Abstract
Introduction
Poor food intake is common among elderly living in nursing homes, leading to micronutrient deficiency (MD). There are no recommendations for the management of MD in malnourished older adults.
Methods
We conducted a single arm, open-label, multicenter interventional study in institutionalized malnourished older adults to describe the effect of a 4-week daily energy and protein dense oral nutritional supplementation (ONS, 600 kcal, 30 g protein per unit) containing 50% of the recommended daily micronutrient intake on micronutrient status. Plasma concentrations of vitamins (A, B9, B12, C, E), magnesium (Mg), selenium (Se) and zinc (Zn), and erythrocyte vitamin B9 were measured at baseline and after 4 weeks.
Results
Forty-six participants completed the study (age 87.4 ± 6.6). At baseline, the most frequent MD were Se (48%), Zn (35%), Mg (24%) and vitamin C (24%). Plasma concentrations of vitamins B9, B12, C and E, Mg, Se and Zn significantly increased and the proportion of subjects with at least one MD decreased (p = 0.006). However, after 4 weeks, 40% of subjects still had at least one MD.
Discussion
ONS consumption improved micronutrient status but did not correct MD in all participants. Our data suggest that the prescription of vitamin, mineral and trace element supplementation should be considered in institutionalized malnourished older adults in addition to high energy and high protein ONS.
Keywords: magnesium, malnutrition, micronutrient, nursing home, older adults, selenium, vitamin, zinc
1. Introduction
Poor food intake is still common among older adults living in nursing homes, leading to protein-energy malnutrition and micronutrient deficiency (1, 2). Protein-energy malnutrition has been extensively addressed, leading to guidelines and recommendations for the nutritional care and oral nutritional supplements (ONS) of older adults, including those living in nursing homes (3, 4). In contrast, the deficiency in micronutrients due to low food intake in older adults living in nursing homes has received little attention.
In nursing homes, a high prevalence of inadequate intake of many vitamins, trace elements and minerals (retinol, vitamins B2, B6, B9, B12, C, E, potassium, calcium, magnesium, copper and zinc) have been reported in several countries (5–11). Residents consuming pureed food seem to be particularly at risk of low micronutrient intake (11, 12). Biological measurements suggest that the most important deficiencies affecting residents are zinc, selenium, vitamins B1, B6, B9, B12 and C (10, 13–15). These deficiencies may lead to various neurological, psychiatric, hematological, and skin disorders affecting quality of life and survival. In addition, numerous micronutrients are essential for the proper functioning of the immune system (16). Especially, zinc deficiency leads to a dysregulation in the adaptive immunity that can result in an increased production of pro-inflammatory cytokines (contributing to inflammaging), as well as a decreased response to vaccination (17). Furthermore, optimal status for zinc and vitamin C are mandatory for the healing of chronic wounds such as pressure sores (18). Magnesium has an important role in maintaining muscle function in aging animals and human. In aged mice, magnesium supplementation promotes muscle regeneration and maintains muscle mass and strength by activating the mammalian target of rapamycin signaling (19). In humans, magnesium intake is inversely associated with sarcopenia (20). Selenium deficiency is also frequent in Western older populations (21). Selenium has antioxidant properties, an active role in endothelial function, and could act as one of key actors of cardiovascular prevention (22). Older adults are especially at risk for vitamin B12 deficiency because of frequently inadequate animal-source food intake (23). Vitamin B12 deficiency is suspected to promote cognitive decline. Lastly, most symptoms of vitamin B9 deficiency overlap with those of vitamin B12 deficiency, including depression and cognitive disorders (24). However, despite potentially serious consequences on the quality of aging, micronutrient deficiencies are difficult to diagnose because clinical symptoms may be unspecific or attributable to aging or comorbidities. In addition, micronutrient plasma measurements are not routinely performed in malnourished subjects. Practitioners may be at loss as how to cope with micronutrient deficiencies as there are no guidelines regarding multi-micronutrient supplementation in malnourished older adults.
Multi-micronutrient supplementation increases micronutrient plasma concentrations in malnourished older adults (13–15, 25, 26), but are not routinely prescribed in nursing home residents. Energy and protein dense ONS that are recommended for malnourished older adults do contain micronutrients, as regulated by country guidelines (27), but it is not clear if the micronutrient content of ONS is high enough to improve or correct micronutrient status. We found three studies describing the change in plasma micronutrient status in nursing home residents after ONS prescription (with no additional micronutrient supplementation) (15, 26, 28). The changes in plasma concentrations of selected micronutrients were measured before and after 10–24 weeks of ONS providing 272–500 kcal, 8.5–15 g of protein, and vitamins, minerals and trace elements in varying quantities. Fiatarone Singh et al. (28) reported no significant change in folates, iron, vitamin D and E. In the second study (26), plasma concentrations of B6, B9 and B12 improved significantly, but only that of B6 and B9 reached the normal range for all residents after 12 weeks. In the third study (15), plasma concentrations of vitamin D, B9 and B12 improved significantly but only B9 levels reached the normal range for all residents. Thus, the micronutrient status of malnourished older adults living in nursing homes receiving ONS needs to be further explored.
The ONS Renutryl Booster (Nestlé Health Science) was especially formulated for older adults, is rich in energy and protein and the composition includes 50% of micronutrient French RDA for older adults (29). We aimed to describe the effect of 1-month energy and protein dense oral nutritional supplementation of this ONS on micronutrient status (magnesium, selenium, zinc, vitamins A, B9, B12, C, and E) in malnourished older adults living in nursing homes.
2. Materials and methods
2.1. Study design, selection of participants and ethics
Between 2012 and 2014, we conducted a single arm, open-label, multicenter interventional study in nursing homes and long-term care facilities. Nineteen facilities agreed to participate in the study: five were investor-owned for-profit facilities, seven were non-profit institutions, and seven were public facilities. During the opening visits of the centers, the medical and paramedical staff of each center were informed to the study protocol and trained for the assessment of anthropometric measures, body composition, physical performances and for the quantification of ONS consumption. Inclusion criteria were age ≥70 years and moderate malnutrition. Following French Health Authority recommendations at the time of the study (30), moderate malnutrition was defined by the presence of at least one of the following criteria: body weight loss ≥5% in 1 month or ≥10% in 6 months, or body mass index (BMI) <21, plasma albumin <35 g/L or Mini Nutritional Assessment—Short Form (MNA-SF) ≤7. Non-inclusion criteria were (a) severe malnutrition, defined by the presence of at least one of the following criteria: body weight loss ≥10% in 1 month or ≥15% in 6 months, BMI <18, plasma albumin <30 g/L; (b) no oral feeding; (c) life expectancy <6 months; (d) inflammatory syndrome with C-reactive protein (CRP) >30 mg/L; (e) stage IV in heart failure according to the New-York Heart Association; (f) chronic kidney disease with estimated glomerular filtration rate <30 ml/min; (g) pulmonary disease requiring long-term oxygen therapy; (h) severe dementia [Mini Mental State Examination score (MMSE) <10] (31); (i) inability to walk 6 m alone with or without a walk-assisting device; (j) treatment within the last 3 months with systemic corticosteroid therapy; (k) enteral or parenteral nutrition, amino acid supplementation, or supplementation within the last 3 months with any ONS or micronutrients (magnesium, zinc, selenium, vitamins A, B9, B12, E, or C); and (l) intolerance to ONS. All study participants (or their legal guardian) were given oral and written information about the study protocol and signed an informed consent prior to inclusion. The study protocol was approved by the Institutional Review Board Comité de Protection des Personnes Ile-de-France—Paris VI as n°63-12, and was registered in the French National Database ID-RCB as 2012-A00740-43.
2.2. Oral nutritional supplementation
The ONS Renutryl Booster® (Nestlé Health Science) used in this study is a 300 ml liquid milk ONS with a high density in energy (2 kcal/ml, 600 kcal for 300 ml) and protein (30 g for 300 ml). The micronutrient composition of this ONS is given in Table 1. The micronutrient composition is in accordance with the European policies (27, 32). Each participant was asked to consume one bottle of the ONS daily for 28 days. Participants could choose among different flavors (vanilla, strawberry, caramel, coffee, carrot cream). They could choose a different flavor each day, and consume the bottle all at once or spread throughout the day. ONS intake was assessed by weighing the bottle the day following delivery. Caregivers from each nursing home were trained to weigh the bottles and complete the compliance chart. Compliance was expressed in percentage (volume weighed/300) per day and expressed as median [Q1–Q3] for the 4 weeks' duration of the study.
Table 1.
Oral nutritional supplement composition (per unit of 300 ml).
| Sweet flavor | Salty flavor | ||
|---|---|---|---|
| Energy | Kcal | 600 | 600 |
| Protein | g | 30 | 30 |
| % | 20% | 20% | |
| Carbohydrate | g | 72 | 72 |
| % | 48.5 | 48.5 | |
| Sucrose | g | 21 | 21 |
| Lactose | g | < 1.5 | < 1.5 |
| Lipid | g | 21 | 21 |
| % | 31.5 | 31.5 | |
| Minerals and trace elements | |||
| Sodium | mg | 285 | 584 |
| Potassium | mg | 720 | 720 |
| Calcium | mg | 687 | 687 |
| Phosphorus | mg | 459 | 459 |
| Magnesium | mg | 150 | 150 |
| Chlorides | mg | 255 | 564 |
| Iron | mg | 5.1 | 5.1 |
| Zinc | mg | 7.5 | 7.5 |
| Copper | μg | 750 | 750 |
| Manganese | μg | 498 | 498 |
| Fluorine | mg | 1.2 | 1.2 |
| Chrome | μg | 63 | 63 |
| Molybdenum | μg | 21 | 21 |
| Selenium | μg | 39 | 39 |
| Iodine | μg | 75 | 75 |
| Vitamins | |||
| A | μg | 351 | 351 |
| D | μg | 5.1 | 5.1 |
| E | mg | 10.2 | 10.2 |
| K | μg | 36 | 36 |
| C | mg | 60 | 60 |
| B1 | mg | 0.6 | 0.6 |
| B2 | mg | 0.8 | 0.8 |
| B5 | mg | 2.5 | 2.5 |
| B6 | mg | 1.11 | 1.11 |
| B12 | μg | 2.4 | 2.4 |
| Niacin | mg | 13.5 | 13.5 |
| Folic acid | μg | 201 | 201 |
| Biotin | μg | 30 | 30 |
| Osmolarity | mOsmol/L | 580 | 530 |
2.3. Clinical data
Clinical data was collected on day 1 and day 29 by a trained investigator. Data included age, gender, comorbidities, cognitive performance (MMSE) and disability for activities of daily living (ADL) (33). The nursing home medical record was used to record comorbidities, including hypertension, diabetes mellitus, cancer, cardiovascular, psychiatric, neurological, and osteoarticular disorders. The number of current medications was recorded as an indicator of comorbidity. Moreover, in order to consider potential drug-nutrient interactions, we determined the proportion of participants taking drugs known to interact with micronutrients availability (proton pump inhibitors, statins, metformin, loop and thiazide diuretics) at baseline (34). We also described what drugs were interrupted or newly prescribed during the participation in the study. Nutritional status was assessed by MNA-SF, BMI and recent weight loss. Participants with severe or moderate decline in food intake in the past 3 months were identified using the first item of the MNA-SF. Physical performance was assessed using the Timed Up and Go (TUG) test (35). Body composition was measured using bioelectrical impedance analysis (Analycor 4/5W analyzer, Spengler, Cachan, France); fat mass and fat free mass were measured in kg at day 1 and day 29.
2.4. Micronutrient assays and laboratory tests
Nursing home nurses drew blood samples of each participant in the morning after a fasting night on day 1 (before the first ONS intake) and on day 29 (the day after the last ONS intake). Five test tubes were collected: (a) two 5-ml dry tubes for albumin, transthyretin, total cholesterol, triglycerides, vitamins A, E, B9, and B12 determinations, (b) two 5-ml heparinized tubes for CRP, magnesium, vitamin C, selenium, and zinc determinations, and (c) one 5-ml ethylenediaminetetraacetic acid (EDTA) tube for erythrocyte folate determination. The test tubes were transported in an isothermal bag at four degrees Celsius to the Clinical Chemistry Laboratory of the Cochin University Hospital within 3 h of collection. For geographically distant centers, the pre-analytical phase was performed in a local laboratory, then the tubes were sent at −80°C to the Biological Chemistry Laboratory of Cochin University Hospital where all tests were performed except for Zn and Se which were analyzed by Cerba Laboratory (ZI Des Bethunes 11 r Equerre, 95310 Saint Ouen l'Aumône, France). Vitamins A, E, and C were measured by reversed-phase high-performance liquid chromatography with fluorometry (vitamins A and E) (36) or ultraviolet (vitamin C) (37) detection. Vitamin E concentrations were corrected for cholesterol and triglyceride levels. Vitamin B9 (serum and erythrocytes) and B12 were assessed by electrogenerated chemiluminescence (38). Magnesium was assessed by colorimetry and the zinc and selenium by Atomic absorption spectrometry (39). To define micronutrient deficiency we used thresholds proposed in the literature (40–45). Moreover, the analytical characteristics of micronutrient assays (including the normal ranges for the laboratory) are shown in Supplementary Table 1.
2.5. Statistical analyses
Continuous variables were expressed as mean and standard deviation (SD) or median with first (Q1) and third (Q3) quartile. Categorical variables were expressed as number and percentage. The clinical variables were compared between values at day 1 and at day 29 using paired Student t-test or Wilcoxon test for paired samples for continuous variables or McNemar test for categorical variables. The primary aim of the study was first to determine the efficacy of ONS consumption for increasing the plasma and erythrocyte concentrations of all measured micronutrients globally, and not for a single one in particular. For this purpose, we used the O'Brien method (46), designed to test the efficacy of interventional clinical trials on multiple endpoints. According to this method, we built a composite score reflecting global micronutrient status, after checking on multi-linearity and correlation between variables (plasma concentration of magnesium, selenium, zinc, vitamins A, B9, B12, C, and E). Multi-normality of the micronutrient plasma concentrations was rejected (Kolmogorov-Smirnov test), and none of the variables were strongly or inversely correlated to others. Thus, the application conditions were met to use the non-parametric O'Brien Rank sum test for paired samples, in order to compare the composite score between day 1 and day 29. This test takes into account the alpha risk inflation in case of multiple comparisons. Then, we compared the change of each micronutrient concentration between day 1 and day 29 using the paired Student t-test. In addition, we compared the proportion of participants with at least one micronutrient deficiency between day 1 and day 29 using the McNemar test. Lastly, for each micronutrient we also gave the proportion of participants presenting a deficiency at day 1 and at day 29 and performed the McNemar test to compare the proportions between day 1 and day 29 when the application condition was respected (at least five discordant pairs in the 2 × 2 contingency table). Considering a power of 0.8 and an alpha risk of 0.05, the minimum sample size was estimated at 36 participants. Considering the risks of missing data and protocol deviations, we aimed to include a minimum of 55 participants. The analysis used the SAS 9.4. software (SAS Institute, Cary, NC, USA). A p-value < 0.05 was considered for significance.
3. Results
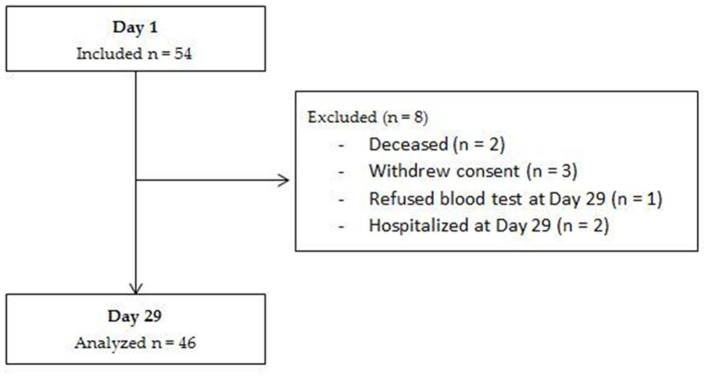
We included 54 participants recruited in 12 of the 19 facilities that had agreed to participate in the study. Inclusions were not simultaneous in the different institutions, with dates ranging from November 2012 to July 2014. Among the included participants, 46 completed the study and were included in the analyses (Figure 1). Participant's characteristics at baseline are presented in Table 2. The mean age was 87 years, and 76% were women. Half of the participants had three or more chronic conditions, mainly cardiovascular, psychiatric, neurological and osteoarticular disorders. Consequently, polypharmacy was frequent with an average of six medications per patient. Ten (22%) participants were treated with proton pump inhibitors, nine (20%) with statins, nine (20%) with loop diuretics, four (9%) with thiazide diuretic, and two (4%) with metformin at baseline. None of these medications were interrupted during the 4-week intervention and none were newly prescribed. All participants were moderately malnourished, in accordance with inclusion criteria, as showed by rates of low BMI, albuminemia, MNA-SF and by weight loss during the last 3 months. Participants consumed a median of 85% (Q1–Q3: 67%−94%) of the ONS volume given during the 4 weeks.
Figure 1.
Flow-chart of the subjects included and analyzed in the study.
Table 2.
Baseline characteristics of the population (n = 46).
| Mean ±SD or n (%) | |
|---|---|
| Women | 35 (76%) |
| Age (year) | 87.4 ± 6.6 |
| Activities of daily living (ADL/6) | 4.5 ± 1.2 |
| Mini mental state examination (/30) | 19.8 ± 5.7 |
| Comorbidities | |
| Psychiatric disorders | 19 (41%) |
| Cardiovascular disorders | 16 (35%) |
| Neurological disorders | 11 (24%) |
| Osteoarticular disorders | 11 (24%) |
| Cancer | 7 (15%) |
| Diabetes | 4 (9%) |
| Polypharmacy (number of drugs) | 6.3 ± 2.9 |
| Proportion of participant treated with | |
| Proton pump inhibitors | 10 (22%) |
| Statins | 9 (20%) |
| Loop diuretic | 9 (20%) |
| Thiazide diuretic | 4 (9%) |
| Metformin | 2 (4%) |
| Nutritional status | |
| MNA-SF (/14) | 8.2 ± 2.0 |
| MNA-SF ≤ 7 | 14 (30%) |
| BMI | 21.6 ± 3.1 |
| BMI < 21 | 29 (63%) |
| Weight loss | 6 (13%) |
| ≥5% in 1 month | 5 (11%) |
| ≥10% in 6 months | 1 (2%) |
| Albuminemia (g/L) | 36.8 ± 4.0 |
| Albuminemia < 35 g/L | 15 (33%) |
| Transthryretinemia (mg/L) | 224 ± 61 |
BMI, body mass index; MNA-SF, Mini Nutritional Assessment—Short Form.
Plasma concentration of all analyzed micronutrients and erythrocyte level of B9 increased significantly from Day 1 to Day 29, with the exception of vitamin A, for which the concentration increased, but not with statistically significance (Table 3). The composite score showed an improvement in global micronutrient status (p < 0.0001). Transthyretinemia also increased significantly (p = 0.001) between day 1 and day 29, while the number of participants with low food intake decreased (p = 0.006) and participants put on a mean of 1.1 kg (p = 0.0001). There were no significant changes in body composition or in physical performance assessed by TUG (Table 3).
Table 3.
Change in micronutrients concentrations and clinical parameters in 4 weeks (n = 46).
| Day 0 | Day 29 | p-value | |
|---|---|---|---|
| Micronutrient (thresholds for deficiency) * | |||
| Magnesium [ < 0.76 mmol/L (41)] | 0.80 ± 0.11 | 0.85 ± 0.09 | < 0.001 |
| Selenium [ < 0.75 μmol/L (40)] | 0.78 ± 0.17 | 1.07 ± 0.24 | < 0.001 |
| Zinc [ < 10.7 μmol/L in female (45) or < 11.3 μmol/L in male (45)] | 11.6 ± 2.1 | 12.2 ± 2.2 | 0.016 |
| Vitamin A [ < 0.7 μmol/L (44)] | 2.02 ± 0.60 | 2.16 ± 0.82 | 0.180 |
| Vitamin E [ < 2.22 μmol/mmol (43)] | 5.1 ± 1.1 | 5.5 ± 1.2 | < 0.001 |
| Vitamin C [ < 4.05 mg/L (40)] | 8.7 ± 4.7 | 10.3 ± 3.9 | 0.005 |
| Vitamin B9 serum [ < 4.0 μg/L (42)] | 5.7 ± 2.0 | 9.0 ± 2.8 | < 0.001 |
| Vitamin B9 erythrocyte [ < 151 μg/L (42)] | 606 ± 184 | 714 ± 778 | < 0.001 |
| Vitamin B12 [ < 203 ng/L (42)] | 388 ± 193 | 410 ± 223 | 0.032 |
| Residents with at least one low micronutrient level | 38 (86) | 17 (40) | < 0.001 |
| Micronutrient deficiencies (number) | 1.7 ± 1.1 | 0.5 ± 0.7 | < 0.001 |
| Composite score | 384 ± 14 | 531 ± 15 | < 0.001 |
| Other biological data | |||
| Albumin (g/L) | 36.8 ± 4.0 | 36.4 ± 4.1 | 0.547 |
| Transthyretin (mg/L) | 224 ± 61 | 243 ± 65 | 0.001 |
| Clinical parameters | |||
| Moderate or severe decline in food intake | 34 (74) | 23 (50) | 0.006 |
| Body weight (kg) | 55.9 ± 10.2 | 57.0 ± 10.3 | < 0.001 |
| Fat mass (kg)** | 19.2 ± 5.8 | 18.8 ± 5.6 | 0.308 |
| Fat free mass (kg)** | 33.9 ± 6.8 | 34.4 ± 6.6 | 0.466 |
| Timed up and go test (seconds) | 19.7 ± 6.8 | 19.8 ± 6.5 | 0.757 |
Data are expressed as mean ± SD or number (percentage). The composite score reflects global micronutrient status (log-transformed plasma concentration of magnesium, selenium, zinc, vitamins A, B9, B12, C and E). A higher score reflects a better micronutrient status.
*Threshold chosen to define micronutrient insufficiency based on literature (reference).
**Missing data for body composition in five participants.
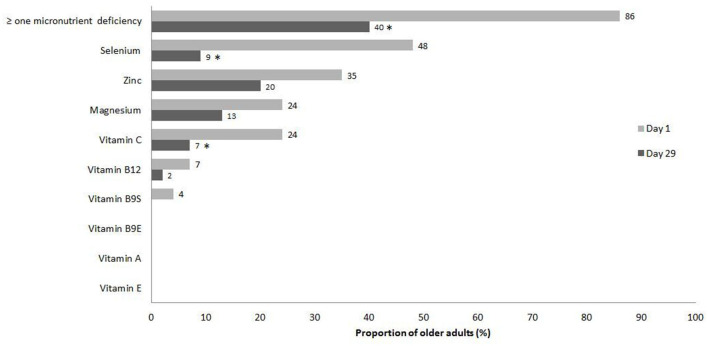
Figure 2 shows the proportion of participants with micronutrients deficiencies at day 1 and day 29. The most frequently observed deficiencies at baseline were selenium (48%), zinc (35%), magnesium (24%) and vitamin C (24%). The proportion of participants that had at least one micronutrient deficiency was reduced from 86 to 40% (p = <0.001) after 4 weeks (Figure 2).
Figure 2.
Proportion of participants with micronutrients deficiencies at day1 and day 29. Barres indicates the proportion of older adults with deficiency at day 1 and day 29. *Statistical significance (p < 0.05) for the comparison between day 1 and day 29 with the McNemar test.
4. Discussion
A first step toward improving management of micronutrient deficiencies in malnourished older adults is to determine if energy and protein dense ONS prescription restores or, at least, improves micronutrient status. We show, in the present study, that multi micronutrient deficiency affected a large majority of nursing home residents and that a 4-week daily intake of an energy and protein dense ONS containing micronutrients significantly improved micronutrient status. However, micronutrient plasma levels did not reach normal levels for all participants within the 4 weeks of the study.
In our study, the most frequently observed deficiencies at baseline were selenium (48%), zinc (35%) and vitamin C (24%), which have antioxidant proprieties. Deficiencies in other antioxidant micronutrients were less prevalent, e.g., 13% for vitamin A and none for vitamin E. In comparison, the prevalence of antioxidant micronutrient deficiencies in other populations of nursing home residents were 38%−68% for selenium, 19%−72% for zinc and 35%−75% for vitamin C (25, 47–49). In another study, baseline zinc plasma concentrations were similar to what we observed (50), but the prevalence of the deficiency was not mentioned. Beta-carotene was low in 15% of residents in another study and there was no deficiency in tocopherol (25), which is very similar to what we observed. Overall, and even though data from the literature are scarce, our results confirm a high prevalence of selenium, zinc and vitamin C deficiency at baseline in nursing home residents.
In our study, ONS intake reduced substantially the prevalence of selenium, zinc, and vitamin C deficiencies, which may have a beneficial effect on the health of this population. In a randomized study (25, 51), older institutionalized adults received daily, for 1 year, a supplementation of either vitamins (beta-carotene, vitamin C, and vitamin E), trace elements (zinc and selenium), both or a placebo. It is important to mention that the doses of antioxidant vitamins and trace elements were within physiological range but higher than those in standard high energy high protein ONS (27, 32). Authors report an increase in the mean plasma levels of the micronutrients and an improvement in antioxidant defense and immune response (52). Micronutrient status (especially zinc and vitamin C), also has an important role in the healing of chronic wounds such as pressure sores (18, 48). The prevalence of pressure ulcers varies from 3 to 32% in nursing homes (53). An ONS enriched in zinc, selenium, vitamin C and E and arginine lead to faster reduction in pressure ulcer area than an isoenergetic and isonitrogenous ONS (54). The design of that study did not allow to differentiate the effect of micronutrients from that of arginine on wound healing, but it may be hypothesized that all nutrients concurred to faster healing (18). Lastly, very low vitamin C plasma concentrations will lead to clinical symptoms of scurvy (55), which would in turn impair the residents' ability to eat correctly.
Magnesium deficiency was observed in 24% of residents in our study, and the prevalence was reduced almost by half after 4 weeks ONS intake. Plasma magnesium has seldom been measured in nursing homes, but was reported to be low in 33% of residents (48). In addition to poor food intake, many conditions were liable to lead to hypomagnesemia, including chronic renal failure and poorly-controlled diabetes, and medication such as loop diuretics and proton pomp inhibitors (56), all of which are highly prevalent in the nursing home setting. Indeed, our data showed that more than 20% of the residents were treated with loop diuretics and 22% with proton pump inhibitors. Chronic hypomagnesemia may be implicated in the pathogenesis of various metabolic disorders such as obesity, insulin resistance, hypertension or low-grade inflammation (56). Magnesium supplementation may thus reduce the cardiovascular risk affecting largely institutionalized older adults. In the short term, magnesium supplementation may reduce the risk of atrial fibrillation (57) that affects 7.5%−17% of nursing home residents (58) and reduce blood pressure (59), that can help reduce the risk of cardiovascular and cerebrovascular events. Selenium, as well as zinc deficiencies are associated with the prevalence and prognosis of heart failure (60–62). Neuropsychiatric disorders are also highly prevalent in nursing homes. Deficiencies in B vitamins were associated with an higher risk of depression, whereas B vitamin-fortified foods consumption was associated with a lower risk (63). Observational studies suggest that the association between malnutrition and depression may be partly explained by multiple micronutrient deficiencies (64). The role of B vitamins in cognition is also debated. Higher concentrations in vitamin B12 and folates are associated with better cognitive status in transversal studies, but not with a reduction of cognitive decline in longitudinal studies (65). Results from intervention trials are heterogeneous, thereby making it difficult to conclude on the efficacy of vitamin B supplementation to prevent cognitive decline (66).
ONS are usually prescribed for more than 1 month and it may be hypothesized that the ONS we tested would result in the correction of all deficiencies if prescribed for a longer duration. However, previously published studies that involved ONS prescription up to 24 weeks also did not completely correct micronutrient status (15, 26, 28). We add to these studies by using an ONS that was specially formulated for older adults, with 50% of RDA for older adults (29). In comparison, previously mentioned studies used ONS with either one third of RDA (28), 25%−175% of RDA with enhanced amounts of antioxidants (15), or no reference to RDA (26). In contrast to the composition of ONS used in other studies, we used a richer ONS (600 kcal and 30 g of protein per unit), a composition that reaches or exceeds both energy and protein levels that are recommended for ONS prescription in malnourished older adults: at least 400 kcal and 30 g of protein/day provided by ONS in addition to regular meals (3).
In our study, the improvement in micronutrient status was favored by a notably high compliance to ONS (88%), as compared to previously reported compliance to ONS (67, 68). This high compliance to the ONS was associated with a significant weight gain in only 4 weeks. In the same time, the proportion of residents with “moderate to severe decline in food intake” significantly decreased. This adds to previously reported data suggesting that ONS consumption has little effect on usual food intake and thus increases total energy intake (3, 67). Lastly, no change in body composition and physical performance (Timed Up and Go test) was observed. This may be partly explained by the short duration of the study. However, our study protocol included no physical activity, and thus had little chance of improving muscle mass and function.
In the present study, non-inclusion criteria included chronic kidney disease with estimated glomerular filtration rate <30 ml/min. Bearing this in mind, the ONS was given to all other residents regardless of renal function. On one hand, high protein intake may contribute to accelerate renal failure in patients with chronic kidney disease on the long term. On the other hand, low protein intake may worsen muscle wasting in malnourished subjects, with short-term consequences. Thus, protein restriction is only recommended (69) in “metabolically stable” patients (i.e., absence of any active inflammatory or infectious diseases, no hospitalization within 2 weeks, absence of poorly controlled diabetes and consumptive diseases such as cancer, absence of antibiotic or immunosuppressive medications, and absence of significant short-term loss of body weight).” In addition, oral protein-energy supplementation is recommended in adults with CKD 3–5D (2D) at risk of or with protein-energy wasting, “a minimum of a 3-month trial of oral nutritional supplements to improve nutritional status if dietary counseling alone does not achieve sufficient energy and protein intake to meet nutritional requirements” (69).
Our study has limitations. First, plasma measurements of vitamins and minerals may not reflect perfectly their whole body status. Second, dietary intake of micronutrients was not assessed: this constitutes a potential confounding factor to interpret the results. Also, in this real-life study, the quality, quantity and timing of the regular meals and the rate of ONS consumption (either all at once or spread throughout the day), which were not recorded, may have had an impact on micronutrient absorption and plasma concentration changes. Third, residents could choose each day to consume either a salty or a sweet flavored ONS, but this choice was not rated. The salty ONS contains more sodium and chloride (Table 1), bringing salt (NaCl) intake from 0.7 g in the sweet flavored ONS to 1.5 g in the salty ONS. Although a higher intake of sodium may favor excretion of calcium in the renal proximal tubule (70), sodium and chloride intake is not known to change micronutrient absorption and metabolism (71). Lastly, we did not assess the clinical consequences of micronutrient deficiencies. This point deserves further studies including a larger population. However, very few clinical interventional studies concerning micronutrients in older adults living in nursing homes have been reported, and most of them have been published more than 20 years ago (13, 25, 26, 28). We add to these studies by reporting baseline and changes in plasma concentrations of eight vitamins and trace elements in response to consumption of a high energy and high protein ONS.
In conclusion, we showed that an ONS containing 50% of recommended daily allowances for vitamin, mineral and trace elements improves micronutrient status, but micronutrient plasma levels did not reach normal levels in all participants after 4 weeks and 40% of them had still at least one micronutrient deficiency. We believe that vitamin, mineral and trace element supplements should be routinely prescribed in combination with energy and protein dense ONS in institutionalized older adults at the beginning of their nutritional care. Further studies will be needed to determine the doses and duration of micronutrient supplementation that are needed to correct micronutrient status in institutionalized older adults. The issue of correcting micronutrient deficiencies should also be addressed in older malnourished hospitalized patients and in those living at home.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Comité de Protection des Personnes Ile-de-France—Paris VI. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization: LC, JG, CA, OB, and AR-S. Methodology: NN, LB, CA, AR-S, and LC. Validation, formal analysis, and data curation: LB, MS, and PC-A. Investigation and project administration: JG and BD. Writing—original draft preparation: AC, AR-S, MS, and PC-A. Writing—review and editing: AC, NN, MS, PC-A, AR-S, OB, CA, BD, and LC. Supervision: AR-S and LC. Funding acquisition: LC. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We would like to thank all the medical practioners that included the residents in the nursing homes: Dr. Amar P. (EHPAD Korian Les Sarments, Suresnes), Dr. Bakouch M. (Clinique La Polysiane, Aubagne), Dr. Bayle C. (EHPAD Péan, Paris), Dr. Carlier J. P. (Résidence des Sapins, Rouen), Dr. Ciuperger S. (Maison Bethlehem et Résidence Laury Munch, Strasbourg), Dr. Djeghloul T. and Dr. Legoue E. (Centre Hospitalier and Maison de Retraite, Sainte-Foy-la-Grande), Dr. Drapeau M. (EHPAD Les Romarins, Villeveyrac), Dr. Kholer D. (Résidence d'Automne, Reims), Dr. Lachamp M. (Centre Gérontologique Départemental, Marseille), Dr. Lambrou I. (EHPAD Caritas, Strasbourg), Dr. Rabatel C. (Maison Le Chatelet, Meudon and Maison Les Poètes, Malakoff), Dr. Nasfi A. (USLD La Roseraie, Neuilly-sur-Marne), Dr. Nkoua M. N. (La Chesnaye, Suresnes), Dr. Plutino M. (Résidence Les Séolanes, Marseille), Dr. Roch B. (Pôle gérontologique Cos Saint Maur, Marseille), and Dr. Villiers-Caron B. (CHG La Filandière Deville-Les-Rouen).
Funding Statement
This research was funded by Nestlé Health Science.
Conflict of interest
LC is a shareholder of the Citrage Company and a member of its Scientific Committee. He received personal fees from Nestlé Health Science for participation in this clinical study. URP 4466 received an unrestricted grant from Nestlé Health Science to perform studies and biological measurements. AR-S declares receiving personal fees from Nestlé Health Science for participation in clinical studies, training courses and congress conferences. OB declares receiving personal fees from Nestlé Health Science for participation in clinical studies. JG was an employee of Nestlé Health Science when conducting the clinical study and an employee of SANOFI when writing this article. LB was employed by Soladis Group. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1249936/full#supplementary-material
References
- 1.Vural Z, Avery A, Kalogiros DI, Coneyworth LJ, Welham SJM. Trace mineral intake and deficiencies in older adults living in the community and institutions: a systematic review. Nutrients. (2020) 12:1072. 10.3390/nu12041072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torbahn G, Sulz I, Großhauser F, Hiesmayr MJ, Kiesswetter E, Schindler K, et al. Predictors of incident malnutrition-a nutritionDay analysis in 11,923 nursing home residents. Eur J Clin Nutr. (2022) 76:382–8. 10.1038/s41430-021-00964-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volkert D, Beck AM, Cederholm T, Cruz-Jentoft A, Goisser S, Hooper L, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr Edinb Scotl. (2019) 38:10–47. 10.1016/j.clnu.2018.05.024 [DOI] [PubMed] [Google Scholar]
- 4.Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition - a consensus report from the global clinical nutrition community. Clin Nutr Edinb Scotl. (2019) 38:1–9. 10.1016/j.clnu.2019.02.033 [DOI] [PubMed] [Google Scholar]
- 5.Assis BS, Jairza JMB-M, Lopes JA, Roriz AKC, Melo AL, Previdell A, et al. Micronutrient intake in elderly living in nursing homes. Nutr Hosp. (2018) 35:59–64. 10.20960/nh.1348 [DOI] [PubMed] [Google Scholar]
- 6.Rakicioglu N, Aksoy B, Tamer F, Yildiz EA, Samur G, Pekcan G, et al. Nutritional status and eating habits of the institutionalised elderly in Turkey: a follow-up study. J Hum Nutr Diet. (2016) 29:185–95. 10.1111/jhn.12320 [DOI] [PubMed] [Google Scholar]
- 7.Lengyel CO, Whiting SJ, Zello GA. Nutrient inadequacies among elderly residents of long-term care facilities. Can J Diet Pract Res. (2008) 69:82–8. 10.3148/69.2.2008.82 [DOI] [PubMed] [Google Scholar]
- 8.Aghdassi E, McArthur M, Liu B, McGeer A, Simor A, Allard JP. Dietary intake of elderly living in Toronto long-term care facilities: comparison to the dietary reference intake. Rejuvenation Res. (2007) 10:301–9. 10.1089/rej.2006.0530 [DOI] [PubMed] [Google Scholar]
- 9.Suominem M, Laine T, Routasalo P, Pitkala KH, Rasanen L. Nutrient content of served food, nutrient intake and nutritional status of residents with dementia in a Finnish nursing home. J Nutr Health Aging. (2004) 8:234–8. [PubMed] [Google Scholar]
- 10.Girodon F, Lombard M, Galan P, Brunet-Lecomte P, Monget AL, Arnaud J, et al. Effect of micronutrient supplementation on infection in institutionalized elderly subjects: a controlled trial. Ann Nutr Metab. (1997) 41:98–107. 10.1159/000177984 [DOI] [PubMed] [Google Scholar]
- 11.Keller HH, Lengyel C, Carrier N, Slaughter SE, Morrison J, Duncan AM, et al. Prevalence of inadequate micronutrient intakes of Canadian long-term care residents. Br J Nutr. (2018) 119:1047–56. 10.1017/S0007114518000107 [DOI] [PubMed] [Google Scholar]
- 12.Vucea V, Keller HH, Morrison JM, Duncan AM, Duizer LM, Lengyel CO, et al. Intake and factors associated with consumption of pureed food in long term care: an analysis of making the most of mealtimes (M3) project. J Nutr Gerontol Geriatr. (2018) 37:59–81. 10.1080/21551197.2018.1470056 [DOI] [PubMed] [Google Scholar]
- 13.van der Wielen RP, van Heereveld HA, de Groot CP, van Staveren WA. Nutritional status of elderly female nursing home residents; the effect of supplementation with a physiological dose of water-soluble vitamins. Eur J Clin Nutr. (1995) 49:665–74. [PubMed] [Google Scholar]
- 14.Grieger JA, Nowson CA, Jarman HF, Malon R, Ackland LM. Multivitamin supplementation improves nutritional status and bone quality in aged care residents. Eur J Clin Nutr. (2009) 63:558–65. 10.1038/sj.ejcn.1602963 [DOI] [PubMed] [Google Scholar]
- 15.Manders M, de Groot CPGM, Blauw YH, Dhonukshe-Rutten RAM, van Hoeckel-Prüst L, Bindels JG, et al. Effect of a nutrient-enriched drink on dietary intake and nutritional status in institutionalised elderly. Eur J Clin Nutr. (2009) 63:1241–50. 10.1038/ejcn.2009.28 [DOI] [PubMed] [Google Scholar]
- 16.Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. Nutrients. (2020) 12:236. 10.3390/nu12010236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maywald M, Rink L. Zinc homeostasis and immunosenescence. J Trace Elem Med Biol. (2015) 29:24–30. 10.1016/j.jtemb.2014.06.003 [DOI] [PubMed] [Google Scholar]
- 18.Munoz N, Posthauer ME, Cereda E, Schols JMGA, Haesler E. The role of nutrition for pressure injury prevention and healing: the 2019 international clinical practice guideline recommendations. Adv Skin Wound Care. (2020) 33:123–36. 10.1097/01.ASW.0000653144.90739.ad [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Wang Q, Zhang Z, Fu R, Zhou T, Long C, et al. Magnesium supplementation enhances mTOR signalling to facilitate myogenic differentiation and improve aged muscle performance. Bone. (2021) 146:115886. 10.1016/j.bone.2021.115886 [DOI] [PubMed] [Google Scholar]
- 20.Yang S-W, Chen Y-Y, Chen W-L. Association between oral intake magnesium and sarcopenia: a cross-sectional study. BMC Geriatr. (2022) 22:816. 10.1186/s12877-022-03522-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ter Borg S, Verlaan S, Hemsworth J, Mijnarends DM, Schols JMGA, Luiking YC, et al. Micronutrient intakes and potential inadequacies of community-dwelling older adults: a systematic review. Br J Nutr. (2015) 113:1195–206. 10.1017/S0007114515000203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopes Junior E, Leite HP, Konstantyner T. Selenium and selenoproteins: from endothelial cytoprotection to clinical outcomes. Transl Res J Lab Clin Med. (2019) 208:85–104. 10.1016/j.trsl.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 23.Allen LH. How common is vitamin B-12 deficiency? Am J Clin Nutr. (2009) 89:693S−6S. 10.3945/ajcn.2008.26947A [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Zhu W, Xing Y, Jia J, Tang Y. B vitamins and prevention of cognitive decline and incident dementia: a systematic review and meta-analysis. Nutr Rev. (2022) 80:931–49. 10.1093/nutrit/nuab057 [DOI] [PubMed] [Google Scholar]
- 25.Girodon F, Blache D, Monget AL, Lombart M, Brunet-Lecompte P, Arnaud J, et al. Effect of a two-year supplementation with low doses of antioxidant vitamins and/or minerals in elderly subjects on levels of nutrients and antioxidant defense parameters. J Am Coll Nutr. (1997) 16:357–65. 10.1080/07315724.1997.10718698 [DOI] [PubMed] [Google Scholar]
- 26.Wouters-Wesseling W, Wouters AEJ, Kleijer CN, Bindels JG, de Groot CPGM, van Staveren WA. Study of the effect of a liquid nutrition supplement on the nutritional status of psycho-geriatric nursing home patients. Eur J Clin Nutr. (2002) 56:245–51. 10.1038/sj.ejcn.1601319 [DOI] [PubMed] [Google Scholar]
- 27.The European Commission . Commission Delegated Regulation (EU) 2016/128 of 25 September 2015 supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as Regards the Specific Compositional and Information Requirements for Food for Special Medical Purposes. [Official Journal of the European Union]. (2015). Available online at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016R0128&rid=1 (accessed January 18, 2023).
- 28.Fiatarone Singh MA, Bernstein MA, Ryan AD, O'Neill EF, Clements KM, Evans WJ. The effect of oral nutritional supplements on habitual dietary quality and quantity in frail elders. J Nutr Health Aging. (2000) 4:5–12. [PubMed] [Google Scholar]
- 29.Martin A. Apports Nutritionnels Conseillés Pour la Population Française. 3rd ed. (2001), p. 307–36.2772360 [Google Scholar]
- 30.>Raynaud-Simon A, Revel-Delhom C, Hébuterne X, French Nutrition and Health Program French Health High Authority . Clinical practice guidelines from the French Health High Authority: nutritional support strategy in protein-energy malnutrition in the elderly. Clin Nutr Edinb Scotl. (2011) 30:312–9. 10.1016/j.clnu.2010.12.003 [DOI] [PubMed] [Google Scholar]
- 31.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–98. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 32.Commission Directive 1999/21/EC of 25 March 1999 on Dietary Foods for Special Medical Purposes (Text with EEA relevance) . Available online at: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:1999L0021:20070119:EN:PDF
- 33.Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv Plan Adm Eval. (1976) 6:493–508. 10.2190/UURL-2RYU-WRYD-EY3K [DOI] [PubMed] [Google Scholar]
- 34.Gröber U, Schmidt J, Kisters K. Important drug-micronutrient interactions: a selection for clinical practice. Crit Rev Food Sci Nutr. (2020) 60:257–75. 10.1080/10408398.2018.1522613 [DOI] [PubMed] [Google Scholar]
- 35.Podsiadlo D, Richardson S. The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. (1991) 39:142–8. 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- 36.Kuhn M, Nakib S, De Bandt JP, Cynober L, Loï C. Simultaneous determination of retinol and alpha-tocopherol in polymeric diets for enteral nutrition. J Chromatogr A. (2008) 1205:186–90. 10.1016/j.chroma.2008.07.076 [DOI] [PubMed] [Google Scholar]
- 37.Rümelin A, Fauth U, Halmágyi M. Determination of ascorbic acid in plasma and urine by high performance liquid chromatography with ultraviolet detection. Clin Chem Lab Med. (1999) 37:533–6. 10.1515/CCLM.1999.086 [DOI] [PubMed] [Google Scholar]
- 38.Zhou YK Li H, Liu Y. Determination of vitamin B12 by chemiluminescence analysis. Yao Xue Xue Bao. (1989) 24:611–7. [PubMed] [Google Scholar]
- 39.Jeffery J, Frank AR, Hockridge S, Stosnach H, Costelloe SJ. Method for measurement of serum copper, zinc and selenium using total reflection X-ray fluorescence spectroscopy on the PICOFOX analyser: validation and comparison with atomic absorption spectroscopy and inductively coupled plasma mass spectrometry. Ann Clin Biochem. (2019) 56:170–8. 10.1177/0004563218793163 [DOI] [PubMed] [Google Scholar]
- 40.Berger MM, Shenkin A, Schweinlin A, Amrein K, Augsburger M, Biesalski H-K, et al. ESPEN micronutrient guideline. Clin Nutr Edinb Scotl. (2022) 41:1357–424. 10.1016/j.clnu.2022.02.015 [DOI] [PubMed] [Google Scholar]
- 41.Schimatschek HF, Rempis R. Prevalence of hypomagnesemia in an unselected German population of 16,000 individuals. Magnes Res. (2001) 14:283−90. [PubMed] [Google Scholar]
- 42.de Benoist B. Conclusions of a WHO technical consultation on folate and vitamin B12 deficiencies. Food Nutr Bull. (2008) 29:S238–244. 10.1177/15648265080292S129 [DOI] [PubMed] [Google Scholar]
- 43.Thurnham DI, Davies JA, Crump BJ, Situnayake RD, Davis M. The use of different lipids to express serum tocopherol: lipid ratios for the measurement of vitamin E status. Ann Clin Biochem. (1986) 23:514–20. 10.1177/000456328602300505 [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization . Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations. World Health Organization; (2011), p. 5. Available online at: https://apps.who.int/iris/handle/10665/85859 (accessed May 21, 2023). [Google Scholar]
- 45.Hotz C, Peerson JM, Brown KH. Suggested lower cutoffs of serum zinc concentrations for assessing zinc status: reanalysis of the second National Health and Nutrition Examination Survey data (1976-1980). Am J Clin Nutr. (2003) 78:756–64. 10.1093/ajcn/78.4.756 [DOI] [PubMed] [Google Scholar]
- 46.O'Brien PC. Procedures for comparing samples with multiple endpoints. Biometrics. (1984) 40:1079–87. 10.2307/2531158 [DOI] [PubMed] [Google Scholar]
- 47.MacDonell SO, Miller JC, Harper MJ, Reid MR, Haszard JJ, Gibson RS, et al. Multiple micronutrients, including zinc, selenium and iron, are positively associated with anemia in new zealand aged care residents. Nutrients. (2021) 13:1072. 10.3390/nu13041072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wörwag M, Classen HG, Schumacher E. Prevalence of magnesium and zinc deficiencies in nursing home residents in Germany. Magnes Res. (1999) 12:181–9. [PubMed] [Google Scholar]
- 49.Carr AC, Zawari M. Does aging have an impact on vitamin C status and requirements? A scoping review of comparative studies of aging and institutionalisation. Nutrients. (2023) 15:915. 10.3390/nu15040915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grieger J, Nowson C, Ackland ML. Anthropometric and biochemical markers for nutritional risk among residents within an Australian residential care facility. Asia Pac J Clin Nutr. (2007) 16:178–86. [PubMed] [Google Scholar]
- 51.Galan P, Preziosi P, Monget AL, Richard MJ, Arnaud J, Lesourd B, et al. Effects of trace element and/or vitamin supplementation on vitamin and mineral status, free radical metabolism and immunological markers in elderly long term-hospitalized subjects. Geriatric Network MIN. VIT. AOX. Int J Vitam Nutr Res. (1997) 67:450–60. [PubMed] [Google Scholar]
- 52.Girodon F, Galan P, Monget AL, Boutron-Ruault MC, Brunet-Lecomte P, Preziosi P, et al. Impact of trace elements and vitamin supplementation on immunity and infections in institutionalized elderly patients: a randomized controlled trial. MIN VIT AOX geriatric network. Arch Intern Med. (1999) 159:748–54. 10.1001/archinte.159.7.748 [DOI] [PubMed] [Google Scholar]
- 53.Anthony D, Alosoumi D, Safari R. Prevalence of pressure ulcers in long-term care: a global review. J Wound Care. (2019) 28:702–9. 10.12968/jowc.2019.28.11.702 [DOI] [PubMed] [Google Scholar]
- 54.Cereda E, Klersy C, Serioli M, Crespi A, D'Andrea F, OligoElement Sore Trial Study Group. A nutritional formula enriched with arginine, zinc, and antioxidants for the healing of pressure ulcers: a randomized trial. Ann Intern Med. (2015) 162:167–74. 10.7326/M14-0696 [DOI] [PubMed] [Google Scholar]
- 55.Raynaud-Simon A, Cohen-Bittan J, Gouronnec A, Pautas E, Senet P, Verny M, et al. Scurvy in hospitalized elderly patients. J Nutr Health Aging. (2010) 14:407–10. 10.1007/s12603-010-0032-y [DOI] [PubMed] [Google Scholar]
- 56.Pelczyńska M, Moszak M, Bogdański P. The role of magnesium in the pathogenesis of metabolic disorders. Nutrients. (2022) 14:1714. 10.3390/nu14091714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lutsey PL, Chen LY, Eaton A, Jaeb M, Rudser KD, Neaton JD, et al. Pilot randomized trial of oral magnesium supplementation on supraventricular arrhythmias. Nutrients. (2018) 10:884. 10.3390/nu10070884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abdel-Latif AK, Peng X, Messinger-Rapport BJ. Predictors of anticoagulation prescription in nursing home residents with atrial fibrillation. J Am Med Dir Assoc. (2005) 6:128–31. 10.1016/j.jamda.2005.01.006 [DOI] [PubMed] [Google Scholar]
- 59.Dominguez L, Veronese N, Barbagallo M. Magnesium and hypertension in old age. Nutrients. (2020) 13:139. 10.3390/nu13010139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bomer N, Grote Beverborg N, Hoes MF, Streng KW, Vermeer M, Dokter MM, et al. Selenium and outcome in heart failure. Eur J Heart Fail. (2020) 22:1415–23. 10.1002/ejhf.1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu X, Huang L, Zhao J, Wang Z, Yao W, Wu X, et al. The relationship between serum zinc level and heart failure: a meta-analysis. BioMed Res Int. (2018) 2018:2739014. 10.1155/2018/2739014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoshihisa A, Abe S, Kiko T, Kimishima Y, Sato Y, Watanabe S, et al. Association of serum zinc level with prognosis in patients with heart failure. J Card Fail. (2018) 24:375–83. 10.1016/j.cardfail.2018.02.011 [DOI] [PubMed] [Google Scholar]
- 63.Moore K, Hughes CF, Hoey L, Ward M, Cunningham C, Molloy AM, et al. B-vitamins in relation to depression in older adults over 60 years of age: the Trinity Ulster Department of Agriculture (TUDA) Cohort Study. J Am Med Dir Assoc. (2019) 20:551–7.e1. 10.1016/j.jamda.2018.11.031 [DOI] [PubMed] [Google Scholar]
- 64.Chrzastek Z, Guligowska A, Sobczuk P, Kostka T. Dietary factors, risk of developing depression, and severity of its symptoms in older adults-A narrative review of current knowledge. Nutrition. (2023) 106:111892. 10.1016/j.nut.2022.111892 [DOI] [PubMed] [Google Scholar]
- 65.Zhang C, Luo J, Yuan C, Ding D. Vitamin B12, B6, or folate and cognitive function in community-dwelling older adults: a systematic review and meta-analysis. J Alzheimers Dis JAD. (2020) 77:781–94. 10.3233/JAD-200534 [DOI] [PubMed] [Google Scholar]
- 66.Ford AH, Almeida OP. Effect of vitamin B supplementation on cognitive function in the elderly: a systematic review and meta-analysis. Drugs Aging. (2019) 36:419–34. 10.1007/s40266-019-00649-w [DOI] [PubMed] [Google Scholar]
- 67.Hubbard GP, Elia M, Holdoway A, Stratton RJ. A systematic review of compliance to oral nutritional supplements. Clin Nutr Edinb Scotl. (2012) 31:293–312. 10.1016/j.clnu.2011.11.020 [DOI] [PubMed] [Google Scholar]
- 68.Grönstedt H, Vikström S, Cederholm T, Franzén E, Luiking YC, Seiger Å, et al. Effect of sit-to-stand exercises combined with protein-rich oral supplementation in older persons: the Older Person's Exercise and Nutrition Study. J Am Med Dir Assoc. (2020) 21:1229–37. 10.1016/j.jamda.2020.03.030 [DOI] [PubMed] [Google Scholar]
- 69.Ikizler TA, Burrowes JD, Byham-Gray LD, Campbell KL, Carrero J-J, Chan W, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis. (2020) 76:S1–S107. 10.1053/j.ajkd.2020.05.006 [DOI] [PubMed] [Google Scholar]
- 70.Hanna RM, Ahdoot RS, Kalantar-Zadeh K, Ghobry L, Kurtz I. Calcium transport in the kidney and disease processes. Front Endocrinol. (2021) 12:762130. 10.3389/fendo.2021.762130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kiela PR, Ghishan FK. Physiology of intestinal absorption and secretion. Best Pract Res Clin Gastroenterol. (2016) 30:145–59. 10.1016/j.bpg.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.