Abstract
Background:
Parent-child separation has been associated with negative mental health across childhood and adulthood, yet little is known about the long-term impacts for cardiovascular health. This systematic review synthesized and evaluated the quality of the literature examining the association between exposures to parent-child separation and cardiometabolic outcomes in adulthood.
Methods:
Following a registered protocol, online databases (Pubmed, PsycInfo, and Web of Science) were searched for relevant studies. Studies were included if they (a) defined the exposure before age 18 as institutionalization, foster care placement, parental incarceration, separation due to parents migrating for economic reasons, or asylum and war; and (b) quantified the association between parent-child separation and cardiometabolic events and diagnoses (e.g., coronary heart disease, diabetes) and risk factors (e.g., body mass index, fat distribution, serum-based metabolic markers, inflammatory markers in adulthood (≥ age 18). Studies lacking an unexposed comparison group were excluded. The risk for bias in each study was assessed with a modified Newcastle-Ottawa Scale.
Results:
Of the 1938 studies identified, 13 met our inclusion criteria. Two of the four studies examining associations between parent-child separation and cardiometabolic events and diagnoses found positive associations with coronary heart disease and diabetes. Amongst the 13 studies examining associations with any type of adult cardiometabolic risk factors, eight of these studies reported at least one positive association. Sub-analyses considering separate reasons for parent-child separation provided clearer insights: War evacuation was associated with hypertension and high blood pressure across four studies from the same cohort; out-of home care experiences largely evidenced null results across five different studies, and two studies on parental incarceration suggested positive associations with elevated inflammation, BMI and blood pressure.
Conclusions:
The connections between parent-child separation and adult cardiometabolic outcomes and risk factors are currently inconsistent. The results may depend on the reason for separation, age of assessment, analytic differences and other psychosocial variables that are often unmeasured in this literature.
Keywords: cardiovascular disease, metabolic disease, metabolic syndrome, parent-child separation, war evacuation, foster care, temporary care, parental incarceration, childhood
Parent-child separation is common and occurs for various reasons, including early institutionalization, placement into foster care, parental incarceration, war evacuation, asylum seeking, and parental economic migration.1–3 Currently, 3.18 to 9.42 million children reside in institutions under depriving conditions worldwide.4 Considering data from the US, each year thousands of children are removed from their parents due to maltreatment, parental loss or unmet needs. In 2021, there were 407,000 children in foster care5, one in twelve children have experienced parental incarceration6, and the number of unaccompanied migrant children seeking asylum peaked.7 Elsewhere, in rural regions of countries including China, Ecuador, and South Africa, 33–40% of children have been separated from one or more parent due to economic migration.2 Importantly, recent systematic reviews indicate that parent-child separation is associated with many psychosocial risk factors (e.g., anxiety, depression, low social support) and poor health behaviors across childhood1–3 and adulthood8. Given that these psychosocial factors are bi-directionally related to the development of cardiometabolic risk factors (e.g., elevated inflammation and blood pressure)9 10, it is important to examine long-term cardiometabolic health outcomes linked to parent-child separation. To date, there has not been a systematic review on studies examining the long-term effects of parent-child separation on cardiometabolic health in adulthood.
Cardiovascular and metabolic diseases are the first and seventh leading causes of death worldwide, respectively.11 There is now recognition that adverse childhood experiences, reflecting a wide range of traumas, can exert cumulative and dose-dependent effects on elevated cardiometabolic risks in adulthood.12 13 Within this work, studies have largely focused on adverse aspects of the childhood family environments.14 15 Less is known about parent-child separation, which reflects an absence of parent-child relationship, and its effect on developmental pathways that shape cardiometabolic health.
Developmental models suggest that elevated cardiometabolic risk among children who experienced separation from parents could be a direct, latent effect that appears after years of disrupted responsivity of biological systems16, and/or may be downstream consequences of maladaptive health behaviors.17–20 Indeed, biobehavioral risk factors that shape elevated cardiometabolic risks, including patterns of stunted growth21 22 and dysregulated neuroendocrine systems23 24 are already apparent in childhood amongst individuals with a history of institutional care. Similarly, left-behind children in China show disrupted neuroendocrine functioning25, stunted growth, unhealthy food preferences, lower physical activity, and higher rates of smoking and alcohol consumption, as documented in a recent review.26 Importantly, not all individuals who experience parental separation display the same health risks. Various contextual factors are known to modify risk and resilient health trajectories among individuals with a history of parent-child separation. These factors include the timing and duration of parent-child separation27–29, as well as ongoing experiences such as the quality of later caregiving experiences30 31 and later stressful events32. Additionally, individual factors, such as sex, have been associated with differential risks among children separated from parent, though these reports are inconsistent.33–35
The main objective of the present review was to systematically evaluate the evidence on the association between parent-child separation and adult cardiometabolic events and diagnoses (e.g., hospitalization, morbidity, and mortality) and cardiometabolic risk factors (e.g., biomarkers such as blood pressure, body mass index, levels of inflammation) in adulthood. For each study, we evaluated the robustness and quality. We also summarized the additional findings on individual or contextual factors, such as sex and age at and duration of separation, that could modify the associations.
Method
Database Search
This review followed PRISMA guidelines for systematic reviews and meta-analyses.40 The review and protocol were registered with PROSPERO (CRD42021246022), and our search strategies were designed by a medical librarian (C.M.). To identify relevant articles, we searched Pubmed (National Library of Medicine, NCBI), PsycInfo (American Psychological Association EBSCO host), and Web of Science Core Collection (Clarivate) from inception through August 11, 2022. Controlled vocabulary terms were included when available and appropriate (see full search strategies in Appendix A).
Inclusion and exclusion criteria.
To be eligible for inclusion, we required that studies include a quantitative description of an association between parent-child separation and cardiometabolic outcomes in adulthood using an observational, case control, or intervention design. Eligible separation exposures included early institutionalization, placement into foster care, parental incarceration, separation when parents migrate for economic reasons, separation due to asylum and war. Studies were required to have at least one outcome within the following categories: cardiovascular or metabolic disease outcomes and death, and cardiometabolic risk factors, such as body mass index, fat distribution, blood pressure, or serum-based metabolic risk markers and inflammatory biomarkers. Studies were excluded if: less than 50% of the participants were 18 years or older at outcome assessment, the study lacked an unexposed comparison group, or the report was not in English. Furthermore, we excluded studies if the measured exposure was parental marital separation or divorce, or parental death, as we deemed these exposures to be qualitatively distinct from the selected exposures. We did not restrict articles based on geography or year of publication.
Data Extraction
Independent reviewers conducted abstract and full-text screening using Covidence, with two individuals (A.T., A.Y., K.E., K.P., S.O., R.K., N.E., N.S.) viewing each record. Disagreements on eligibility were discussed and addressed via group consensus. For each of the identified qualifying studies, two reviewers (A.T., R.K., L.B., K.E.) then extracted data related to study design, sample size, sample characteristics (e.g., percent male, mean age, race/ethnicity), exposure (i.e., reason for parent-child separation) and age of separation, cardiometabolic outcome or risk factor, covariates, tested moderators and mediators, measure(s) of association, and results. These same coauthors conducted citation searches of the articles retrieved via database searches to identify additional articles.
Assessment of Study Quality and Risk of Bias
The quality and risk of bias of each study were assessed using a modified version of the Newcastle–Ottawa Scale for cohort studies (Appendix B).41 This scale consists of 8 items and uses a star system to provide descriptive information on three quality domains: (a) selection of study groups (e.g., representativeness of the group exposed to parent-child separation); (b) comparability of study groups (e.g., whether the non-exposed group matched on geographic location, age, and sex, and whether analyses adjusted for covariates); and (c) ascertainment of the exposure and outcome (e.g., whether the outcomes were objectively assessed or subjective reports, the temporal interval between exposure and outcome, and sample attrition). Two independent reviewers examined each study (A.T paired with either L.B., R.K., or K.E.) and discrepancies were resolved through discussions with a third reviewer (N.S.). Each study received up to a maximum of 9 stars.
Results
Search Results and Included Studies
Our search identified 1938 unique studies for screening. Full texts were further reviewed for 33 studies; of these, 13 met inclusion criteria (Figure 1). Table 1 summarizes the studies, characteristics of samples, designs, and methods. There were 10 cohort studies: four studies were part of the Helsinki birth cohort29 35 42 43, two studies used data from the National Longitudinal Study of Adolescent Health (AddHealth)34 44, one study used data from the 1970 British Cohort Study45, two study used data from the National Child Development Study46 47, one study compared data from the Midwest Evaluation of the Adult Functioning of Former Foster Youth to an age-matched non-exposed comparison sample from AddHealth.44 Of these 10 cohort studies, 9 used prospective reports or record data to measure the exposure of separation 29 35 42–47 and only one used retrospective reports.34 Cross-sectional designs were used in 3 studies.48–50 The sample sizes ranged from 44 to 12,915 participants (median= 2514). Four studies took place in the US, four in Finland, three in the UK, one in Australia, and one in Luxembourg. The nature of parent-child separation varied and included: Out-of-home care (n=5)45–47 49 50, foster care (n=1)44, war evacuation (n=3)29 35 42, parental incarceration (n=2)34 51, multiple of these exposures (n=1)43, and other reasons (e.g., parental desertion and later adoption) (n=1).48 The age of exposure ranged from age 0.6 months to 17 years among the prospective studies28–30 34 35 42–46 51–53, and from 0 months to 18 years among the cross-sectional studies.48–50 The outcomes were assessed in young adulthood (18–34 years, n= 4)34 44 48 51, adulthood (35 to 59 years, n= 3)45–47, older adulthood (60 years and above, n=4)29 35 42 43, or across a large age range (18–65+ years, n= 2).49 50
Figure 1.
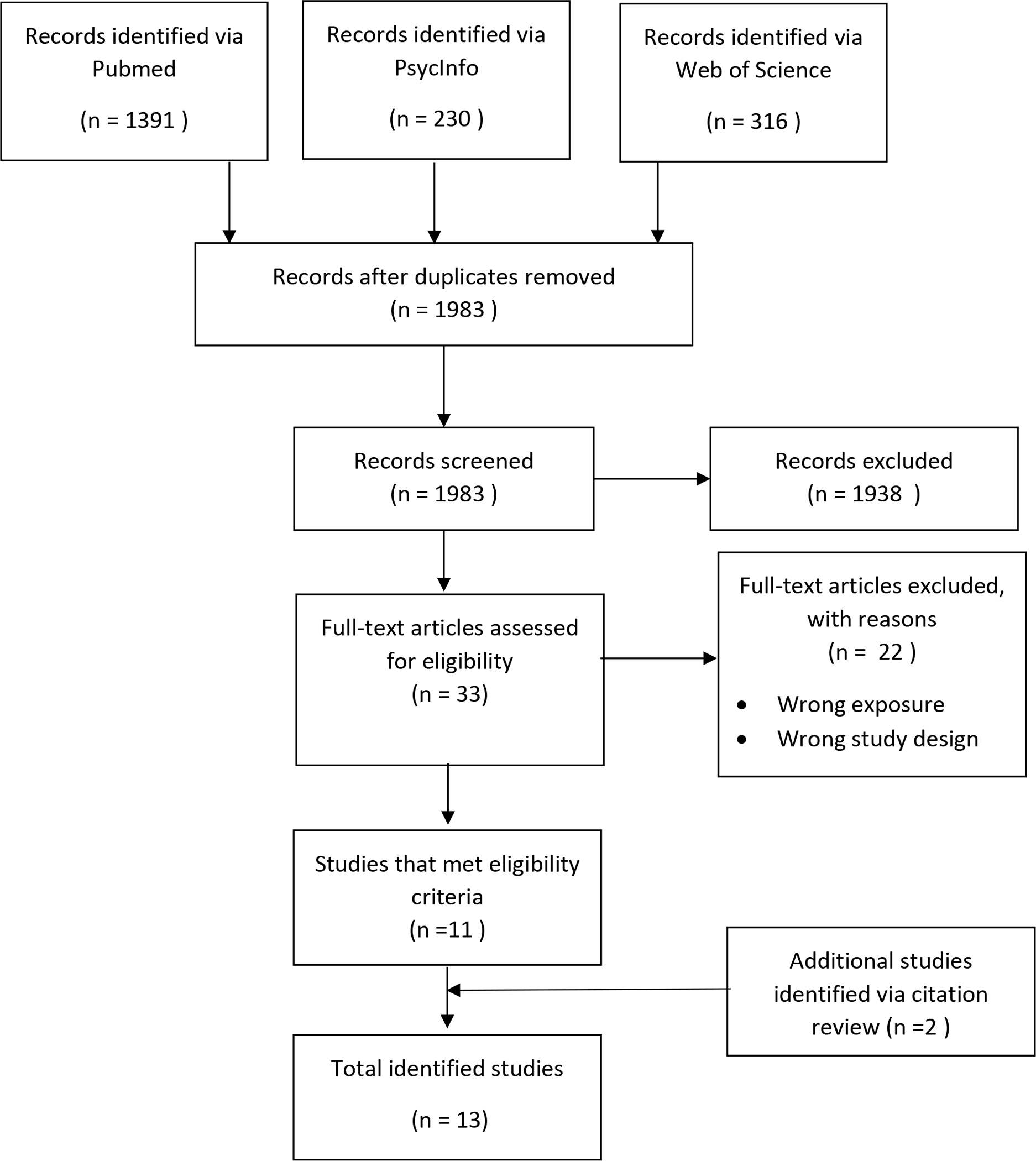
PRISMA diagram of study selection.
Table 1.
Summary of studies, characteristics of samples, designs, and methods
| Number of studies | % | |
|---|---|---|
|
| ||
| Year of publication | ||
| Before 2010 | 2 | 15.4% |
| 2010–2015 | 4 | 30.8% |
| 2016–2022 | 7 | 53.8% |
| Study design | ||
| Cohort studies with prospective measures of exposures | 9 | 69.2% |
| Chort studies with retrospective measures of exposures | 1 | 7.7% |
| Cross-sectional studies with retrospective measures of exposures | 3 | 23.1% |
| Sample size | ||
| N< 100 | 1 | 7.7% |
| 100> N <999 | 1 | 7.7% |
| N > 1000 | 11 | 84.6% |
| Location of study | ||
| United States | 4 | 30.8% |
| Europe | 8 | 61.5% |
| Australia | 1 | 7.7% |
| Sample age at adult outcome assessment | ||
| Young adulthood (ages 18–34) | 4 | 30.8% |
| Adulthood to middle adulthood (ages 35–59) | 3 | 23.1% |
| Older adulthood (ages 60+) | 4 | 30.8% |
| Large range in adulthood (between 18–65) | 2 | 15.4% |
| Type of parent-child separation | ||
| War evacuation | 3 | 23.1% |
| Foster care, out of home care, temporary care, adoption, and unspecified separation | 7 | 53.8% |
| Parental incarceration | 2 | 15.4% |
| Multiple | 1 | 7.7% |
| Method to measure parent-child separation | ||
| Self-report | 7 | 53.8% |
| Record | 1 | 7.7% |
| Both | 5 | 38.5% |
Quality.
Overall, the quality of studies was high with a mean quality rating of 6.31 (SD=2.36) (Table 2; see ratings of individual studies in Appendix C). Ten of the studies included representative exposed groups. All 13 studies used control groups that were matched on geographic location, age, and sex, and the majority (n=12) adjusted for sex and age in the adult assessment in analyses; of these, 5 studies additionally adjusted for early childhood socio-economic status (see Tables 3 and 4). Nine of the 13 studies included ascertained exposures of parent-child separation, using record data (n=1)42 or a combination of record and self-report (n=5)29 35 44 47 54; the other studies used self-report alone (n=7).34 45 46 48–51 In ascertaining outcomes, the majority (n=10) used objective assessments of cardiometabolic events (e.g., hospitalization, mortality) or diagnoses from records, or biomarkers (see Tables 3 and 4). Furthermore, 6 of the 9 cohort studies with prospective measures of the exposure and follow-up either had low attrition, included statistical descriptions ensuring that the resulting sample was representative of the original cohort, or included a random sampling technique to select a subset for further assessments.
Table 2.
Summary of quality ratings for included studies.
| M (SD) | N (%) | |
|---|---|---|
|
| ||
| Overall quality rating (possible range: 0 to 9) | 6.31 (2.36) | |
| Representative exposed group | 10 (76.9%) | |
| Representative non-exposed group (e.g., matched for age, sex, location) | 13 (100%) | |
| Ascertained exposure (e.g., record data) | 9 (69.2%) | |
| Outcome was not present at the beginninga | 10 (76.9%) | |
| Comparability of cohorts: controlled for basic variables (e.g., sex, age) | 12 (92.3%) | |
| Comparability of cohorts: controlled for additional early childhood experiences (e.g., childhood SES) | 5 (38.5%) | |
| Rigorous outcome assessment (non-self report and/or record linkage) | 10 (76.9%) | |
| Contains some longitudinal follow-up | 9 (69.2%) | |
| Adequate follow-up (e.g., complete follow-up or ensured representativeness of resulting sample) | 6 (46.2%) | |
Note. See Appendices B and C for details about each item and ratings of the individual study.
Longitudinal and/or screened for cardiometabolic conditions.
Table 3.
Summary of findings from studies examining metabolic and cardiovascular diagnoses and events.
| Study | Design | Sample description | Type of separation | Type of outcomes & Age(s) at outcome | Covariates | Findings (main effects, moderation by sex, age at and duration of separation) | Effect size(s) for main effects |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Ahrens et al., 2014 | Prospective | Sub-sample from the Midwest Evaluation of the Adult Functioning of Former Foster Youth (n= 596), and a non-exposed, economically-secure group matched for age (n=1461) drawn from the National Longitudinal Study of Adolescent Health (AddHealth) | Foster care; self-port and record | Diabetes; Self-report Age range: 25–26 | Sex Race, ethnicity Age at adult assessment Attained education level in adulthood Economic insecurity at adult assessment Past or current pregnancy | No difference in reports of diabetes between foster care group and economically-secure control group. Moderation by sex: none. | NA |
| Alastalo et al., 2009 | Prospective | Simple random sample of the Helsinki Birth Cohort: Separated group (n=320), non-separated group (n=1683) | War evacuation; self-report and record | Cardiovascular disease Type 2 diabetes Chronic diseases (via meds); self-report use of medication and physician diagnoses Mean age: 62 | Sex Father’s occupation status in childhood Age at adult assessment Attained education level in adulthood | War evacuees displayed higher risk for any cardiovascular disease and type 2 diabestes. Moderation by sex: not significant. Effects of age at evacuation: not significant. Effects of duration of evacuation: Positive relations between longer period of evacuation and greater cardiovascular disease risks. | ORs= 1.5 (CI= 1.0 to 2.0) to 1.7 (CI=1.1, 2.4) |
| Alastalo et al., 2012 | Prospective | The Helsinki Birth Cohort: separated group (n=1726), non-separated group (n=11189) | War evacuation; record | Medication for CHD Hospital admission or death for coronary event or stroke Cardiovascular mortality; record Mean age: 64 | Analyses were stratified by year of birth and by sex Adjusted for father’s occupation status in childhood | War evacuation associated with greater use of medication for CHD, though no association with the other outcomes. Effects of age at evacuation: Early childhood period (ages 4–7) associated with greater risk for CHD medication only. Effects of duration of separation: Risk for CHD was higher if subjects were separated 1–3 years compared to non-separated. Effect not seen for those separated for <1 or >3 years. | HRs= .94 (CI= .72, 1.21) to 1.29 (CI= 1.04, 1.59) |
| Xie et al., 2021 | Prospective | Data from the National Child Development Study in the UK: Separated group (n=420), non-separated group (n=10740) | Out of home care; parent-report | Diabetes; self-report Mean age: 42 | Sex Childhood SES Health conditions | No differences in self-report daibetes in bivariate and adjusted analyses. | OR= .50 (CI= .10, 1.5) |
Note. CHD= Coronary heart disease. ORs= odds ratios. HRs= hazard ratios. CI= confidence interval. NA= not available because the effect sizes and/or information that could be used to calculate effect sizes are not available in the article.
Table 4.
Summary of findings for cardiometabolic risk factors.
| Study | Design | Sample description | Type of separation | Type of cardiometabolic risk factors & Age(s) at outcome | Covariates | Findings (main effects, moderation by sex, age at and duration of separation) | Effect size(s) for main effects |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Ahrens et al., 2014 | Prospective | Sub-sample from the Midwest Evaluation of the Adult Functioning of Former Foster Youth (n= 596), and a non-exposed, economically-secure group matched for age (n=1461) drawn from the National Longitudinal Study of Adolescent Health (AddHealth) | Foster care; self-port and record | BMI Dyslipidemia Hypertension Any cardiovascular risk factor; Self-report Age range: 25–26 |
Sex Race, ethnicity Age at adult assessment Attained education level in adulthood Economic insecurity at adult assessment Past or current pregnancy |
Foster care group associated with higher BMI, hypertension, and having any cardiovascular risk factors compared to an economically-secure control group. Moderation by sex: Stronger relations among females in the foster care group. |
OR for any cardiovascular risk= 2.2 (CI= 1.76, 2.76) d for BMI= .23 |
| Boch et al., 2015 | Prospective | Data from AddHealth (Waves 1 and 4): separated group exposed to maternal incarceration (n=374), control group (n=11469); Separated group exposed to paternal incarceration (n=1257), non-separated group (n=9559) | Parental incarceration; Retrospective self-report | hs-CRP Age range: 24–34 |
Separate models run for young adult females and males Race and ethnicity Foreign birth Lived in two-biological-parent household Public assistance Parent education level Sibling in AddHealth Age at adult assessment Use of anti-inflammatory medication Ever been in jail/prison History of physical, sexual, and emotional abuse by caregiver before age 18 Current pregnancy Hormonal contraceptives Father died Subclinical conditions Infectious/inflammatory conditions |
Females exposed to paternal incarceration had elevated hs-CRP compared to females who did not experience paternal incarceration; no difference evident for males. Maternal incarceration was not significantly related to cardiometabolic outcomes in either females or males. |
OR in females for CRP: 1.24 (CI= .92, 1.65) to 1.37 (CI= 1.07, 1.76) OR in males for CRP: 1.08 (CI= .80, 1.45) to 1.27 (CI= .79, 2.05) |
| Alastalo et al., 2009 | Prospective | Simple random sample of the Helsinki Birth Cohort: Separated group (n=320), non-separated group (n=1683) | War evacuation; self-report and record | Total cholesterol HDL, LDL Triglycerides ApoA, ApoB Apoprotein(a) BMI Waist circumference Hypertension Blood pressure (SBP, DBP) Blood pressure medication; combination of self-report & measures Mean age: 62 |
Sex Father’s occupation status in childhood Age at adult assessment Attained education level in adulthood |
War evacuees displayed higher blood pressure, apoprotein(a), and hypertension. Moderation by sex: not significant. Effects of age at evacuation: not significant. Effects of duration of evacuation: not significant. |
d for blood pressure= .01 to .30 d for lipids= .00 to .14 Cohen’s d for BMI & wasit circumference= .06 to .08 |
| Alastalo et al., 2012 | Prospective | The Helsinki Birth Cohort: separated group (n=1726), non-separated group (n=11189) | War evacuation; record | Medication for hypertension; record Mean age: 64 |
Analyses were stratified by year of birth and by sex Adjusted for father’s occupation status in childhood |
War evacuation was not associated with greater use of medication for hypertension. Effects of age at evacuation: Toddlerhood period (ages 2–4) were associated with lower risk of hypertension. Effects of duration of separation: No association with medication for hypertension. |
OR for hypertension= .89 (CI= .78, 1.00) |
| Alastalo et al., 2013 | Prospective | Simple random sample of the Helsinki Birth Cohort restricted to the non-obese: Separated group (n=192), non-separated group (n=1169) | War evacuation; self-report and record | Blood pressure (SBP, DBP); measured Mean age: 61 |
Sex Father’s occupation status in childhood Age at adult assessment Attained education level in adulthood |
War evacuation was related to higher SBP. Effects of age at evacuation: Higher blood pressure associated with separation in early childhood (4 – 7 years). Effects of duration of separation are dependent on sex: Female war evacuees with < 1 year of separation had higher blood pressure relative to male war evacuees with < 1 year of separation, Males with > 2 years of separation had higher blood pressure than females with > 2 years of separation. |
D for blood pressure= .08 to .31 |
| Suarez et al., 2017 | Prospective | Subsample of the Helsinki Birth Cohort: Separated group (n=273), non-separated group (n=1455) | Wartime separation from both parents; self-report and record | Impaired glucose tolerance Impaired fasting glucose 4 additional measures of glucose 5 measures of insulin sensitivity and secretion Mean age: 62 |
None | In bivariate analyses, separated subjects had higher likelihood of impaired glucose tolerance compared to non-separated subjects. No other significant relations emerged. |
d for glucose = .07 to 8.7 d for insulin= .00 to .09 |
| Batty et al, 2021 | Prospective | 1970 British Cohort Study: Separated group (n=371), non-separated group (n=8210) | Out-of-Home care; parent-report | BM Systolic blood pressure HDL Triglycerides Glycated hemoglobin CRP; measured Age range: 46–48 |
Sex Maternal age at birth Pre-adult hospital visits Age 10 behavioral problems and paternal social class |
No difference between separated and non-separated group for any outcomes. | |
| Cooley et al., 2018 | Cross-sectional | Convenience sample recruited in an urban primary care clinic serving low income, un/under insured individuals in the United States: separated group (n=49), non-separated group (n=76) | Out-of-Home placement care; self-report | BMI; record Age range: 18–65 |
None | No difference between separated and non-separated groups in BMI. | d for BMI= .20 |
| de Mestral et al., 2020 | Prospective | Data from the National Child Development Study in the UK: separated group (n=322), non-separated group (n=7690) | Out-of-Home placement care; self-report and record | Blood pressure (SBP, DBP) Heart rate BMI, wait-to-hip ratio Lipids and cholesterols (triglycerides, HDL, LDL) HbA1c Inflammatory markers (CRP, fibrinogen, Immunoglobulin E) Blood clotting markers (D-dimer, tPA, vWF); measured Age range: 44–45 |
Sex Childhood SES composite Early life health (childhood hospitalizations, disability, internalizing and externalizing symptoms) SBP and DBP adjusted in analyses examining hypertension. |
Separated subjects had lower BMI than non-separated. Associations with other outcomes were not significant. Effects of age at separation: not significant. Effects of duration of separation: not significant. |
NA |
| Xie et al., 2021 | Prospective | Data from the National Child Development Study in the UK: Separated group (n=420), non-separated group (n=10740) | Out of home care; parent-report | Obesity High blood pressure; self-report Mean age: 42 |
Sex Childhood SES Health conditions |
No differences in self-reported obesity or high blood pressure in bivariate and adjusted analyses. | ORs across obesity and high blood pressure= .8 (CI= .50 1.1) to 1.2 (CI=.80, 1.70) |
| Hengesch et al., 2018 | Cross-sectional | Subsample of the EpiPath cohort in Luxembourg: Separated group (n=22) non-separated group (n=22). Exclusion criteria: cold intolerance, current medication, heavy smoking, >30 g alcohol consumption daily, illicit drug use in prior 3 months, BMI <19 or >30. | Separation from parents in early life, institutionalization, and subsequent adoption; self-report | Heart rate Mean arterial pressure (MAP)Reactivity of heart rate & Reactivity of MAP to cold pressor test, and paced auditory serial addition task; measured Age range: 19–30 |
Sex | Separated group had lower baseline mean arterial pressure (MAP) compared to non-separated group, but no significant difference in changes in MAP. Moderation by sex: not significant. |
NA |
| Roettger et al., 2022 | Prospective | Data from the Mater Hospital-University of Queensland Study on Pregnancy; Analyses at age 21: Separated group ≤ age 5 (n=41) and non-separated group (n=2277), separated group ≤ age 14 (n=121) and non-separated group (n=2393); Analyses at age 30: Separated group group ≤ age 5 (n=19) and non-separated group (n=1532), separated group ≤ age 14 (n=83) and non-separated group (n=1580); | Parental incarceration of the mother and partner (i.e., biological or non-biological father) ≤ age 5 and ≤ age 14; Parent report | BMI Waist circumference Blood pressure (SBP, DBP); measured Age 21 and 30 | Sex Maternal education Child birth weight Ethnicity Pregnancy status at adult assessments |
Results for early parental imprisonment before age 5 was related to higher BMI, SBP, DBP, and waist circumference at age 30. The relation between parental imprisonment before age 5 and higher BMI was already apparent at age 21. Results for parental imprisonment before age 14 was related to higher BMI at age 30 but not at age 21. In analyses that separated females and males, the relations were stronger in females. |
d for early parental imprisonmentacross body mass and blood presure= .16 to .62 d for for later parental imprisonmentacross body mass and blood presure= .02 to .21 |
| Schneider et al., 2009 | Cross-sectional | Sub-sample of the California Women’s Health Survey: Separated group (n=368), non-separated group (n=9240) | Out-of-Home placement care; self-report | Obesity; self-report Age range: 18–65 |
Age Race/ethnicity |
Separated subjects had greater risk of obesity than non-separated subjects. | OR for obesity= 1.26 (CI= 1.00, 1.60) |
Note. BMI= body mass index. HDL= high density lipoprotein. LDL= low density lipoprotein. SBP= systolic blood pressure. DBP= diatolic blood pressure. CRP= c-reactive protein. hs-CRP= high sensitivity C-reactive protein. MAP= mean arterial pressure. D-dimer= Fibrin D-dimer. vWF= von Willebrand factor. tPA= tissue-type plasminogen activator (tPA). ORs= odds ratios. NA= not available because the effect sizes and/or information that could be used to calculate effect sizes are not available in the article.
Associations between Parent-Child Separation and Cardiometabolic Outcomes in Adulthood
Across the 13 studies29 34 35 42–51, there were a total of 33 cardiometabolic outcomes (Appendix D). The individual studies often used multiple indices for a single construct (e.g., various indices of insulin and glucose to examine insulin and glucose sensitivity; multiple indices of lipids, and body size), and there was little overlap in the specific measures that could be summarized across studies. Below, we discuss the findings examining effects of parent-child separation on (a) cardiovascular and metabolic events and diagnoses and (b) cardiometabolic risk factors, followed by (c) a discussion of findings linked to age and duration of parent-child separation.
Cardiovascular and Metabolic Diagnoses and Events
Table 3 summarizes four studies35 42 44 46 that examined cardiovascular and metabolic diagnoses (e.g., coronary heart disease [CHD] and diabetes) and events (e.g., hospitalization and mortality) in adulthood. We discuss the findings grouped by the reasons for parent-child separation.
War evacuation.
Two35 42 of these studies were prospective studies from the Helsinki cohort examining effects of war evacuation on cardiometabolic events and diagnoses in older adulthood (i.e., ages 62–64). The two studies differed in sample size and method, as the earlier study41 contained a smaller sub-sample (n=2003) and used a combination of self-report and record data for both the outcomes and exposure, whereas the later study54 used a larger sample (n=12,915) and record data only. Despite methodological differences, the two studies both reported higher risk of type II diabetes, CHD, and the use of medications for CHD in war evacuees compared to the non-exposed group in older adulthood, accounting for sex, age at adult assessment, and father’s occupation status in childhood as a proxy of childhood SES.35 42 However, there were no differences observed for hospitalization or mortality related to CHD.42
Out-of-Home Care.
The third study used a prospective design and data from the National Child Development Study from the UK to examine the effects of out-of-home care reported by parents on self-report diabetes in mid-adulthood at age 42.46 The results were based on a large sample (n=11,160) and adjusted for sex, childhood SES, and health conditions, though no differences emerged for risk of diabetes between the out-of-home care and non-separated groups.
Foster Care.
One prospective study compared foster youth (n=596) from the Midwest Evaluation of the Adult Functioning of Former Foster Youth to an age-matched non-exposed and economically secured group (n=1416) from AddHealth.44 Foster care experience was prospectively reported by participants in interviews conducted every two years before age 17 and diabetes was self-reported at age 25. The findings showed no difference in self-report diabetes between the foster care group compared to the economically-secure control group, adjusting for sex, age, and adult education attainment.
Cardiometabolic Risk Factors
Table 4 summarizes all 13 studies29 34 35 42–51 that examined cardiometabolic risk factors. We discuss the findings grouped by the reasons for parent-child separation.
War evacuation.
Four29 35 42 43 studies from the Helsinki cohort examined effects of war evacuation on adult cardiometabolic risk factors using prospective designs. The studies varied in sample sizes, methods in measuring the exposure (i.e., self-report and record data vs. record data alone) and investigated different cardiometabolic risk factors. The earliest study (n=2003) used a combination of self-report and record data to identify individuals with exposure to war evacuation and measured cardiometabolic biomarkers, including measures of body mass, lipids, blood pressure, and medication for hypertentsion.35 The findings showed that war evacuees displayed higher blood pressure, apoprotein(a), and hypertension by age 62. The second study contained a larger sample and used record data to identify exposures to war evacuation as well as the use of medication for hypertension in older adulthood, among other previously reported outcomes.42 In the larger sample, the prevalence of medication for hypertension was comparable between war evacuees and the non-separated group. In the third study, a more stringent exclusion criterion was applied to define a small subsample (n=1361) who were not obese (BMI < 30 kg/m2); the exposure of war evacuation was identified using both self-report and record data and the outcomes of blood pressure in older adulthood were measured. Ruling out obesity, the main result showed that war evacuees showed higher systolic blood pressure (SBP), but not diastolic blood pressure (DBP) at age 64 than the non-separated group.29 The fourth study focused on metabolic risk factors in a sub-sample (n=1728) who completed various measures of glucose and insulin functioning and sensitivity in older adulthood at age 62; The exposure of war evacuation was identified through self-report and record data.43 The results showed higher prevalence of impaired glucose tolerance amongst war evacuees compared to the non-separated group, though no differences were observed for insulin sensitivity and functioning.43 Overall, the Helsinki cohort studies offer evidence that exposure to war evacuation in childhood may increase the risks of hypertension, higher blood pressure, and glucose intolerance, which align with the higher prevalence of CHD and diabetes reported amongst these individuals.
Parental incarceration.
Two34 51 studies examined exposure to parental incarceration and reported positive associations with cardiometabolic risk factors. One large cohort study used data from AddHealth in the US (n=10,816) to test associations between retrospective reports of parental incarceration and inflammation (i.e., C-reactive protein; CRP) in young adulthood (age 28).34 The results differed by gender, with females exposed to paternal incarceration showing elevated CRP, while this association was not observed in males. Moreover, maternal incarceration was not associated with CRP in either males or females.34 The second cohort study used a large Australian cohort (n=2515) to examine parent-reported incarceration of the mother and partner (i.e., biological or non-biological father) at two developmental periods, including early childhood (before age 5) and before adolescence (before age 14) and relations with measures of body mass and blood pressure in young adulthood, ages 21 and 30.51 The results showed similar relations across gender, such that early parental imprisonment before age 5 was related to higher BMI at both ages 21 and 30, and SBP, DBP, and waist circumference at age 30. Similarly, parental incarceration in the later developmental period (< age 14) was related to higher BMI at age 30, but not related to the other measures.
Foster care.
One study compared foster youth (n=596) from the Midwest Evaluation of the Adult Functioning of Former Foster Youth to an age-matched non-exposed and economically secured group (n=1416) from AddHealth.44 Foster care experience was prospectively reported by participants in interviews conducted every two years before age 17 and cardiometabolic risk factors were obtained through self-report at age 25. The findings showed that the foster care group reported higher BMI, hypertension, and having any cardiovascular risk factors compared to the economically-secure control group in young adulthood, adjusting for sex, age, and adult education attainment. Additional analyses stratified by sex showed stronger relations in females than males. However, the self-report nature of the cardiometabolic assessment is an obvious weakness of the study.
Out-of-home care.
Five45 47 49 50 55 studies examined separation due to out-of-home care. This includes three relatively large cohort studies from the UK45 47 55 that largely reported null associations between prospective reports of out-of-home care and cardiometabolic risk factors in mid-adulthood. Two studies from the UK used data from the National Child Development Study47 55: One examined out-of-home care from age 0 to 16 using record and self-report data in the sample (n=8012), who completed an assessment for various metabolic (i.e., various measures of glucose, glucose intolerance, insulin secretion and sensitivity) and inflammatory markers at age 44. The results showed that out-of-home care in childhood was generally unrelated to various metabolic measures and inflammatory markers, adjusting for sex, age, childhood SES and other covariates.46 Though there was one unexpected association, in which out-of-home care associated with lower BMI. The other study from this sample (n=11,160) examining prospective reports of out-of-home care up to age 17 documented similar null relations with self-reported obesity and high blood pressure at age 42. A third study from the UK used a large sample (n=8581) from the 1970 British Cohort Study to examine relations between parent-report out-of-home care in childhood and relations with a comprehensive assessment of cardiometabolic risk factors at age 46 to 48. The results adjusting for sex, childhood SES, and other behavioral problems, also showed null associations with all of the cardiovascular risk factors that were examined (i.e., BMI, SBP, HDL, triglycerides, glycated hemoglobin, CRP).45
Furthermore, two cross-sectional studies from the US examined retrospective self-reports of out-of-home placements in childhood and associations with body mass (i.e., BMI or obesity) across a large age range in adulthood (ages 18–65)49 50 which produced mixed results. One study used a large sample (n=9608) from the California Women’s Health Survey and reported positive associations between self-reported out-of home care in childhood and self-reported obesity among women.49 Several clear limitations of this study, beyond the cross-sectional design, included potential bias in self-reports of adult obesity and an exclusively female sample. The other cross-sectional study used a small convenience sample (n=125) of low income and/or uninsured individuals recruited from a clinic in south-eastern US reported null associations with BMI extracted from records.50 Given that the sampling method in this study was biased toward lower socioeconomic characteristics, it is likely that the non-exposed group would show poor health since they were seeking consultation or treatment at the clinic. As such, the findings from this study may not be representative.
Institutionalization followed by adoption.
One cross-sectional study used a small subsample (n= 44) of the Epigenetic Effects of Early Life Adverse Events on Adult Pathology (EpiPath) cohort to examine institutionalization and subsequent adoption in infancy (< 6 months of age) in relation to baseline and reactivity of mean arterial pressure (MAP) and heart rate to a cold pressor test in young adulthood (ages 19–30).48 The separated group showed lower baseline MAP compared to the non-separated group, but no differences in MAP reactivity nor heart rate measures.48
Effects of Age and Duration of Parent-Child Separation
Five29 35 42 47 51 cohort studies examined the effects of age of parent-child separation and four29 35 42 47 cohort studies examined the duration of parent-child separation on adult cardiometabolic risks. Generally, studies either stated exploratory aims or hypothesized that earlier age and longer duration of exposure would relate to increased cardiometabolic risks. However, mixed results were reported across studies.
Age at separation.
Mixed findings on age at separation were reported within the Helsinki cohort, as one study found no effects of age of separation due to war evacuation on cardiovascular disease35, whereas two studies reported that early childhood separation (between ages 4–7) was related to use of medication for coronary heart disease42 and blood pressure.29 Notably, all three studies used prospective record data or a combination of record and self-report data; as such, the mixed results are unlikely caused by the objectivity of exposure. Instead, the different results among the Helsinki studies could be due to sample size differences and different analytic approaches. Additionally, data in the National Child Development Study from the UK suggested null associations between age of out-of-home care and multiple cardiometabolic risk factors.47 Another study using an Australian cohort examined age at parental incarceration and found that earlier age at separation before age 5, as opposed to separation before age 14, had stronger relations with higher BMI, SBP, DBP, and waist circumference at age 30.51
Duration of separation.
Mixed results also appeared for the duration of separation. Within the Helsinki cohort examining cardiometabolic outcomes in late adulthood, one report suggested longer duration of parent-child separation was related to greater cardiovascular disease risks35; however, a second report showed non-linear associations, such that individuals with 1–3 years of separation showed greater risks compared to the non-exposed group42, whereas associations were not evident among participants with < 1 year or > 3 years of separation.42 In a third report from the same cohort, the relations depended on participants’ sex, with female war evacuees who endured less than one year of parent-child separation having higher blood pressure.29 Lastly, the National Child Development Study from the UK found null associations between the duration of out-of-home care and various cardiometabolic risk factors in mid-adulthood.47 The mixed findings call into question whether associations between the duration of separation and cardiometabolic risks exist; if they exist, it is unclear what the shape of association might be (i.e., linear, non-linear).
Discussion
The main goal of this systematic review was to evaluate the evidence on the association between parent-child separation and adult cardiometabolic outcomes (e.g., events and diagnoses) and risk factors (e.g., blood pressure, body mass index, inflammation). Within studies, we also evaluated the findings for the age and duration of the exposure whenever possible. Here, we first discuss the findings on cardiometabolic outcomes and risk factors, followed by findings on age and duration of parent-child separation.
Cardiometabolic Events and Diagnoses and Risk Factors
This review revealed only four35 42 44 46 studies which have examined cardiovascular and metabolic events and diagnoses. The effect sizes across the four studies were null to small (ORs= .50 to 1.7). Two35 42 of the four35 42 44 46 studies which had reported positive associations between CHD and diabetes were part of the Helsinki cohort following war evacuees into older adulthood. These long-term longitudinal studies were able to provide more objective data from medical records as well as self-report, since the participants were assessed in older adulthood (≥ age 60). In contrast, the other two studies on foster and out-of-home care which showed null results with cardiometabolic diagnoses used self-report in young to mid-adulthood.44 46
A larger set of studies examined cardiometabolic risk factors. Eight29 34 35 42–44 49 51 of the thirteen29 34 35 42–51 studies examining cardiometabolic risk factors reported at least one positive association with parent-child separation. For the most frequently used measures, such as body mass and blood pressure or hypertension, the effect sizes ranged from small to large (e.g., cohen’s d= .06 to .62). However, the results were quite mixed given that many studies did not use overlapping cardiometabolic measures. To provide a clearer picture of the results, we considered specific reasons for parent-child separation. The four studies on war evacuation consistently indicated hypertension, high blood pressure, and glucose intolerance as risk factors for CHD and diabetes in older adulthood29 35 42 43; however, all of the studies on war evacuation used slightly different samples from the same Helsinki cohort assessed in older adulthood, which limits generalization. Two studies on parental incarceration across childhood and adolescence suggested elevated inflammation (i.e., CRP), body mass, and blood pressure as risk factors, though there were no overlapping measures used across the two studies that could provide a sense of reliability.34 51 The results examining out-of-home and foster care were largely mixed. For out-of-home care experiences, the majority of studies evidenced null results for all cardiometabolic risk factors in four45–47 49 of the five45–47 49 50 studies. Notably, the studies on out-of-home care included several high quality large cohorts with prospective reports of the exposure, and measured a comprehensive set of cardiometabolic risk factors in adulthood. Furthermore, one study on foster care across childhood and adolescence reported positive associations with self-reported BMI and hypertension in young adulthood.44 In contrast, another study from the Epipath cohort examining institutionalization and adoption in early infancy revealed negative results, as the separated group exhibited lower baseline mean arterial pressure than the non-separated group48. Notably, the timing and duration of exposure in this study (under 6 months of age) are quite different from those reported amongst studies on foster and out-of-home care, which often consist of longer durations of adversities and more unstable caregiving environments throughout childhood and adolescence. The sample size was also smaller with stringent exclusion criteria, excluding a host of variables linked to poor health (e.g., current use of medication, substance- and alcohol- use, and BMI <19 or >30), which could limit the generalizability of the results.
The null and inconsistent findings could be a result of various factors, including the substantial differences in age of assessments for the outcomes (e.g., younger to older adulthood), various types of cardiometabolic risk factors and biomarkers, diverse reasons for separation, and analytic approaches. The main associations between parent-child separation and adult cardiometabolic outcomes could vary by age, as some evidence suggests that the effect sizes of associations between early stressors and cardiometabolic risks increase as individuals age56, though stronger associations have also been found in mid- relative to late- life.57 58 While some elevated cardiometabolic risk factors could be observed as early as childhood, cardiovascular events and diagnoses typically emerge in mid to older adulthood. These diagnoses require long-term follow-up to be objectively documented through medical records. However, amongst studies assessing cardiovascular and metabolic diagnoses in early and mid-adulthood, all have relied on self-report data, which could be biased or inaccurate44 46.
The null and inconsistent findings also could be due to unmeasured psychosocial and caregiving variables. In examining the diverse reasons for separation, we note that some reasons for parent-child separation are confounded with difficult childhood family environments, such as poverty and maltreatment. However, only five of the included studies accounted for the socioeconomic environment in childhood and adulthood. Studies which do not account for individuals’ socioeconomic environments could inflate the associations between parent-child separation and cardiometabolic findings. Additionally, the groups exposed to parent-child separation likely experienced much lower standard of living in childhood compared to comparison groups. As such, it would be important for future studies to use an appropriate comparison group and/or control for the childhood socioeconomic environments.
Similarly, it is also likely that children with experiences of foster and out-of-home care or parental incarceration would enter a study with more psychosocial dysfunction, trauma, and experiences of poor-quality caregiving environments. However, the included studies typically did not account for the child’s caregiving environment nor psychosocial experiences prior to and after parent-child separation. Only one study included covariates linked to a history of psychosocial experiences, such as a history of child maltreatment and whether the child lived in a stable home with two biological parents34. The caregiving experiences vary and serve as important variables in predicting whether children will have long-term negative or positive outcomes, because the heterogeneous reasons for parent-child separation may not necessarily reflect an adverse event. For instance, parent-child separation due to removal from a dysfunctional home or institution into a supportive foster home could be a positive experience depending on the caregiving quality of the new home. In contrast, parent-child separation due to war evacuation is assumed to adversely affect children because it disrupts “normal” caregiving environments and psychosocial development. A possible reason why parent-child separation was related to poorer cardiovascular health in the Helsinki studies may be because the children who were separated in Finland at the time had exposures to multiple traumas (e.g., parents killed in war; their homes were bombed, and many evacuated to another country with a different language). Future studies should collect additional information linked to caregiving quality and psychosocial experiences prior to, during, and after parent-child separation when possible to have a clearer understanding of the effects of parent-child separation.
Age and Duration of Parent-Child Separation
In examining the effects of age and duration of the exposure to parent-child separation, mixed results of positive, inverse, and null associations were reported across five studies. 29 35 42 47 51 Studies that have examined the duration of separation showed findings of linear35 and non-linear42 associations, suggesting that prolonged duration of separation, as well as brief periods of separation, were associated with worse outcomes. The inconsistent findings could be related to various analytic choices, as some studies used arbitrary age categories of separation to indicate early and later childhood29, rather than using continuous measures of age. The reference group also varied, as the comparisons were not always made within the group exposed to parent-child separation (i.e., early compared to later age of separation), but between various categories of age at separation compared to a non-exposed group.42 Furthermore, the duration of separation may be less relevant and depend on the psychosocial and caregiving experiences prior to and after separation. Future longitudinal studies are needed to examine age and duration of separation and include measures of important variables, such as quality of the caregiving environment.
The current state of the literature limits the present systematic review in several ways. First, heterogeneity in the exposures and outcomes, as well as the limited number of studies with similar outcomes, rendered it impossible to conduct a meta-analysis and to evaluate which outcomes were most strongly associated with parent-child separation. Second, although there was heterogeneity in the types of exposures, the extant literature was largely limited to foster or temporary care, and war evacuation. Thus, the long-term cardiometabolic outcomes of children exposed to separation due to other reasons, such as parental incarceration, parental economic migration, unaccompanied migrant children, and deportation remain unknown, even though the mental health effects of these exposures have been documented.8 Third, although we aimed to summarize whether the evidence is moderated by sex, developmental timing, and duration of the exposure, few studies tested these differences. Fourth, our search was limited to studies reported in English. Last, the evidence was limited as there was a relatively small set of studies; some studies also consisted of small sample sizes; and several prospective studies came from the same cohort. While we acknowledge that large-scale long-term longitudinal studies are difficult to conduct, additional studies in diverse cohorts with appropriately powered sample sizes are needed to extend generalizability. Future studies are needed to test main effects on cardiometabolic outcomes, plausible behavioral and biological mechanisms that could indirectly influence these relations, and contextual and individual factors.
In conclusion, the available evidence examining the long-term consequences of parent-child separation on cardiometabolic health in adulthood is inconsistent and may depend on the reasons for separation and other psychosocial experiences of the child. Given the ubiquity of children experiencing separation from their parents and the immense economic burden of cardiovascular diseases, it remains a priority for future studies to evaluate the link between parent-child separation and long-term health outcomes, as well as biological mechanisms that can be reversed.59 Advances in these areas could inform policies and interventions that aim to reduce the burden of this common form of childhood experience.
Support:
This project was supported by a seed grant from the Reversibility Network, a National Institute on Aging (NIA)-funded network of researchers whose mission is to advance research around remediating the effects of early life adversities (ELA) in mid and later life (Grant: R24AG065174). In addition, Drs. Tang and Slopen were supported by the National Institutes of Health Grant (1R01HL151848-01). Dr. Yazawa was financially supported by Japan Society for the Promotion of Science Research Fellowship for Young Scientists (JP21J01171).
Appendix A: Search Strategies
PubMed (National Library of Medicine, NCBI)
(“Infant”[Mesh] OR “Child”[Mesh] OR “Adolescent”[Mesh] OR neonat*[tiab] OR newborn*[tiab] OR new born*[tiab] OR infant[tiab] OR infants[tiab] OR infant’s[tiab] OR infancy[tiab] OR pre school[tiab] OR preschool[tiab] OR preschooler*[tiab] OR school age*[tiab] OR schoolage*[tiab] OR child*[tiab] OR boy[tiab] OR boys[tiab] OR girl[tiab] OR girls[tiab] OR youth[tiab] OR youths[tiab] OR minors[tiab] OR preadolescen*[tiab] OR prepubescen*[tiab] OR prepubert*[tiab] OR preteen*[tiab] OR adolescen*[tiab] OR pubert*[tiab] OR pubescen*[tiab] OR teen*[tiab] OR pediatric*[tiab] OR paediatric*[tiab])
AND
(“Child, Abandoned”[Mesh] OR “Family Separation”[Mesh] OR “Maternal Deprivation”[Mesh] OR “Paternal Deprivation”[Mesh] OR “Prisoners”[Mesh] OR “Child, Institutionalized”[Mesh] OR “Institutionalization”[Mesh:NoExp] OR “Orphanages”[Mesh] OR “Foster Home Care”[Mesh] OR “Child, Foster”[Mesh] OR abandoned[tiab] OR abandonment[tiab] OR child parent separation*[tiab] OR child separation*[tiab] OR childhood separation*[tiab] OR family separation*[tiab] OR father child separation*[tiab] OR maternal separation*[tiab] OR mother child separation*[tiab] OR parental separation*[tiab] OR parent child separation*[tiab] OR temporary separation[tiab] OR economic migration[tiab] OR left behind[tiab] OR maternal migration[tiab] OR parental migration[tiab] OR paternal migration[tiab] OR separated children[tiab] OR unaccompanied children[tiab] OR unaccompanied minors[tiab] OR ((migrant*[tiab] OR migration[tiab] OR refugee*[tiab]) AND (separat*[tiab] OR unaccompanied[tiab])) OR parental absence[tiab] OR maternal absence[tiab] OR paternal absence[tiab] OR maternal deprivation*[tiab] OR parental deprivation*[tiab] OR paternal deprivation*[tiab] OR criminal justice[tiab] OR imprison*[tiab] OR incarcerat*[tiab] OR prisoner*[tiab] OR institutionalization*[tiab] OR institutionalized[tiab] OR orphanage*[tiab] OR ((war[tiab] OR wartime[tiab]) AND (evacu*[tiab] OR separat*[tiab] OR unaccompanied[tiab])) OR (asylum[tiab] AND (separat*[tiab] OR unaccompanied[tiab])) OR foster care[tiab] OR foster child*[tiab] OR foster youth*[tiab] OR foster home*[tiab] OR out of home care[tiab] OR substitute care[tiab])
AND
(“Cardiovascular Diseases”[Mesh:NoExp] OR “Heart Diseases”[Mesh:NoExp] OR “Myocardial Infarction”[Mesh] OR “Cerebrovascular Disorders”[Mesh:NoExp] OR “Brain Infarction”[Mesh:NoExp] OR “Cerebral Infarction”[Mesh] OR “Stroke”[Mesh] OR cardiometabolic[tiab] OR cardiovascular[tiab] OR cerebral infarction*[tiab] OR cerebrovascular[tiab] OR heart disease*[tiab] OR myocardial infarction*[tiab] OR stroke[tiab] OR “Arteriosclerosis”[Mesh:NoExp] OR “Atherosclerosis”[Mesh] OR “Coronary Artery Disease”[Mesh] OR “Ischemia”[Mesh:NoExp] OR “Myocardial Ischemia”[Mesh:NoExp] OR “Brain Ischemia”[Mesh:NoExp] OR “Carotid Intima-Media Thickness”[Mesh] OR “Vascular Calcification”[Mesh] OR “Ankle Brachial Index”[Mesh] OR ankle brachial index[tiab] OR arteriosclerosis[tiab] OR atherosclerosis[tiab] OR atherosclerotic[tiab] OR coronary artery calcification[tiab] OR coronary artery calcium[tiab] OR coronary artery disease[tiab] OR coronary calcium score[tiab] OR intima media thickness[tiab] OR ischemia[tiab] OR ischemic[tiab] OR vascular calcification[tiab] OR “Blood Pressure”[Mesh] OR “Hypertension”[Mesh:NoExp] OR blood pressure[tiab] OR hypertension[tiab] OR hypertensive[tiab] OR “Cholesterol”[Mesh:NoExp] OR “Cholesterol, LDL”[Mesh] OR “Lipoproteins, LDL”[Mesh] OR “Cholesterol, HDL”[Mesh] OR “Lipoproteins, HDL”[Mesh] OR “Triglycerides”[Mesh] OR “Hyperlipidemias”[Mesh:NoExp] OR “Hypercholesterolemia”[Mesh] OR “Hyperlipoproteinemias”[Mesh] OR “Hypertriglyceridemia”[Mesh] OR cholesterol[tiab] OR high density lipoprotein*[tiab] OR hypercholesterolemia[tiab] OR hyperlipidemia[tiab] OR hyperlipoproteinemia[tiab] OR hypertriglyceridemia[tiab] OR low density lipoprotein*[tiab] OR triglyceride*[tiab] OR “Diabetes Mellitus, Type 2”[Mesh] OR “Metabolic Syndrome”[Mesh] OR “Blood Glucose”[Mesh] OR “Glucose Tolerance Test”[Mesh] OR “Glycated Hemoglobin A”[Mesh] OR “hemoglobin A1c protein, human” [Supplementary Concept] OR diabetes[tiab] OR glucose[tiab] OR glycated hemoglobin[tiab] OR glycosylated hemoglobin[tiab] OR hba1c[tiab] OR hemoglobin a1c[tiab] OR metabolic syndrome[tiab] OR “Inflammation”[Mesh:NoExp] OR “Acute-Phase Proteins”[Mesh] OR “C-Reactive Protein”[Mesh] OR “Cytokines”[Mesh:NoExp] OR “Interleukin-6”[Mesh] OR “Interleukin-10”[Mesh] OR “Tumor Necrosis Factor-alpha”[Mesh] OR “Fibrinogen”[Mesh] OR acute-phase protein*[tiab] OR c reactive protein[tiab] OR cytokines[tiab] OR fibrinogen[tiab] OR inflammation[tiab] OR inflammatory[tiab] OR interleukin[tiab] OR tumor necrosis factor[tiab] OR “Obesity”[Mesh:NoExp] OR “Obesity, Morbid”[Mesh] OR “Obesity, Abdominal”[Mesh] OR “Overweight”[Mesh:NoExp] OR “Body Mass Index”[Mesh] OR “Body Weight”[Mesh:NoExp] OR “Weight Gain”[Mesh:NoExp] OR “Adiposity”[Mesh] OR “Body Fat Distribution”[Mesh] OR “Skinfold Thickness”[Mesh] OR “Waist Circumference”[Mesh] OR “Waist-Height Ratio”[Mesh] OR “Waist-Hip Ratio”[Mesh] OR adiposity[tiab] OR body fat[tiab] OR body mass index[tiab] OR body weight[tiab] OR fat distribution[tiab] OR fat mass[tiab] OR obese[tiab] OR obesity[tiab] OR over weight[tiab] OR overweight[tiab] OR skin fold[tiab] OR skinfold[tiab] OR waist circumference[tiab] OR waist height ratio*[tiab] OR waist hip ratio*[tiab] OR waist to hip ratio*[tiab] OR weight gain*[tiab])
NOT
(Editorial[ptyp] OR Letter[ptyp] OR Case Reports[ptyp])
NOT
(“Animals”[Mesh] NOT “Humans”[Mesh])
PsycInfo (American Psychological Association, Ebsco host)
Search Options
Remove: Apply equivalent subjects”
Document type: Journal Article
S1)
DE “Early Experience”
OR
Age Group (AG)=
Neonatal OR Infancy OR Preschool Age OR School Age OR Adolescence
OR
TITLE OR ABSTRACT OR KEYWORDS:
infancy OR “pre school” OR preschool OR preschooler* OR “school age*” OR schoolage* OR child* OR boy OR boys OR girl OR girls OR youth OR youths OR minors OR preadolescen* OR prepubescen* OR prepubert* OR preteen* OR adolescen* OR pubert* OR pubescen* OR teen* OR pediatric* OR paediatric*
S2)
DE “Abandonment” OR DE “Family Separation”
OR
TITLE OR ABSTRACT OR KEYWORDS:
abandoned OR abandonment OR ((child* N3 separation*) AND (child* N6 (parent* OR maternal OR mother* OR paternal OR father*))) OR ((children N5 separated) AND (children N8 (parent* OR mother* OR father*))) OR (separation* N3 (family OR families)) OR (economic N3 migration*) OR “left behind” OR “maternal migration” OR “parental migration” OR “paternal migration” OR (children N3 unaccompanied) OR (minors N3 unaccompanied)
S3)
DE “Mother Absence” OR DE “Parental Absence” OR DE “Father Absence”
OR
TITLE OR ABSTRACT OR KEYWORDS:
“maternal deprivation*” OR “parental deprivation*” OR “paternal deprivation*” OR “parental absence” OR “maternal absence” OR “paternal absence”
S4)
(DE “Criminal Justice” OR DE “Incarceration” OR DE “Prisoners” OR DE “Prisoners of War”) AND (DE “Parents” OR DE “Mothers” OR DE “Fathers”)
OR
TITLE OR ABSTRACT OR KEYWORDS:
((“criminal justice” OR imprison* OR incarcerat* OR prison*) N8 (parent* OR “paternal” OR father* OR “maternal” OR mother*))
S5)
DE “Institutionalization” OR DE “Orphanages”
OR
TITLE OR ABSTRACT OR KEYWORDS:
institutionalization* OR institutionalized OR orphanage*
S6)
(DE “Asylum Seeking” OR DE “Political Asylum”) AND (DE “Parents” OR DE “Mothers” OR DE “Fathers”)
OR
TITLE OR ABSTRACT OR KEYWORDS:
((war OR wartime) N8 (evacu* OR separat* OR unaccompanied)) OR (asylum N8 (separat* OR unaccompanied))
S7)
DE “Foster Care” OR DE “Foster Children”
OR
TITLE OR ABSTRACT OR KEYWORDS:
“foster care” OR “foster child*” OR “foster youth*” OR “foster home*” OR “out of home care” OR “substitute care”
S8)
DE “Cardiovascular Disorders” OR DE “Heart Disorders” OR DE “Myocardial Infarctions” OR DE “Cerebrovascular Disorders” OR DE “Cerebrovascular Accidents”
OR
TITLE OR ABSTRACT OR KEYWORDS:
cardiovascular OR cardiometabolic OR “heart disease*” OR “myocardial infarction*” OR “cerebral infarction*” OR cerebrovascular OR stroke
S9)
DE “Atherosclerosis” OR DE “Arteriosclerosis” OR DE “Ischemia” OR DE “Cerebral Ischemia”
OR
TITLE OR ABSTRACT OR KEYWORDS:
atherosclerosis OR atherosclerotic OR arteriosclerosis OR “coronary artery disease” OR “coronary artery calcification” OR “coronary calcium score” OR “coronary artery calcium” OR “intima media thickness” OR ischemia OR ischemic OR “vascular calcification”
S10)
DE “Blood Pressure” OR DE “Hypertension”
OR
TITLE OR ABSTRACT OR KEYWORDS:
“blood pressure” OR hypertension OR hypertensive
S11)
DE “Cholesterol” OR DE “Lipoproteins”
OR
TITLE OR ABSTRACT OR KEYWORDS:
cholesterol OR “high density lipoprotein*” OR “low density lipoprotein*” OR triglyceride* OR hyperlipidemia OR hypercholesterolemia OR hyperlipoproteinemia OR hypertriglyceridemia
S12)
DE “Type 2 Diabetes” OR DE “Metabolic Syndrome” OR DE “Blood Sugar”
OR
TITLE OR ABSTRACT OR KEYWORDS:
diabetes OR “metabolic syndrome” OR glucose OR “glycated hemoglobin” OR “glycosylated hemoglobin” OR HbA1c OR “hemoglobin a1c”
S13)
DE “Cytokines” OR DE “Inflammation” OR DE “Interleukins” OR DE “Tumor Necrosis Factor”
OR
TITLE OR ABSTRACT OR KEYWORDS:
acute phase protein*” OR “c reactive protein” OR fibrinogen OR inflammation OR inflammatory OR interleukin OR “tumor necrosis factor”
S14)
DE “Body Fat” OR DE “Body Mass Index” OR DE “Body Weight” OR DE “Obesity” OR DE “Overweight”
OR
TITLE OR ABSTRACT OR KEYWORDS:
adiposity OR “body fat” OR “body mass index” OR “body weight” OR “fat distribution” OR “fat mass” OR “over weight” OR overweight OR “skin fold” OR skinfold OR “waist circumference” OR “waist height ratio*” OR “waist hip ratio*” OR “waist to hip ratio*” OR “weight gain*”
S1 AND (S2 OR S3 OR S4 OR S5 OR S6 OR S7) AND (S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14)
Web of Science, Core Collection - (Clarivate)
Edition: Social Sciences Citation Index
Advanced search: other options = exact search
Exclude Document Types:
Proceedings Papers, Editorial Materials, Meeting Abstracts
Search all in TOPIC:
#1)
“new born*” OR newborn* OR infant OR infants OR “infant’s” OR “infancy” OR “pre school” OR “preschool” OR preschooler* OR “school age*” OR schoolage* OR child* OR “boy” OR “boys” OR “girl” OR “girls” OR youth OR youths OR minors OR preadolescen* OR prepubescen* OR prepubert* OR preteen* OR adolescen* OR pubert* OR pubescen* OR teen* OR pediatric* OR paediatric*
#2)
abandoned OR abandonment OR ((child* NEAR/3 separation*)) AND (child* NEAR/6 (parent* OR “maternal” OR mother* OR “paternal” OR father*)) OR ((children NEAR/4 separated) AND (children NEAR/7 (parent* OR mother* OR father*))) OR (separation* NEAR/3 (family OR families)) OR (economic NEAR/3 migration*) OR “left behind” OR “maternal migration” OR “parental migration” OR “paternal migration” OR (children NEAR/3 unaccompanied) OR (minors NEAR/3 unaccompanied) OR ((migrant* OR migration OR refugee*) NEAR/3 (separat* OR unaccompanied))
#3)
“maternal deprivation*” OR “parental deprivation*” OR “paternal deprivation*” OR “parental absence” OR “maternal absence” OR “paternal absence”
#4)
((“criminal justice” OR imprison* OR incarcerat* OR prison*) NEAR/8 (parent* OR paternal OR father* OR maternal OR mother*))
#5)
institutionalization* OR institutionalized OR orphanage*
#6)
((war OR wartime OR evacuee* OR evacuated OR evacuation*) NEAR/8 (separated OR separation OR unaccompanied)) OR (asylum NEAR/8 (separated OR separation OR unaccompanied))
#7)
“foster care” OR “foster child*” OR “foster youth*” OR “foster home*” OR “out of home care” OR “substitute care”
#8)
cardiovascular OR cardiometabolic OR “heart disease*” OR “myocardial infarction*” OR “cerebral infarction*” OR cerebrovascular OR stroke
#9)
atherosclerosis OR atherosclerotic OR arteriosclerosis OR “coronary artery disease” OR “coronary artery calcification” OR “coronary calcium score” OR “coronary artery calcium” OR “intima media thickness” OR ischemia OR ischemic OR “vascular calcification”
#10)
“blood pressure” OR hypertension OR hypertensive
#11)
cholesterol OR “high density lipoprotein*” OR “low density lipoprotein*” OR triglyceride* OR hyperlipidemia OR hypercholesterolemia OR hyperlipoproteinemia OR hypertriglyceridemia
#12)
diabetes OR metabolic syndrome OR glucose OR glycated hemoglobin OR glycosylated hemoglobin OR HbA1c OR “hemoglobin a1c”
#13)
“acute phase protein*” OR “c reactive protein” OR fibrinogen OR inflammation OR inflammatory OR interleukin OR tumor necrosis factor
#14)
adiposity OR “body fat” OR “body mass index” OR “body weight” OR “fat distribution” OR “fat mass” OR “over weight” OR overweight OR “skin fold” OR skinfold OR “waist circumference” OR “waist height ratio*” OR “waist hip ratio*” OR “waist to hip ratio*” OR “weight gain*”
#1 AND (#2 OR #3 OR #4 OR #5 OR #6 OR #7) AND (#8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14)
1938 Total Unique records for screening
Appendix B. Adapted Newcastle-Ottawa Quality Assessment Scale for cohort studies
Instructions:
There are 8 items listed below that guides the process in assessing the quality of each article
A study can be awarded a maximum of one star (or asterisk) for each numbered item
When the study meets the threshold indicating a “good” design/ method (this is reflected by an asterisk below), give that study one star for that criterion in the excel spreadsheet. For criterion 5, “Comparability”, the study could receive up to two stars.
Selection:
- Representativeness of the exposed cohort
- truly representative of the average child separated from parents (e.g., due to foster care, incarcerated parent, war evacuation, economic migration…) in the geographic location*
- somewhat representative of the average child separated from parents in the geographic location
- selected group of users (e.g., nurses, volunteers)
- no description of the derivation of the cohort
- Selection of the non-exposed cohort
- drawn from the same geographic location as the exposed cohort*
- drawn from a different source/geographic location but matched or controlled for age and sex*
- drawn from a different source/geographic location
- no description of the derivation of the non-exposed cohort
- Ascertainment of exposure
- Secure record (e.g, administrative records)*
- Prospective report or interview*
- Retrospective report
- No description
- Demonstration that outcome of interest was not present at start of study
- Longitudinal and screened for cardiometabolic conditions*
- Longitudinal*
- Cross-sectional
Comparability
- Comparability of cohorts on the basis of the design or analysis (up to 2 stars can be awarded)
- study controls for sex and age at the outcome assessment or study directly matched exposed and non-exposed groups based on sex and age *
- controls for any childhood precursors of cardiometabolic conditions (e.g., any variable that measures or are proxies of childhood socioeconomic environment, or family environment)
Outcome
- Assessment of outcome
- Measured; Non self-report*
- Record linkage*
- Self report
- No description
- Was follow-up long enough for outcomes to occur
- yes (any temporal interval between reported exposure and follow-up, prospective measures of parent-child separation followed by subsequent measures of cardiometabolic health) *
- no (cross-sectional study, or a prospective study that retrospectively measured childhood parent-child separation in adulthood at the same time as the outcome was measured)
- Adequacy of follow up of cohorts
- complete follow up - all subjects accounted for*
- subjects lost to follow up unlikely to introduce bias results - small number lost (less than 30% loss at follow- up), or description that the attritted sample is representative of the original cohort and not different in outcome variable (i.e., the ones who did not return are not more likely to have poor cardiometabolic health or diseases)*
- follow up rate <30% and no description of those lost
- no statement
Note. Adapted from: Wells, G. A, Shea, B., O’Connel, D. et al. The Newcastle-Ottawa scale (NOS) for assessing the quailty of nonrandomised studies in meta-analyses. http://www.Ohri.ca/programs/clinical_epidemiology/oxford.htm 2009 Feb 1
Appendix C.
Quality ratings for each study.
| Study | 1. Representativeness of exposed | 2. Selection of non-exposed | 3. Exposure ascertainment | 4. Outcome not present at beginning | 5. Comparability of cohorts | 6. Outcome assessment | 7. Follow-up long enough | 8. Adequacy of follow-up | Total within each study |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Ahrens et al. 2014. | * | * | * | * | * | * | * | 7 | |
| Alastalo et al. 2009. | * | * | * | * | * | * | * | * | 8 |
| Alastalo et al., 2012. | * | * | * | * | * | * | * | * | 8 |
| Alastalo et al. 2013. | * | * | * | * | * | * | * | * | 8 |
| Batty et al. 2021 | * | * | * | * | ** | * | * | 8 | |
| Boch 2015. | * | * | * | ** | * | * | 6 | ||
| Cooley 2018. | * | * | 2 | ||||||
| de Mestral 2019. | * | * | * | * | ** | * | * | 7 | |
| Hengesch 2018. | * | * | * | 3 | |||||
| Rotteger et al. 2022. | * | * | * | * | ** | * | * | 8 | |
| Schneider 2009. | * | * | 2 | ||||||
| Suarez 2017. | * | * | * | * | * | * | * | * | 8 |
| Xie et al., 2022. | * | * | * | * | ** | * | 7 | ||
Note. Ratings can range from 0 to 9. Comparability can receive up to 2 points, all other criterion can receive up to 1 point.
Appendix D.
Summary of 33 cardiometabolic outcomes and results across the 11 studies.
| Ahrens 2014 | Alastalo 2009 | Alastalo 2012 | Alastalo 2013 | Batty 2021 | Boch 2015 | Cooley 2018 | de Mestral 2019 | Hengesch 2018 | Roettger 2022 | Schneider 2009 | Suarez 2017 | Xie 2021 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Age at outcome | 25–26 | 62 | 64 | 61 | 46–48 | 24–34 | 18–65 | 44–45 | 19–30 | 21 & 30 | 18–65 | 62 | 42 |
| Reason for separation | FC | war | war | war | OHC | PI | OHC | OHC | Inst, adopt | PI | OHC | war | OHC |
| Outcomes: | |||||||||||||
| mortality due to CHD | − | ||||||||||||
| hospital admission for CV event | − | ||||||||||||
| cardiovascular disease | + | ||||||||||||
| BMI | + | − | − | − | * | + | |||||||
| obesity | + | − | |||||||||||
| Hypertension | + | + | |||||||||||
| Blood Pressure | + | + | − | − | + | − | |||||||
| apoprotein(a)/lipoprotein(a) | + | ||||||||||||
| apoproteinB | − | ||||||||||||
| apoproteinA | − | ||||||||||||
| Dyslipidemia | − | ||||||||||||
| total cholesterol | − | ||||||||||||
| HDL | − | − | − | ||||||||||
| LDL | − | − | |||||||||||
| Triglycerides | − | − | − | ||||||||||
| composite of cardiovascular risk factora | + | ||||||||||||
| hsCRP, CRP | − | +F | |||||||||||
| heart rate | − | − | |||||||||||
| heart rate reactivity | − | ||||||||||||
| waist-to-hip ratio | − | ||||||||||||
| waist circumference | − | + | |||||||||||
| mean arterial pressure | * | ||||||||||||
| MAP reactivity | − | ||||||||||||
| Diabetes | − | + | − | ||||||||||
| HbA1c, glycated hemoglobin | − | − | |||||||||||
| impaired glucose tolerance | + | ||||||||||||
| impaired fasting glucose tolerance | − | ||||||||||||
| 4 other measures of glucose | − | ||||||||||||
| 5 measures of insulin sensitivity and secretion | − | ||||||||||||
| Medication use for blood pressure | − | ||||||||||||
| Medication use for chronic diseases | − | ||||||||||||
| Medication use for CHD | + | ||||||||||||
| Medication use for hypertension | − | − | |||||||||||
Note. For the results: + (positive sign)= separated > non-separated.
(asterisk)= separated < non-separated. −(negative sign)= null association.
cardiovascular risk composite includes dyslipidemia, hypertension, diabetes, smoker, and BMI>=30.
F= found in females only.
FC= foster care. OFC= out of home care. PI= parental incarceration. Inst= institutionalization. Adopt= adoption. CV= cardiovascular. CHD= Coronary heart disease. BMI= body mass index. HDL= high density lipoprotein. LDL= low density lipoprotein. hsCRP= high sensitivity C-reactive protein. MAP= mean arterial pressure.
References
- 1.Waddoups AB, Yoshikawa H, Strouf K. Developmental Effects of Parent–Child Separation. Annual Review of Developmental Psychology 2019;1:387–410. [Google Scholar]
- 2.Fellmeth G, Rose-Clarke K, Zhao C, et al. Health impacts of parental migration on left-behind children and adolescents: a systematic review and meta-analysis. The Lancet 2018;392(10164):2567–82. doi: 10.1016/S0140-6736(18)32558-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schapiro NA, Kools SM, Weiss SJ, et al. Separation and Reunification: The Experiences of Adolescents Living in Transnational Families. Current Problems in Pediatric and Adolescent Health Care 2013;43(3):48–68. doi: 10.1016/j.cppeds.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 4.Zeanah CH, Humphreys KL. Global prevalence of institutional care for children: a call for change. The Lancet Child & Adolescent Health 2020 [DOI] [PubMed] [Google Scholar]
- 5.Administration of Children and Families. National Data Shows Number of Children in Foster Care Decreases for the Third Consecutive Year 2021 [Available from: https://www.acf.hhs.gov/media/press/2021/national-data-shows-number-children-foster-care-decreases-third-consecutive-year.
- 6.Gotsch K. Families and mass incarceration. The Sentencing Project 2018 [Available from: https://www.sentencingproject.org/publications/6148/ accessed 8/11/2020 2020.
- 7.US Department of Health and Human Services. Latest Unaccompanied Children Data – FY2021. 2021
- 8.Seker S, Boonmann C, Gerger H, et al. Mental disorders among adults formerly in out-of-home care: a systematic review and meta-analysis of longitudinal studies. European Child & Adolescent Psychiatry 2021:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larkin KT, Chantler PD. Stress, depression, and cardiovascular disease. Cardiovascular implications of stress and depression: Elsevier; 2020:1–12. [Google Scholar]
- 10.Levine GN, Cohen BE, Commodore-Mensah Y, et al. Psychological health, well-being, and the mind-heart-body connection: a scientific statement from the American Heart Association. Circulation 2021;143(10):e763–e83. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. The top 10 causes of death 2018. [Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death accessed 8/3/2020.
- 12.Dong M, Giles WH, Felitti VJ, et al. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation 2004;110(13):1761–66. [DOI] [PubMed] [Google Scholar]
- 13.Brown DW, Anda RF, Tiemeier H, et al. Adverse childhood experiences and the risk of premature mortality. American journal of preventive medicine 2009;37(5):389–96. [DOI] [PubMed] [Google Scholar]
- 14.Loucks EB, Almeida ND, Taylor SE, et al. Childhood family psychosocial environment and coronary heart disease risk. Psychosomatic medicine 2011;73(7):563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almeida ND, Loucks EB, Kubzansky L, et al. Quality of parental emotional care and calculated risk for coronary heart disease. Psychosomatic medicine 2010;72(2):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin 2011;137(6):959–97. doi: 10.1037/a0024768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kajeepeta S, Gelaye B, Jackson CL, et al. Adverse childhood experiences are associated with adult sleep disorders: a systematic review. Sleep Med 2015;16(3):320–30. doi: 10.1016/j.sleep.2014.12.013 [published Online First: 2015/03/18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Raffeld MR, Slopen N, et al. Childhood adversity and insomnia in adolescence. Sleep Med 2016;21:12–8. doi: 10.1016/j.sleep.2016.01.011 [published Online First: 2016/07/28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russell SJ, Hughes K, Bellis MA. Impact of childhood experience and adult well-being on eating preferences and behaviours. BMJ Open 2016;6(1):e007770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes K, Bellis MA, Hardcastle KA, et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. The Lancet Public Health 2017;2(8):e356–e66. doi: 10.1016/S2468-2667(17)30118-4 [DOI] [PubMed] [Google Scholar]
- 21.Johnson DE, Tang A, Almas AN, et al. Caregiving disruptions affect growth and pubertal development in early adolescence in institutionalized and fostered Romanian children: A randomized clinical trial. The Journal of pediatrics 2018;203:345–53. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reid BM, Miller BS, Dorn LD, et al. Early growth faltering in post-institutionalized youth and later anthropometric and pubertal development. Pediatric research 2017;82(2):278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLaughlin KA, Sheridan MA, Tibu F, et al. Causal effects of the early caregiving environment on development of stress response systems in children. Proceedings of the National Academy of Sciences 2015:201423363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koss KJ, Mliner SB, Donzella B, et al. Early adversity, hypocortisolism, and behavior problems at school entry: A study of internationally adopted children. Psychoneuroendocrinology 2016;66:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang D-d, Fang J, Zhang L, et al. Pubertal recalibration of cortisol reactivity following early life parent-child separation. Journal of Affective Disorders 2021;278:320–26. [DOI] [PubMed] [Google Scholar]
- 26.Račaitė J, Lindert J, Antia K, et al. Parent emigration, physical health and related risk and preventive factors of children left behind: a systematic review of literature. International journal of environmental research and public health 2021;18(3):1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu W, Zhang A, He H, et al. Inflammatory burden in adolescents with prolonged parent-child separation. Brain, behavior, and immunity 2021;98:257–62. [DOI] [PubMed] [Google Scholar]
- 28.Bevan K, Kumari M. Maternal separation in childhood and hair cortisol concentrations in late adulthood. Psychoneuroendocrinology 2021;130:105253. [DOI] [PubMed] [Google Scholar]
- 29.Alastalo H, Räikkönen K, Pesonen A, et al. Early life stress and blood pressure levels in late adulthood. Journal of Human Hypertension 2013;27(2):90–94. [DOI] [PubMed] [Google Scholar]
- 30.Savolainen K, Eriksson JG, Kananen L, et al. Associations between early life stress, self-reported traumatic experiences across the lifespan and leukocyte telomere length in elderly adults. Biological Psychology 2014;97:35–42. doi: 10.1016/j.biopsycho.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 31.Tyrka AR, Wier L, Price LH, et al. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biological psychiatry 2008;63(12):1147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang A, Wade M, Fox NA, et al. The prospective association between stressful life events and inflammation among adolescents with a history of early institutional rearing. Development and Psychopathology 2020;32(5):1715–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roettger ME, Boardman JD. Parental incarceration and gender-based risks for increased body mass index: evidence from the National Longitudinal Study of Adolescent Health in the United States. American Journal of Epidemiology 2012;175(7):636–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boch SJ, Ford JL. C-reactive protein levels among US adults exposed to parental incarceration. Biological research for nursing 2015;17(5):574–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alastalo H, Räikkönen K, Pesonen A-K, et al. Cardiovascular health of Finnish war evacuees 60 years later. Annals of medicine 2009;41(1):66–72. [DOI] [PubMed] [Google Scholar]
- 36.Joseph JJ, Golden SH. Cortisol dysregulation: the bidirectional link between stress, depression, and type 2 diabetes mellitus. Annals of the New York Academy of Sciences 2017;1391(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kivimäki M, Steptoe A. Effects of stress on the development and progression of cardiovascular disease. Nature Reviews Cardiology 2018;15(4):215. [DOI] [PubMed] [Google Scholar]
- 38.Xu C, Wang Z, Su X, et al. Association between leucocyte telomere length and cardiovascular disease in a large general population in the United States. Scientific reports 2020;10(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamura Y, Takubo K, Aida J, et al. Telomere attrition and diabetes mellitus. Geriatrics & Gerontology International 2016;16(S1):66–74. doi: 10.1111/ggi.12738 [DOI] [PubMed] [Google Scholar]
- 40.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wells G, Shea B, O’Connell D, et al. Newcastle-Ottawa quality assessment scale cohort studies, 2014.
- 42.Alastalo H, Räikkönen K, Pesonen A-K, et al. Cardiovascular morbidity and mortality in Finnish men and women separated temporarily from their parents in childhood—a life course study. Psychosomatic Medicine 2012;74(6):583–87. [DOI] [PubMed] [Google Scholar]
- 43.Suarez A, Lahti J, Kajantie E, et al. Early life stress, FKBP5 polymorphisms, and quantitative glycemic traits. Psychosomatic medicine 2017;79(5):524–32. [DOI] [PubMed] [Google Scholar]
- 44.Ahrens KR, Garrison MM, Courtney ME. Health outcomes in young adults from foster care and economically diverse backgrounds. Pediatrics 2014;134(6):1067–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Batty GD, Hamer M. Public care during childhood and biomedical risk factors in middle age: the 1970 British Cohort Study. American Journal of Epidemiology 2021;190(1):176–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xie T, De Mestral C, Batty GD. Association of public care in childhood with social, criminal, cognitive, and health outcomes in middle-age: five decades of follow-up of members of the 1958 birth cohort study. J Epidemiol Community Health 2021;75(3):289–96. [DOI] [PubMed] [Google Scholar]
- 47.de Mestral C, Bell S, Hamer M, et al. Out-of-home care in childhood and biomedical risk factors in middle-age: National birth cohort study. American Journal of Human Biology 2020;32(3):e23343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hengesch X, Elwenspoek MM, Schaan VK, et al. Blunted endocrine response to a combined physical-cognitive stressor in adults with early life adversity. Child abuse & neglect 2018;85:137–44. [DOI] [PubMed] [Google Scholar]
- 49.Schneider R, Baumrind N, Pavao J, et al. What happens to youth removed from parental care?: Health and economic outcomes for women with a history of out-of-home placement. Children and Youth Services Review 2009;31(4):440–44. [Google Scholar]
- 50.Cooley ME, Thompson HM, Murray H. Health outcomes of medically and economically vulnerable adults: a comparison of former foster youth and nonfoster youth. Family & community health 2018;41(3):159–67. [DOI] [PubMed] [Google Scholar]
- 51.Roettger ME, Houle B, Najman J, et al. Parental imprisonment as a risk factor for cardiovascular and metabolic disease in adolescent and adult offspring: A prospective Australian birth cohort study. SSM-Population Health 2022;18:101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pesonen A-K, Räikkönen K, Feldt K, et al. Childhood separation experience predicts HPA axis hormonal responses in late adulthood: a natural experiment of World War II. Psychoneuroendocrinology 2010;35(5):758–67. [DOI] [PubMed] [Google Scholar]
- 53.Power C, Atherton K, Strachan DP, et al. Life-course influences on health in British adults: effects of socio-economic position in childhood and adulthood. International journal of epidemiology 2007;36(3):532–39. [DOI] [PubMed] [Google Scholar]
- 54.Suarez A, Lahti J, Czamara D, et al. The epigenetic clock and pubertal, neuroendocrine, psychiatric, and cognitive outcomes in adolescents. Clinical epigenetics 2018;10(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shields GS, Deer LK, Hastings PD, et al. Adiposity, inflammation, and working memory: Evidence for a vicious cycle. Brain, Behavior, & Immunity-Health 2021;13:100202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chiang JJ, Lam PH, Chen E, et al. Psychological stress during childhood and adolescence and its association with inflammation across the lifespan: A critical review and meta-analysis. Psychological Bulletin 2022;148(1–2):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robert SA, Li LW. Age variation in the relationship between community socioeconomic status and adult health. Research on aging 2001;23(2):234–59. [Google Scholar]
- 58.House JS, Kessler RC, Herzog AR. Age, socioeconomic status, and health. The Milbank Quarterly 1990:383–411. [PubMed] [Google Scholar]
- 59.Reiss D, Nielsen L, Godfrey K, et al. Midlife reversibility of early-established biobehavioral risk factors: A research agenda. Developmental psychology 2019;55(10):2203. [DOI] [PMC free article] [PubMed] [Google Scholar]


