Abstract

CuWO4 is a ternary semiconductor oxide with excellent visible light harvesting properties up to 550 nm and stability at high pH values, which make it a suitable material to build photoanodes for solar light conversion to hydrogen via water splitting. In this work, we studied the photoelectrochemical (PEC) performance of transparent CuWO4 electrodes with tunable light absorption and thickness, aiming at identifying the intrinsic bottlenecks of photogenerated charge carriers in this semiconductor. We found that electrodes with optimal CuWO4 thickness exhibit visible light activity due to the absorption of long-wavelength photons and a balanced electron and hole extraction from the oxide. The PEC performance of CuWO4 is light-intensity-dependent, with charge recombination increasing with light intensity and most photogenerated charge carriers recombining in bulk sites, as demonstrated by PEC tests performed in the presence of sacrificial agents or cocatalysts. The best-performing 580 nm thick CuWO4 electrode delivers a photocurrent of 0.37 mA cm–2 at 1.23 VSHE, with a 7% absorbed photon to current efficiency over the CuWO4 absorption spectrum.
Keywords: photoelectrocatalysis, ternary oxides, solar water splitting, solar light, renewable energy
1. Introduction
Photoelectrochemical (PEC) water splitting produces clean O2 and H2 from water by exploiting sunlight directly.1 The oxygen evolution reaction requires anodic semiconductor materials that are resistant to harsh reaction conditions. Moreover, an ideal material needs a narrow band gap to absorb a considerable portion of visible light and guarantee a solar to hydrogen efficiency above 10%, which is the lower limit for industrialization.2,3
Binary metal oxide-based photoelectrodes, such as WO3 and α-Fe2O3,4,5 initially attracted interest as visible-light-active materials. WO3 has a high electron diffusion length and mobility,4,6−8 and optimized electrodes allow almost complete absorbed photons to current conversion efficiency. However, WO3 has poor chemical stability in neutral or basic pH and a relatively wide band gap (2.6–2.7 eV). On the other hand, hematite (Fe2O3) has an intriguing narrow band gap of 2.0 eV with potential solar to hydrogen conversion efficiency (ηSHE) up to 15% (Table 1),9 but limited charge carrier lifetime (below 1 ns in undoped Fe2O3)10,11 and mobility, which translate into suboptimal PEC performances.
Table 1. Commonly Used Semiconductor Oxide Photoanodes.
| semiconductor | BGa/eV | Jmaxb/mA cm–2 | ηSHEc |
|---|---|---|---|
| WO3 | 2.6 | 4 | 5% |
| BiVO4 | 2.4 | 7 | 8% |
| CuWO4 | 2.2 | 10 | 12.5% |
| Fe2O3 | 2.0 | 12.5 | 15% |
| CuMoWO4 | 2.0 | 12.5 | 15% |
bang gap (BG).
maximum current density (Jmax).
solar to hydrogen efficiency (ηSHE) of semiconductor photoanodes assembled in a two-electrode tandem PEC cell, assuming complete light absorption and conversion of photons with energy larger than the band gap and 100% faradaic efficiency to H2 and O2.
More recently, ternary oxides such as BiVO4 with an intermediate band gap of 2.4 eV emerged as highly efficient materials for photoelectrocatalytic applications.12−16 State-of-the-art BiVO4 photoanodes show close-to-unity incident photon to current efficiency up to 480 nm.17 However, the band gap of BiVO4 is slightly larger than that desired for optimal photoanode materials. Indeed, a charge carrier recombination-free BiVO4 electrode (i.e., with an incident photon to current efficiency close to 100% up to the absorption onset at 520 nm) would lead to only 7–8% solar energy to hydrogen conversion, which implies that BiVO4 should be used in combination with narrower band gap materials in complex multilayer photoanodes.18
Ternary CuWO4 oxide has a band gap of 2.2 eV, and it could achieve an ηSHE of 12.5%.19 Furthermore, the CuWO4 band gap can be narrowed by substituting tungsten with molybdenum in quaternary CuWxMoyO4. For example, CuW0.5Mo0.5O4 has a band gap of 2.0 eV, corresponding to an absorption edge of 650 nm.20−22 This tungstate family is stable in neutral and slightly basic pH because of the overlap between Cu 3d orbitals with O 2p orbitals.23,24 This hybridization is also responsible for the negative shift of the CuWO4 valence band compared to that of binary WO3.25 Moreover, CuWO4 is suitable to assemble heterojunction systems with BiVO4,26,27 likewise WO3.28−32
In prior studies, we investigated the charge carrier dynamics in transparent CuWO4 electrodes and found that charge carriers mostly recombine within a 10 ps time scale, with only ca. 10% of them surviving longer than 1 ns to potentially run water oxidation.33 In this work, we sought to experimentally study the intrinsic limits that hamper CuWO4 photoactivity by employing a suite of PEC experiments to understand the factors shaping its performance. We prepared a series of thin-film CuWO4 electrodes through a citrate-based aqueous solution method and tuned the CuWO4 thickness to increase visible light exploitation of the photoanodes. To seek information on the charge carrier recombination processes occurring in bulk and surface CuWO4 and on their implication in the overall PEC performance, we tested the electrodes under front- and back-side irradiation in both simulated solar light and monochromatic irradiation experiments. We also varied the light intensity to study the effect of the charge carrier density on the PEC activity and found that the PEC performance of CuWO4 is light-intensity-dependent, with charge recombination increasing with light intensity, and that most photoproduced charge carriers recombine in bulk sites.
2. Experimental Section
2.1. Materials
The following chemicals were employed in the present work: copper(II) nitrate trihydrate (99% purity, Sigma-Aldrich), ammonium metatungstate hydrate (NH4)6H2W12O40·xH2O (99% purity, Fluka), citric acid (99% purity, Sigma-Aldrich), deionized water, boric acid (99.5% purity, Sigma-Aldrich), and KOH (99.5% purity, Sigma-Aldrich). All chemicals were used as received, with no further purification.
2.2. Photoelectrodes Preparation
The CuWO4 thin films were prepared following a previously reported procedure.33 Specifically, a 0.5 M solution of CuWO4 was prepared by dissolving 14 mmol of citric acid in 5.3 mL of ethanol and 2.4 mL of deionized H2O, followed by the addition of 5 mmol of Cu(NO3)2·3H2O under vigorous stirring and a stoichiometric amount of ammonium metatungstate hydrate. Fluorine-doped tin oxide (FTO) glass (Pilkington Glass, TEC-7, thickness of 2 mm) was used as conductive glass to prepare the FTO/CuWO4 electrodes. The FTO glass was cleaned under sonication for 30 min in a soap-water solution, rinsed thoroughly with water, sonicated for 30 min in ethanol, and then dried in air. After cleaning, the electrodes were prepared by spin coating 100 μL of the CuWO4 solution on FTO, at 4000 rpm for 30 s. Then, the CuWO4 film was preannealed at 250 °C for 10 min and then annealed at 550 °C for 1 h. Prior to any test, the photoanodes were treated for about 30 s in a 0.5 M HCl aqueous solution to eventually eliminate any trace of CuO, then washed with distilled water and dried in air. The deposition, annealing, and HCl cleaning steps were repeated up to six times to prepare multilayer electrodes with the desired thickness and light absorption properties.
The NiFeOx cocatalyst was deposited onto the photoelectrodes employing a hydrothermal method,34 by soaking the CuWO4 electrode into an aqueous solution containing NiCl2 (0.2 mM) and FeCl3 (29.8 mM) as Ni2+ and Fe3+ sources in a closed vessel for 45 min at 100 °C.
2.3. Optical, Morphological, Structural, and Photoelectrochemical Tests
The absorption spectra of the CuWO4 thin films on the electrodes were recorded in transmission mode with a Jasco V-650 spectrophotometer. The crystalline phase of the photoactive materials was determined by XRD analysis of the deposited thin films, using a Philips PW 1830/40 X-ray powder diffractometer equipped with a Cu tube at 40 kV and 40 mA. Top-view and cross-sectional field emission scanning electron microscopy (FESEM) images were acquired employing a LEO 1430 scanning electron microscope operating at a 10 kV accelerating voltage and an 8 mm working distance.
PEC measurements were performed using a homemade cell and an Autolab PGSTAT 12 potentiostat controlled by the NOVA software. In a typical setup, the electrode was used as the working electrode, a Pt wire was used as the counter electrode, and Ag/AgCl (3.0 M in NaCl) was used as the reference electrode. The photoanodes were tested under both back-side irradiation (through the FTO/CuWO4 interface) and front-side irradiation (through the CuWO4/FTO interface). The light source was an Oriel, Model 81172 Solar Simulator equipped with an AM 1.5 G filter. In simulated solar light irradiation experiments, the light intensity was measured with a Thorlab PM200 power meter equipped with a Si power head (S130VC) and set at 100 mW cm–2 (1 sun). In PEC experiments under simulated solar light with different intensities, a neutral light attenuator filter was employed to obtain 25 and 50 mW cm–2 intensities (0.25 and 0.5 sun, respectively) and a quartz lens was used to increase the intensity to 150 and 200 mA cm–2 (1.5 and 2 suns, respectively).
PEC experiments were carried out in a 0.1 M potassium borate (KBi) buffer solution at pH 9 unless otherwise stated. The potential vs Ag/AgCl was converted into the standard hydrogen electrode (SHE) scale using the following equation
The incident photon-to-current efficiency (IPCE) was measured using a 300 W Lot-Oriel Xe lamp equipped with a Lot-Oriel Omni-λ 150 monochromator and a Thorlabs SC10 automatic shutter. A 1.23 V bias vs SHE (VSHE) was applied, and the current was measured with a 10 nm step within the 350 to 600 nm wavelength range. The incident light power was measured at each wavelength by using a calibrated Thorlabs S130VC photodiode connected to a Thorlabs PM200 power meter. The IPCE at each wavelength was calculated using the following equation
where Jλ is the photocurrent density (mA cm–2) and Pλ (mW cm–2) is the power of the monochromatic light at wavelength λ (nm).
The internal quantum efficiency (IQE) at each wavelength was calculated by combining the IPCE curve with the absorption (A) spectrum of each photoanode
3. Results
3.1. Characterization of CuWO4 Multilayer Photoanodes
We started by studying the morphology of the CuWO4 photoanodes by field emission scanning electron microscopy (FESEM). Top-view images (Figure 1A,B) reveal that CuWO4 progressively covers the FTO substrate uniformly. Two deposited layers provide a continuous coating film consisting of oval-shaped nanostructures (Figure S1 in the Supporting Information). Bigger grains form in thicker electrodes (Figure 1B).
Figure 1.
Field emission scanning electron microscopy (FESEM) top views of (A) 2 layers and (B) 5 layers of CuWO4. Inset in (B): cross section of the CuWO4 film obtained by depositing 5 layers. (C) XRD patterns of the CuWO4 electrodes. The diffraction signals of WO3 (JCPDF 89–4476), CuWO4, and underlying FTO are identified with red bars, black arrows, and asterisks, respectively. (D) Absorption spectra of the CuWO4 photoanodes with increasing semiconductor thickness. Inset: scheme of the optical transitions in CuWO4. (E) Thickness of the electrodes with 1–6 CuWO4 layers, from cross-sectional FESEM images (left column) and calculated from the absorption coefficient at 420 nm (right column). (F) Extinction coefficient vs wavelength of a 5-layered CuWO4 electrode.
Cross-sectional images (inset of Figures 1B and S2) show that the CuWO4 coating is compact along its whole thickness. Therefore, we could estimate the thickness of the photoactive layer in electrodes prepared with 1, 2, 3, and 5 CuWO4 deposition cycles (Figure 1E).
The phase purity of the CuWO4 films was assessed through XRD analysis and comparison with the Bragg reflections from JCPDF 72–0616, which revealed a triclinic structure (Figure 1C) and the absence of the WO3 phase in all electrodes. Although no CuO phase is detectable in the XRD patterns, we washed the electrodes with diluted HCl to dissolve possible CuO impurity traces.
Then, we studied the optical properties of the electrodes by absorption spectroscopy in transmittance mode (Figure 1D). The multilayer films are optically transparent, with the absorbance in the UV region steeply increasing below 450 nm, without obvious absorption peaks consistently with the indirect forbidden d–d transition occurring in this Mott–Hubbard semiconductor,35−37 possibly originating light scattering at wavelengths longer than 550 nm.
The main optical transition of CuWO4 is assigned to the photoexcitation of electrons from the valence band, composed of O 2p states partly mixed with Cu 3d states, to the conduction band—empty Cu 3d levels (inset of Figure 1D).33 A ca. 2.1 eV energy gap separates the band edges. CuWO4 has an additional band of empty states at higher energy, i.e., at about 5 eV above the top of the valence band, consisting of W 5d and Cu 3d states. At wavelengths above 550 nm, scattering appears in the absorption spectrum of thicker films, possibly originating from the bigger grains observed in Figure 1B.
We combined the thickness values obtained from cross-sectional FESEM images and the absorption spectrum of CuWO4 to estimate the absorption coefficient of CuWO4 films at 420 nm (the edge between UV and visible light), using the following equation
where α420 (cm–1) is the absorption coefficient of CuWO4 at 420 nm, A420 is the absorption at 420 nm, and d is the average thickness in cm calculated from the cross-sectional FESEM images. From the A420 vs film thickness plot (see Figure S3), we calculated the absorption coefficient for CuWO4 at 420 nm, α420 = (1.65 ± 0.14) 104 cm–1. This value is rather low compared to other semiconductor oxides, consistent with the indirect nature of the CuWO4 band gap. For instance, it is 4-fold lower than the α420 value of BiVO4 (6.7 × 104 cm–1),38 and this implies that to absorb the same amount of 420 nm photons, a CuWO4 electrode needs to be 4 times thicker than a BiVO4 electrode. Interestingly the absorption coefficient for CuWO4 at 420 nm is also ca. 2-fold lower compared to CuW0.5Mo0.5O4 (3.41 × 104 cm–1),20 which supports the hypothesis that Mo doping introduces additional optical transitions in CuWO4.
The α420 for CuWO4 value was then used to evaluate the thickness of the prepared CuWO4 films from their absorbance at 420 nm. These thickness values are reported in Figure 1E. For convenience, hereafter, we identify the electrodes with different CuWO4 thicknesses as CuWO4:X, with X referring to the CuWO4 thickness in nanometers (rounded to the nearest ten).
We also calculated the extinction coefficient of CuWO4 at all wavelengths, based on the overall absorption spectrum of
the thinnest electrode (which is moderately affected by scattering),
as 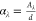 , where Aλ is the absorbance over the 300–800 nm interval and d is the film thickness. The so estimated extinction coefficient
of CuWO4 vs wavelength is shown in Figure 1F.
, where Aλ is the absorbance over the 300–800 nm interval and d is the film thickness. The so estimated extinction coefficient
of CuWO4 vs wavelength is shown in Figure 1F.
3.2. Photoresponse of CuWO4 Multilayer Photoanodes
We performed PEC experiments under monochromatic and simulated solar light irradiation to probe the photoresponse of the CuWO4 electrodes with increasing thickness of the semiconductor layer. The experiments under simulated solar light provide information on their performance under conditions close to field application, while PEC tests performed under monochromatic irradiation provide information on the irradiation wavelength-dependent behavior of photoactive materials. We also tested the electrodes under back-side and front-side irradiation, i.e., with the light reaching CuWO4 through the FTO or the electrolyte, respectively. Indeed the comparison between the PEC performance in the two modes proved diagnostic for identifying electron or hole transport as a limiting process in the illuminated semiconductor.39,40
Linear sweep voltammetry (LSV) tests performed under simulated solar light irradiation in 0.1 M borate buffer solution (KBi buffer) evidence that the photocurrent vs voltage increases with the CuWO4 layer thickness (Figure 2A,B), up to a 580 nm thick CuWO4 photoactive layer. Under back-side irradiation, the electrodes generate a larger photocurrent than under front-side irradiation (Figure 2C). For instance, the photocurrent of the CuWO4:580 electrode under back irradiation is ≥2x than in the front-side mode.
Figure 2.
Linear sweep voltammetry (LSV) curves recorded with CuWO4 multilayer photoanodes under (A) back-side or (B) front-side simulated solar light irradiation (100 mW cm–2) in 0.1 M KBi buffer solution at pH 9. The labels within the figures refer to the thickness of the CuWO4 layers, in nm. (C) Current density at 1.23 VSHE as a function of the CuWO4 thickness, under back- and front-side irradiation (empty and full symbols, respectively). (D) Overall photon flux in the AM 1.5 solar spectrum (black trace) and number of photons absorbed per second by CuWO4:580 (blue area). The dashed vertical line marks the CuWO4 absorption edge.
Indeed, under front-side irradiation, most electrons are photogenerated far from the electron extraction site (the contact with the FTO layer). The lower photocurrent, compared to back-side irradiation, could indicate that electrons in CuWO4 can travel only short distances because they undergo recombination with photogenerated holes within the film thickness, before reaching the extraction site. With the thinnest CuWO4:80 electrode, similar photocurrents are recorded under front- and back-side irradiation (Figure 2A,B), demonstrating that photopromoted electrons can diffuse efficiently at least within a ca. 80 nm distance.
The best-performing CuWO4:580 electrode generates a photocurrent density of 0.37 mA cm–2 at 1.23 VSHE under back-side irradiation. This value is in line with previous records for CuWO4-based electrodes prepared with different synthetic strategies (Table 2).41,42 However, it is far from the maximum photocurrent expected for the 2.2 eV band gap of CuWO4. Indeed, assuming that all absorbed photons in the CuWO4:580 electrode (Figure 2D) are completely converted into current would lead to a 5.6 mA cm–2 theoretical maximum photoresponse under 1 sun illumination. Thus, the photogenerated current is only 6.7% of this maximum value, which implies that more than 90% of the photogenerated charge carriers undergo recombination.
Table 2. Photoelectrochemical Performances of Literature Benchmark CuWO4 Photoanodes Prepared with Different Synthetic Approaches.
| Jg | stability |
|||||
|---|---|---|---|---|---|---|
| synthetic method | electrolyte | thickness/nm | / mA cm–2 | Time / min | Jfinh / mA cm-2–2jfinh | ref |
| spin-coating | 0.1 M KPi (pH 7) | 580 | 0.37 | 120 | 0.37 | this work |
| one step-hya | 0.2 M KPi (pH 7)e | ∼1500 | 0.38 | 50 | 0.3 | (43) |
| ST – hyb | 0.1 M KPi (pH 7) | ≥1000 | ∼0.3 | 60 | ∼5%i | (44) |
| ST – hy | 0.1 M KPi (pH 7) | ≥1000 | 0.34 | 240 | ∼35%i | (45) |
| ST – hy | 1 M KBi (pH 9)f | ≥1000 | 0.4 | 60 | 0.39 | (29) |
| ST – hy | 0.1 M KPi (pH 7) | 2000 | 0.42 | n/a | n/a | (46) |
| ST – hy | 0.1 M KPi (pH 7) | ≥1000 | 0.35 | 240 | 0.26 | (47) |
| spin-coating | KPi (pH 7) | 500 | 0.47 | 600 | 0.4 | (48) |
| e-depositionc | 0.1 M KPi (pH 7) | 2–3000 | 0.18 | 600 | ∼50%i | (25) |
| ALDd | 1 M KBi (pH 9.0) | 80 | ∼0.15 | n/a | n/a | (41) |
| ST - hy | 0.1 M Kpi (pH 7) | ≥1000 | 0.35 | 60 | ∼17%i | (22) |
| spin coating | 0.1 M Na2SO4 (pH 6.8) | 800 | 0.27 | 2.5 | 0.21 | (49) |
| spray pyrolysis | 0.1 M KPi (pH 7) | 1.5–2000 | 0.19 | n/a | n/a | (50) |
| spin coating | KPi/NaCl 0.1/0.14 M | 250 | 0.38 | n/a | n/a | (51) |
| ST – hy | 0.1 M KPi (pH 7) | ≥1000 | 0.38 | 240 | 0.39 | (52) |
one-step hydrothermal.
sacrificial template–hydrothermal.
electrodeposition.
atomic layer deposition.
potassium phosphate buffer.
potassium borate buffer.
current density at 1.23 VSHE,
current density at 1.23 VSHE at the end of the stability test,
current density percent drop at the end of the stability test (in this case the stability is at a voltage ≥1.23 VSHE).
Figure 3 shows the incident photon to current efficiency (IPCE) and internal quantum efficiency (IQE) curves recorded with the CuWO4 photoanodes under back- and front-side irradiation at an applied bias of 1.23 VSHE. All IPCE curves monotonously decrease with increasing incident wavelength without any defined peak or steep IPCE feature (Figure 3A,C). This suggests the presence of a single electronic transition with broad rather than sharp band edges within the investigated light energy. The IQE values (Figure 3B,D), which account for the number of absorbed photons which generate photocurrent and is equal to the charge transport efficiency,53 are larger than the IPCE values. However, in this photoelectrode series, the IQE differs slightly from the corresponding IPCE values because of the relatively high electrode absorbance, especially in the UV region where the electrodes completely absorb the incident light. On the other hand, under visible light irradiation, when the electrodes absorb less than 50% of the incident light, the IQE is noticeably larger than the IPCE (Figure S4). The tests performed under monochromatic irradiation with the thickest electrodes pinpoint a CuWO4 photocurrent onset at ca. 550–560 nm (we measured IPCE at high applied bias, 1.73 VSHE, to increase the photocurrent signal close to the band gap excitation edge; see Figures S5 and S6), in good agreement with the reported 2.2–2.3 eV band gap of CuWO4.
Figure 3.
(A, C) IPCE and (B, D) IQE analyses of CuWO4 multilayer photoanodes under (A, B) back-side and (C, D) front-side monochromatic irradiation, at 1.23 VSHE.
Under back-side irradiation, the CuWO4 electrodes generate larger IPCEs than under front-side irradiation, implying that electron transport issues limit the overall photoresponse of this material and substantiating the behavior observed in LSV tests.39
Notably, under back-side irradiation, in LSV tests CuWO4:580 outperforms the other electrodes (Figure 2A), while in IPCE, the thickest CuWO4:680 electrode is best performing (Figure 3A). This difference relies on both the different light intensities (100 mW cm–2 in LSV analyses vs a few mW cm–2 in IPCE) and the poor electron transport of CuWO4. Under back-side irradiation and the low light intensity employed in IPCE analyses, most photons are absorbed close to the FTO glass where electron extraction toward the external circuit easily occurs. However, a larger light intensity (e.g., under simulated solar light irradiation) generates a larger amount of excited electrons deep in the CuWO4 film, far from FTO, at distances exceeding the mean electron diffusion within CuWO4. These electrons are more likely to recombine with holes and limit the overall photocurrent in the thickest electrode.
3.3. Light Intensity Dependence
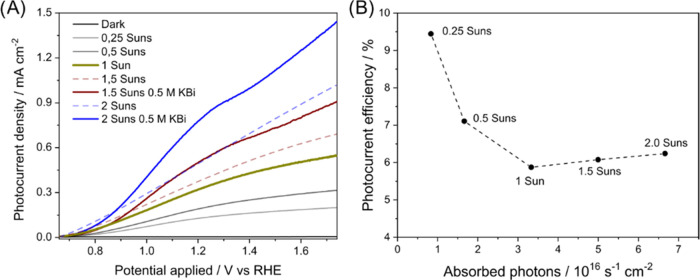
Employing the best-performing CuWO4:580 photoelectrode, we evaluated the effect on photoactivity of the light intensity under back-side irradiation, to get further information on the charge separation efficiency within the CuWO4 electrodes. For intensities larger than 1 sun, the measurements were carried out with the electrode in contact with a 0.5 M KBi electrolyte solution because the 0.1 M KBi electrolyte employed in LSV analyses under 1 sun irradiation (Figure 2) was insufficiently conductive to sustain photocurrents larger than 0.5 mA cm–2 (see dashed lines in Figure 4A).
Figure 4.
(A) LSV curves recorded with the CuWO4:580 photoelectrode under different irradiation intensities in contact with 0.1 M (0–1 sun) or 0.5 M (1.5–2 sun) (continuous lines) KBi solutions. The two dashed lines are the LSV curves recorded under 1.5 and 2 sun irradiation in contact with 0.1 M KBi solutions. (B) Photocurrent efficiency at 1.23 VSHE vs absorbed photons per unit time at different irradiation intensities.
The photocurrent response of the CuWO4:580 photoelectrode increases with increasing light intensity (Figure 4A), though this increase is not linear. We thus calculated the absorbed photons to current efficiency (photocurrent efficiency) over the full solar spectrum at different light intensities by dividing the number of photopromoted electrons exiting the CuWO4 film at 1.23 VSHE by the total number of absorbed photons (Figure 2D, integrated blue area of the absorbed photons per nm). Figure 4B reports the photocurrent efficiency values vs the amount of absorbed photons per unit time.
The photocurrent efficiency decreases with increasing light intensity from 0.25 to 1 sun and stabilizes at ca. 6% for an illumination intensity greater than 1 sun (∼3 × 1016 s–1 cm–2). The drop from 0.25 to 1 sun is consistent with an increased charge carrier recombination at high photon flux, as found when comparing IPCE and LSV results, and could be related to inefficient bulk charge extraction at high light intensity.
3.4. Water Oxidation Kinetics of CuWO4
The PEC characterization of CuWO4 photoanodes points to a photoactivity up to 550–560 nm and severe charge carrier recombination due to limited electron mobility in the bulk. To collect further information about this weak point of CuWO4 photoefficiency, we sought to investigate the oxidation reaction and stability of this semiconductor. The results of such an analysis are reported in Figure 5.
Figure 5.
(A) Chopped chronoamperometry under 1 sun irradiation of an 80 nm thick CuWO4 photoanode (black line) and a 75 nm thick BiVO4 photoanode (red line), under back-side irradiation at 1.23 VSHE. The green (light on) and blue (light off) boxes display expanded portions of the photocurrent curve. (B) Chronoamperometric stability test of the CuWO4:580 electrode under simulated solar light irradiation in 0.1 M KBi. (C) Chopped chronoamperometry under 1 sun irradiation of the CuWO4:580 photoanode in the presence (red line) or in the absence (black line) of 0.5 M H2O2. (D) Chopped LSV curves recorded with a bare (black line) and NiFeOx-modified (red line) CuWO4:80 photoanode under monochromatic irradiation at 420 nm; the light intensity is 9 mW cm–2.
We first compared the performance of CuWO4 to that of an oxide affected by intrinsic sluggish surface water oxidation kinetics. We chose bismuth vanadate because we extensively studied it in previous studies and for its relatively poor photocatalytic activity in the oxygen evolution reaction.54 As shown in Figure 5A, the photocurrent response of undoped BiVO4 at 1.23 VSHE rapidly decreases by more than 60% within a few seconds after the beginning of irradiation. This behavior is usually ascribed to surface hole accumulation due to the slow hole transfer across the semiconductor/electrolyte interface and consequent charge carrier recombination.
Moreover, when the light is switched off, a negative capacitive current appears for BiVO4 due to the consumption of accumulated surface holes via recombination with electrons from the external circuit (a negative current indicates a reductive process taking place at the working electrode). On the other hand, the CuWO4 photoanode shows a sharp photocurrent onset and negligible photocurrent decrease under irradiation, and a sharp decrease when the light is turned on and off, respectively (Figure 5A). These behaviors point to low hole accumulation at the CuWO4 surface; that is, consumption of surface holes occurs right after they reach the electrode/electrolyte interface. We also checked the stability of the CuWO4 electrode and found a negligible activity drop during a 2 h test (see Figure 5B), supporting the low hole accumulation at the surface and the chemical stability of this semiconductor.
To probe the nature of the electron–hole recombination, we performed PEC experiments in the presence of a more feasible oxidation reaction. To do so, we tested the CuWO4:580 electrode with an electrolyte containing H2O2 as an electron donor. Hydrogen peroxide is much more easily oxidizable than water (+0.68 VSHE vs 1.23 VSHE for water) and has a higher reaction rate (H2O2 to H2O is a 2-hole process, while H2O to O2 is a 4-hole process).24 The current doubled to 0.67 mA cm–2 at 1.23 VSHE in the presence of H2O2 (Figure 5C). However, this increase is quite limited compared to BiVO4, for which photocurrent increases from a few μA to mA in contact with electron-donor-containing solutions.55,56 The moderate photocurrent increase in CuWO4 suggests that the few holes reaching the photocatalyst surface can very efficiently undergo oxidation reactions (i.e., water or electron donor oxidation). This experimental evidence rules out sluggish surface electron transfer as the main performance bottleneck in these photoactive films and indicates that the low PEC efficiency of CuWO4 stems far from the surface catalytic sites.
To further check this hypothesis, we deposited a nickel–iron oxyhydroxide water oxidation catalyst on top of CuWO4. NiFeOx ad-layers usually enhance the PEC performance of semiconductors which are limited by poor water oxidation kinetics (e.g., BiVO4 or Ta3O5) or by low chemical stability (Si, or CdTe).54,57,58 PEC analyses showed no performance enhancement for the NiFeOx-modified CuWO4 photoanode compared to the bare one (Figure 5D). The negligible effect of the NiFeOx cocatalyst confirms the intrinsic activity of surface CuWO4 sites and that, therefore, photogenerated charge carriers recombine far from the semiconductor/electrolyte interface.
4. Discussion
The spin coating preparation technique employed here offers a simple way to tune the CuWO4 thickness and reach a desirable trade-off between visible light absorption and electron–hole separation, resulting in optimal PEC performances. The highest current density achieved (0.37 mA cm–2 with the 580 nm thick CuWO4 electrode) aligns with the benchmark literature reports (Table 2). The thickness of the best-performing photoanode (580 nm) is similar to that of transparent electrodes prepared by Tian et al. with a spin-coating synthesis (500 nm),48 suggesting that the optimal balance between absorption and charge separation in undoped, bulk, and polycrystalline CuWO4 is within 5–600 nm. Recently, facet control along the 100 crystal facet showed improved conductivity and enhanced current density (0.38 mA cm–2) in bulk 1.5 μm thick CuWO4 films.43
Nanostructuring provides efficient charge carrier transport.59 Indeed, CuWO4 electrodes with nanoflake morphology22,29,44−47,52 facilitate hole extraction from the oxide flakes because their 30–60 nm width is within the hole diffusion length in CuWO4. However, the film thickness (≥1 μm) exceeds electron diffusion in CuWO4 and likely limits the PEC performance to ∼0.4 mA cm–2.
Doping and mild reduction via hydrogenation of semiconductors offer further control of the defectivity and charge carrier density. For example, Mo doping in BiVO4 improves PEC performances by increasing electron mobility, disfavoring recombination, and enhancing charge separation.60,61 These strategies induce similar chemical and electronic effects in CuWO4 and translate into efficiency improvements (from 0.30 to 0.39 mA cm–2 for hydrogenation,44 from 0.35 to 0.62 mA cm–2 for Mo doping,22 from 0.27 to 0.42 mA cm–2 for Fe doping,49,50 from 0.38 to 0.57 mA cm–2 for fluorine doping).52
The moderate photocurrent increase observed with CuWO4 in the presence of hole scavengers such as H2O2 (Figure 5C) indicates that few holes reach the electrode/electrolyte interface, where they are rapidly consumed at surface catalytic sites.41 This could be behind the slight photocurrent increase reported in previous work with Co-based oxygen evolution cocatalysts (from 0.34 to 0.42 mA cm–2 with CoPi,45 from 0.32 to 0.54 with CoIrOx,47 and from 0.4 to 0.5 mA cm–2 with Co3O4).48 In this work, we observed that the PEC performance of CuWO4 photoanodes is unmodified after coating them with NiFeOx. The lack of change in photoactivity could rely on the ineffective junction between the two materials. These findings further support the fact that Co-based oxygen evolution catalysts are more suitable to enhance the activity of CuWO4 than Ni- and Fe-based ones. The simple synthesis reported here could offer a good platform to further enhance the CuWO4 performance via these synthetic and postsynthetic approaches.
5. Conclusions
CuWO4 photoanodes are good candidates for PEC water splitting applications. In fact, CuWO4 is very photostable and, compared with other semiconductor oxides, has a high intrinsic activity of the surface catalytic sites for water oxidation. On the other hand, PEC tests under front- and back-side irradiation confirm the low mobility of photoelectrons in CuWO4 and indicate that more than 90% of photogenerated charge carriers recombine in the bulk, which heavily limits the photon to current efficiency of CuWO4. The higher quantum efficiencies at low light intensities (e.g., IPCE or below 1 sun) indicate that charge separation becomes less efficient at high photogenerated charge carrier densities and that charge recombination occurs mainly in bulk at defects or interface states. Therefore, CuWO4 electrode optimization requires reducing this internal charge recombination through: (i) nanostructuring, to facilitate electron extraction toward FTO; (ii) defect engineering, to minimize the presence of recombination centers; and (iii) doping the crystalline structure, to act on the electronic states of the material and modulate its charge transport properties.
Acknowledgments
This work was supported by the Cariplo Foundation through the Project 2021-0664 entitled “Carbon dioxide conversion into energy-rich molecules with tailored catalysts” (CO2EnRich). I.G. acknowledges the European Union’s Horizon 2020 research and innovation programme under Marie Sklodowska-Curie grant (agreement no. 846107).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsaem.3c01608.
Field emission electron microscopy images, absorbance vs thickness plot, IPCE and IQE plot for the CuWO4-350 electrode, IPCE at 1.73 VSHE (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Hisatomi T.; Kubota J.; Domen K. Recent Advances in Semiconductors for Photocatalytic and Photoelectrochemical Water Splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. 10.1039/C3CS60378D. [DOI] [PubMed] [Google Scholar]
- Bard A. J.; Fox M. A. Artificial Photosynthesis: Solar Splitting of Water to Hydrogen and Oxygen. Acc. Chem. Res. 1995, 28, 141–145. 10.1021/ar00051a007. [DOI] [Google Scholar]
- Cowan A. J.; Barnett C. J.; Pendlebury S. R.; Barroso M.; Sivula K.; Grätzel M.; Durrant J. R.; Klug D. R. Activation Energies for the Rate-Limiting Step in Water Photooxidation by Nanostructured α-Fe2O3 Nd TiO2. J. Am. Chem. Soc. 2011, 133, 10134–10140. 10.1021/ja200800t. [DOI] [PubMed] [Google Scholar]
- Alexander B. D.; Kulesza P. J.; Rutkowska I.; Solarska R.; Augustynski J. Metal Oxide Photoanodes for Solar Hydrogen Production. J. Mater. Chem. 2008, 18, 2298–2303. 10.1039/b718644d. [DOI] [Google Scholar]
- Tilley S. D.; Cornuz M.; Sivula K.; Grätzel M. Light-Inducedwater Splitting with Hematite: Improved Nanostructure and Iridium Oxide Catalysis. Angew. Chem., Int. Ed. 2010, 49, 6405–6408. 10.1002/anie.201003110. [DOI] [PubMed] [Google Scholar]
- Wang H.; Lindgren T.; He J.; Hagfeldt A.; Lindquist S. E. Photolelectrochemistry of Nanostructured WO3 Thin Film Electrodes for Water Oxidation: Mechanism of Electron Transport. J. Phys. Chem. B 2000, 104, 5686–5696. 10.1021/jp0002751. [DOI] [Google Scholar]
- Reyes-Gil K. R.; Wiggenhorn C.; Brunschwig B. S.; Lewis N. S. Comparison between the Quantum Yields of Compact and Porous WO3 Photoanodes. J. Phys. Chem. C 2013, 117, 14947–14957. 10.1021/jp4025624. [DOI] [Google Scholar]
- Li H.; Lin C.; Yang Y.; Dong C.; Min Y.; Shi X.; Wang L.; Lu S.; Zhang K. Boosting Reactive Oxygen Species Generation Using Inter-Facet Edge Rich WO3 Arrays for Photoelectrochemical Conversion. Angew. Chem., Int. Ed. 2023, 62, e202210804 10.1002/anie.202210804. [DOI] [PubMed] [Google Scholar]
- Prévot M. S.; Sivula K. Photoelectrochemical Tandem Cells for Solar Water Splitting. J. Phys. Chem. C 2013, 117, 17879–17893. 10.1021/jp405291g. [DOI] [Google Scholar]
- Huang Z.; Lin Y.; Xiang X.; Rodríguez-Córdoba W.; McDonald K. J.; Hagen K. S.; Choi K.-S.; Brunschwig B. S.; Musaev D. G.; Hill C. L.; Wang D.; Lian T. In Situ Probe of Photocarrier Dynamics in Water-Splitting Hematite (α-Fe2O3) Electrodes. Energy Environ. Sci. 2012, 5, 8923. 10.1039/c2ee22681b. [DOI] [Google Scholar]
- Cherepy N. J.; Liston D. B.; Lovejoy J. A.; Deng H.; Zhang J. Z. Ultrafast Studies of Photoexcited Electron Dynamics in γ- and r-Fe2O3 Semiconductor Nanoparticles. J. Phys. Chem. B 1998, 5647, 770–776. 10.1021/jp973149e. [DOI] [Google Scholar]
- Kim T. W.; Choi K. S. Nanoporous BiVO4 Photoanodes with Dual-Layer Oxygen Evolution Catalysts for Solar Water Splitting. Science 2014, 343, 990–994. 10.1126/science.1246913. [DOI] [PubMed] [Google Scholar]
- Park Y.; Mc Donald K. J.; Choi K. S. Progress in Bismuth Vanadate Photoanodes for Use in Solar Water Oxidation. Chem. Soc. Rev. 2013, 42, 2321–2337. 10.1039/C2CS35260E. [DOI] [PubMed] [Google Scholar]
- Polo A.; Lhermitte C. R.; Dozzi M. V.; Selli E.; Sivula K. Hydrogenation of ZnFe2O4 Flat Films: Effects of the Pre-Annealing Temperature on the Photoanodes Efficiency for Water Oxidation. Surfaces 2020, 3, 93–104. 10.3390/surfaces3010009. [DOI] [Google Scholar]
- Lee D. K.; Lee D.; Lumley M. A.; Choi K. S. Progress on Ternary Oxide-Based Photoanodes for Use in Photoelectrochemical Cells for Solar Water Splitting. Chem. Soc. Rev. 2019, 48, 2126–2157. 10.1039/C8CS00761F. [DOI] [PubMed] [Google Scholar]
- Lin C.; Dong C.; Kim S.; Lu Y.; Wang Y.; Yu Z.; Gu Y.; Gu Z.; Lee D. K.; Zhang K.; Park J. H. Photo-Electrochemical Glycerol Conversion over a Mie Scattering Effect Enhanced Porous BiVO4 Photoanode. Adv. Mater. 2023, 35, 2209955 10.1002/adma.202209955. [DOI] [PubMed] [Google Scholar]
- Pihosh Y.; Turkevych I.; Mawatari K.; Uemura J.; Kazoe Y.; Kosar S.; Makita K.; Sugaya T.; Matsui T.; Fujita D.; Tosa M.; Kondo M.; Kitamori T. Photocatalytic Generation of Hydrogen by Core-Shell WO3/BiVO4 Nanorods with Ultimate Water Splitting Efficiency. Sci. Rep. 2015, 5, 11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H.; Jang J. W.; Jo Y. H.; Abdi F. F.; Lee Y. H.; Van De Krol R.; Lee J. S. Hetero-Type Dual Photoanodes for Unbiased Solar Water Splitting with Extended Light Harvesting. Nat. Commun. 2016, 7, 13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard N.; Chang Y.; Deangelis A.; Higgins S.; Braun A. A Nanocomposite Photoelectrode Made of 2.2 EV Band Gap Copper Tungstate (CuWO4) and Multi-Wall Carbon Nanotubes for Solar-Assisted Water Splitting. Int. J. Hydrogen Energy 2013, 38, 3166–3176. 10.1016/j.ijhydene.2012.12.104. [DOI] [Google Scholar]
- Polo A.; Nomellini C.; Grigioni I.; Dozzi M. V.; Selli E. Effective Visible Light Exploitation by Copper Molybdo-Tungstate Photoanodes. ACS Appl. Energy Mater. 2020, 3, 6956–6964. 10.1021/acsaem.0c01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. C.; Ping Y.; Galli G. A.; Choi K. S. Synthesis, Photoelectrochemical Properties, and First Principles Study of n-Type CuW1-XMoxO4 Electrodes Showing Enhanced Visible Light Absorption. Energy Environ. Sci. 2013, 6, 2440–2446. 10.1039/c3ee40827b. [DOI] [Google Scholar]
- Yang J.; Li C.; Diao P. Molybdenum Doped CuWO4 Nanoflake Array Films as an Efficient Photoanode for Solar Water Splitting. Electrochim. Acta 2019, 308, 195–205. 10.1016/j.electacta.2019.04.044. [DOI] [Google Scholar]
- Lhermitte C. R.; Bartlett B. M. Advancing the Chemistry of CuWO4 for Photoelectrochemical Water Oxidation. Acc. Chem. Res. 2016, 49, 1121–1129. 10.1021/acs.accounts.6b00045. [DOI] [PubMed] [Google Scholar]
- Uemura Y.; Ismail A. S. M.; Park S. H.; Kwon S.; Kim M.; Niwa Y.; Wadati H.; Elnaggar H.; Frati F.; Haarman T.; Höppel N.; Huse N.; Hirata Y.; Zhang Y.; Yamagami K.; Yamamoto S.; Matsuda I.; Katayama T.; Togashi T.; Owada S.; Yabashi M.; Halisdemir U.; Koster G.; Yokoyama T.; Weckhuysen B. M.; De Groot F. M. F. Femtosecond Charge Density Modulations in Photoexcited CuWO4. J. Phys. Chem. C 2021, 125, 7329–7336. 10.1021/acs.jpcc.0c10525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yourey J. E.; Bartlett B. M. Electrochemical Deposition and Photoelectrochemistry of CuWO4, a Promising Photoanode for Water Oxidation. J. Mater. Chem. 2011, 21, 7651–7660. 10.1039/c1jm11259g. [DOI] [Google Scholar]
- Grigioni I.; Abdellah M.; Corti A.; Dozzi M. V.; Hammarström L.; Selli E. Photoinduced Charge-Transfer Dynamics in WO3/BiVO4 Photoanodes Probed through Midinfrared Transient Absorption Spectroscopy. J. Am. Chem. Soc. 2018, 140, 14042–14045. 10.1021/jacs.8b08309. [DOI] [PubMed] [Google Scholar]
- Selim S.; Francàs L.; García-Tecedor M.; Corby S.; Blackman C.; Gimenez S.; Durrant J. R.; Kafizas A. WO3/BiVO4: Impact of Charge Separation at the Timescale of Water Oxidation. Chem. Sci. 2019, 10, 2643–2652. 10.1039/C8SC04679D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilli S. K.; Deutsch T. G.; Furtak T. E.; Brown L. D.; Turner J. A.; Herring A. M. BiVO4/CuWO4 Heterojunction Photoanodes for Efficient Solar Driven Water Oxidation. Phys. Chem. Chem. Phys. 2013, 15, 3273–3278. 10.1039/c2cp44577h. [DOI] [PubMed] [Google Scholar]
- Ye W.; Chen F.; Zhao F.; Han N.; Li Y. CuWO4 Nanoflake Array-Based Single-Junction and Heterojunction Photoanodes for Photoelectrochemical Water Oxidation. ACS Appl. Mater. Interfaces 2016, 8, 9211–9217. 10.1021/acsami.6b03176. [DOI] [PubMed] [Google Scholar]
- Grigioni I.; Corti A.; Dozzi M. V.; Selli E. Photoactivity and Stability of WO3/BiVO4 Photoanodes: Effects of the Contact Electrolyte and of Ni/Fe Oxyhydroxide Protection. J. Phys. Chem. C 2018, 122, 13969–13978. 10.1021/acs.jpcc.8b01112. [DOI] [Google Scholar]
- Selim S.; Francàs L.; García-Tecedor M.; Corby S.; Blackman C.; Gimenez S.; Durrant J. R.; Kafizas A. WO3/BiVO4 : Impact of Charge Separation at the Timescale of Water Oxidation. Chem. Sci. 2019, 10, 2643–2652. 10.1039/C8SC04679D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigioni I.; Dozzi M. V.; Selli E. Photoinduced Electron Transfer in WO3/BiVO4 Heterojunction Photoanodes: Effects of the WO3 Layer Thickness. J. Phys.: Condens. Matter 2020, 32, 014001 10.1088/1361-648X/ab440b. [DOI] [PubMed] [Google Scholar]
- Grigioni I.; Polo A.; Dozzi M. V.; Ganzer L.; Bozzini B.; Cerullo G.; Selli E. Ultrafast Charge Carrier Dynamics in CuWO4 Photoanodes. J. Phys. Chem. C 2021, 125, 5692–5699. 10.1021/acs.jpcc.0c11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L.; Zhao J.; Li H.; Park J.; Cho I. S.; Han H. S.; Zheng X. One-Step Hydrothermal Deposition of Ni:FeOOH onto Photoanodes for Enhanced Water Oxidation. ACS Energy Lett. 2016, 1, 624–632. 10.1021/acsenergylett.6b00303. [DOI] [Google Scholar]
- Thang H. V.; Albanese E.; Pacchioni G. Electronic Structure of CuWO4 : Dielectric-Dependent, Self-Consistent Hybrid Functional Study of a Mott-Hubbard Type Insulator. J. Phys.: Condens. Matter 2019, 31, 145503 10.1088/1361-648X/aaff3e. [DOI] [PubMed] [Google Scholar]
- Sorenson S.; Driscoll E.; Haghighat S.; Dawlaty J. M. Ultrafast Carrier Dynamics in Hematite Films: The Role of Photoexcited Electrons in the Transient Optical Response. J. Phys. Chem. C 2014, 118, 23621–23626. 10.1021/jp508273f. [DOI] [Google Scholar]
- Dey S.; Ricciardo R. A.; Cuthbert H. L.; Woodward P. M. Metal-to-Metal Charge Transfer in AWO4 (A = Mg, Mn, Co, Ni, Cu, or Zn) Compounds with the Wolframite Structure. Inorg. Chem. 2014, 53, 4394–4399. 10.1021/ic4031798. [DOI] [PubMed] [Google Scholar]
- Grigioni I.; Polo A.; Dozzi M. V.; Stamplecoskie K. G.; Jara D. H.; Kamat P. V.; Selli E. Enhanced Charge Carrier Separation in WO3/BiVO4 Photoanodes Achieved via Light Absorption in the BiVO4 Layer. ACS Appl. Energy Mater. 2022, 5, 13142–13148. 10.1021/acsaem.2c02597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y.; Tsubota T.; Mooij L. P. A.; Van De Krol R. Highly Improved Quantum Efficiencies for Thin Film BiVO4 Photoanodes. J. Phys. Chem. C 2011, 115, 17594–17598. 10.1021/jp203004v. [DOI] [Google Scholar]
- Abdi F. F.; Van De Krol R. Nature and Light Dependence of Bulk Recombination in Co-Pi-Catalyzed BiVO4 Photoanodes. J. Phys. Chem. C 2012, 116, 9398–9404. 10.1021/jp3007552. [DOI] [Google Scholar]
- Gao Y.; Hamann T. W. Quantitative Hole Collection for Photoelectrochemical Water Oxidation with CuWO4. Chem. Commun. 2017, 53, 1285–1288. 10.1039/C6CC09029J. [DOI] [PubMed] [Google Scholar]
- Shadabipour P.; Raithel A. L.; Hamann T. W. Charge-Carrier Dynamics at the CuWO4/Electrocatalyst Interface for Photoelectrochemical Water Oxidation. ACS Appl. Mater. Interfaces 2020, 12, 50592–50599. 10.1021/acsami.0c14705. [DOI] [PubMed] [Google Scholar]
- Chen L.; Li W.; Qiu W.; He G.; Wang K.; Liu Y.; Wu Q.; Li J. Oriented CuWO4 Films for Improved Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2022, 14, 47737–47746. 10.1021/acsami.2c13002. [DOI] [PubMed] [Google Scholar]
- Hu D.; Diao P.; Xu D.; Xia M.; Gu Y.; Wu Q.; Li C.; Yang S. Copper(II) Tungstate Nanoflake Array Films: Sacrificial Template Synthesis, Hydrogen Treatment, and Their Application as Photoanodes in Solar Water Splitting. Nanoscale 2016, 8, 5892–5901. 10.1039/C5NR09210H. [DOI] [PubMed] [Google Scholar]
- Li C.; Guo B.; Peng B.; Yue C.; Diao P. Copper Tungstate (CuWO4) Nanoflakes Coupled with Cobalt Phosphate (Co-Pi) for Effective Photoelectrochemical Water Splitting. Int. J. Electrochem. Sci. 2019, 14, 9017–9029. 10.20964/2019.09.74. [DOI] [Google Scholar]
- Liu Z.; Song Q.; Zhou M.; Guo Z.; Kang J.; Yan H. Synergistic Enhancement of Charge Management and Surface Reaction Kinetics by Spatially Separated Cocatalysts and p-n Heterojunctions in Pt/CuWO4/Co3O4 Photoanode. Chem. Eng. J. 2019, 374, 554–563. 10.1016/j.cej.2019.05.191. [DOI] [Google Scholar]
- Li C.; Diao P. Boosting the Activity and Stability of Copper Tungsten Nanoflakes toward Solar Water Oxidation by Iridium-Cobalt Phosphates Modification. Catalysts 2020, 10, 913 10.3390/catal10080913. [DOI] [Google Scholar]
- Tian C. M.; Jiang M.; Tang D.; Qiao L.; Xiao H. Y.; Oropeza F. E.; Hofmann J. P.; Hensen E. J. M.; Tadich A.; Li W.; Qi D. C.; Zhang K. H. L. Elucidating the Electronic Structure of CuWO4 Thin Films for Enhanced Photoelectrochemical Water Splitting. J. Mater. Chem. A 2019, 7, 11895–11907. 10.1039/C8TA12070F. [DOI] [Google Scholar]
- Sun Y.; Du F.; Xie D.; Yang D.; Jiao Y.; Jia L.; Fan H. Improved Water Oxidation via Fe Doping of CuWO4 Photoanodes: Influence of the Fe Source and Concentration. Chin. Phys. B 2020, 29, 127801 10.1088/1674-1056/aba9cb/meta. [DOI] [Google Scholar]
- Bohra D.; Smith W. A. Improved Charge Separation via Fe-Doping of Copper Tungstate Photoanodes. Phys. Chem. Chem. Phys. 2015, 17, 9857–9866. 10.1039/C4CP05565A. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Yilmaz P.; Ansari J. O.; Khan F. F.; Binions R.; Krause S.; Dunn S. Incorporation of Ag Nanowires in CuWO4 for Improved Visible Light-Induced Photoanode Performance. J. Mater. Chem. A 2015, 3, 9638–9644. 10.1039/C4TA07213H. [DOI] [Google Scholar]
- Li C.; Diao P. Fluorine Doped Copper Tungsten Nanoflakes with Enhanced Charge Separation for Efficient Photoelectrochemical Water Oxidation. Electrochim. Acta 2020, 352, 136471 10.1016/j.electacta.2020.136471. [DOI] [Google Scholar]
- Nair V.; Perkins C. L.; Lin Q.; Law M. Textured Nanoporous Mo:BiVO4 Photoanodes with High Charge Transport and Charge Transfer Quantum Efficiencies for Oxygen Evolution. Energy Environ. Sci. 2016, 9, 1412–1429. 10.1039/C6EE00129G. [DOI] [Google Scholar]
- Seabold J. A.; Choi K. S. Efficient and Stable Photo-Oxidation of Water by a Bismuth Vanadate Photoanode Coupled with an Iron Oxyhydroxide Oxygen Evolution Catalyst. J. Am. Chem. Soc. 2012, 134, 2186–2192. 10.1021/ja209001d. [DOI] [PubMed] [Google Scholar]
- Polo A.; Grigioni I.; Magni M.; Facibeni A.; Dozzi M. V.; Selli E. Unravelling the Bulk and Interfacial Charge Transfer Effects of Molybdenum Doping in BiVO4 Photoanodes. Appl. Surf. Sci. 2021, 556, 149759 10.1016/j.apsusc.2021.149759. [DOI] [Google Scholar]
- Grigioni I.; Stamplecoskie K. G.; Selli E.; Kamat P. V. Dynamics of Photogenerated Charge Carriers in WO3/BiVO4 Heterojunction Photoanodes. J. Phys. Chem. C 2015, 119, 20792–20800. 10.1021/acs.jpcc.5b05128. [DOI] [Google Scholar]
- Zhao J.; Cai L.; Li H.; Shi X.; Zheng X. Stabilizing Silicon Photocathodes by Solution-Deposited Ni-Fe Layered Double Hydroxide for Efficient Hydrogen Evolution in Alkaline Media. ACS Energy Lett. 2017, 2, 1939–1946. 10.1021/acsenergylett.7b00597. [DOI] [Google Scholar]
- Higashi T.; Nishiyama H.; Suzuki Y.; Sasaki Y.; Hisatomi T.; Katayama M.; Minegishi T.; Seki K.; Yamada T.; Domen K. Transparent Ta3N5 Photoanodes for Efficient Oxygen Evolution toward the Development of Tandem Cells. Angew. Chem., Int. Ed. 2019, 58 (8), 2300–2304. 10.1002/anie.201812081. [DOI] [PubMed] [Google Scholar]
- Su J.; Feng X.; Sloppy J. D.; Guo L.; Grimes C. A. Vertically Aligned WO3 Nanowire Arrays Grown Directly on Transparent Conducting Oxide Coated Glass: Synthesis and Photoelectrochemical Properties. Nano Lett. 2011, 11, 203–208. 10.1021/nl1034573. [DOI] [PubMed] [Google Scholar]
- Polo A.; Dozzi M. V.; Grigioni I.; Lhermitte C.; Plainpan N.; Moretti L.; Cerullo G.; Sivula K.; Selli E. Multiple Effects Induced by Mo6+ Doping in BiVO4 Photoanodes. Sol. RRL 2022, 2200349 10.1002/solr.202200349. [DOI] [Google Scholar]
- Ding K.; Chen B.; Fang Z.; Zhang Y.; Chen Z. Why the Photocatalytic Activity of Mo-Doped BiVO4 Is Enhanced: A Comprehensive Density Functional Study. Phys. Chem. Chem. Phys. 2014, 16, 13465–13476. 10.1039/c4cp01350f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.