1038/345593a0. [DOI] [PubMed] [Google Scholar]
- 32.Paradis H, Gaudreau P, Brazeau P, Langelier Y. Mechanism of inhibition of herpes simplex virus (HSV) ribonucleotide reductase by a nonapeptide corresponding to the carboxyl terminus of its subunit 2. J Biol Chem. 1988;263:16045–16050. [PubMed] [Google Scholar]
- 33.Pelosi E, Hicks K A, Sacks S L, Coen D M. Heterogeneity of a herpes simplex virus clinical isolate exhibiting resistance to acyclovir and foscarnet. Adv Exp Med Biol. 1992;312:151–158. doi: 10.1007/978-1-4615-3462-4_15. [DOI] [PubMed] [Google Scholar]
- 34.Pottage J C, Jr, Kessler H A. Herpes simplex virus resistance to acyclovir: clinical relevance. Infect Agents Dis. 1995;4:115–124. [PubMed] [Google Scholar]
- 35.Reichard P. Interactions between deoxyribonucleotide and DNA synthesis. Annu Rev Biochem. 1988;57:349–374. doi: 10.1146/annurev.bi.57.070188.002025. [DOI] [PubMed] [Google Scholar]
- 36.Spector T, Averett D R, Nelson D J, Lamne C U, Morrison R W, Jr, St. Clair M H, Furman P A. Potentiation of antiherpetic activity of acyclovir by ribonucleotide reductase inhibition. Proc Natl Acad Sci USA. 1985;82:4254–4257. doi: 10.1073/pnas.82.12.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spector T, Harrington J A, Porter D J T. Herpes and human ribonucleotide reductases. Inhibition by 2-acetylpyridine 5-[(2-chloroanilino)-thiocarbonyl] thiocarbonohydrazone (348U87) Biochem Phamacol. 1991;42:91–96. doi: 10.1016/0006-2952(91)90685-x. [DOI] [PubMed] [Google Scholar]
- 38.Spector T, Harrington J A, Morrison R, Jr, Lambe C U, Nelson D J, Averett D R, Biron K, Furman P A. 2-Acetylpyridine 5-[(dimethylamino)thiocarbonyl]-thiocarbonohydrazone (A1110U), a potent inactivator of ribonucleotide reductases of herpes simplex and varicella zoster viruses and a potentiator of acyclovir. Proc Natl Acad Sci USA. 1989;86:1051–1055. doi: 10.1073/pnas.86.3.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spector T, Lobe D C, Ellis M N, Blumenkopf T A, Szczech G M. Inactivators of the herpes simplex virus ribonucleotide reductase: hemotological profiles and in vivo potentiation of the antiviral activity of acyclovir. Antimicrob Agents Chemother. 1992;36:934–937. doi: 10.1128/aac.36.5.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suhnel J. Evaluation of synergism or antagonism for the combined action of antiviral agents. Antivir Res. 1990;13:23–40. doi: 10.1016/0166-3542(90)90042-6. [DOI] [PubMed] [Google Scholar]
- 41.Trybala E, Bergstrom T, Svennerholm B, Jeansson S, Glorioso J C, Olofsson S. Localization of a functional site on herpes simplex virus type 1 glycoprotein C involved in binding to cell surface hepran sulphate. J Gen Virol. 1994;75:743–752. doi: 10.1099/0022-1317-75-4-743. [DOI] [PubMed] [Google Scholar]
- 42.Yamada Y, Kimura H, Morishima T, Daikoku T, Maeno K, Nishiyama Y. The pathogenicity of ribonucleotide reductase-Null mutants of herpes simplex virus type 1 in mice. J Infect Dis. 1991;164:1091–1097. doi: 10.1093/infdis/164.6.1091. [DOI] [PubMed] [Google Scholar]