Abstract
This review aimed to assess the diagnostic utility of fecal calprotectin (FCP) for identifying organic gastrointestinal disease (OGID) in patients undergoing colonoscopy for gastrointestinal discomfort or active progression of inflammatory bowel disease (IBD). Studies published between January 2013 and December 2022 evaluating the clinical efficacy of FCP for differentiating OGID against functional gastrointestinal disease (FGID) were identified using PubMed, Cochrane, and Scopus databases. Clinical diagnostic studies involving individuals with lower gastrointestinal symptoms; using FCP as a diagnostic biomarker either in primary, secondary, or tertiary healthcare centers conducted either prospectively or retrospectively using stool samples (index test), contrasting FCP with a reference test, such as colonoscopy, or endoscopy, and assessed using enzyme-linked immunosorbent assay were reviewed. The included studies were subjected to the revised Quality Assessment of Diagnostic Accuracy Studies for assessing the methodological quality by two independent authors. An initial literature search yielded 545 articles rendering 417 records after removing the duplicate records. After reading the abstracts and titles, 89 articles were eligible for full-text screening. The qualitative synthesis resulted in 20 articles. The efficient use of FCP for differentiating IBD from irritable bowel syndrome was investigated in 15 studies.Two of the included studies assessed the diagnostic ability of FCP to distinguish OGID from FGID, two studies utilized patients with ulcerative colitis, and one study involved patients with Crohn’s disease. Overall study quality was high for 65% of studies,moderate for 25% of studies, and low for 10% of studies. The review outlined the diagnostic accuracy of non-invasive FCP assessment for OGID in various clinical scenarios and in individuals of various ages. FCP is used as a tool for screening and monitoring in clinical practice for determining the need of further comprehensive investigations, thereby reducing the redundant use of invasive techniques.
Keywords: organic gastrointestinal disease, inflammatory bowel disease, fecal calprotectin, endoscopy, elisa
Introduction and background
Distinguishing organic gastrointestinal disease (OGID), such as inflammatory bowel disease (IBD), from functional gastrointestinal disease (FGID), such as irritable bowel syndrome (IBS), constitutes one of the diagnostic difficulties encountered, especially in patients with mild disease conditions, as both categories of diseases possess many similar clinical manifestations [1,2]. The key difference between OGID and FGID is inflammatory conditions. Chronic FGIDs are idiopathic gastrointestinal motility disorders that are more common than OGIDs [3,4]. IBD is an organic condition with multifaceted pathogenesis and numerous factors that may be responsible for developing the condition, including intestinal dysbiosis, state of oxidative stress, altered immune system responses within the gastrointestinal tract, and epigenetics [5].
The two most common types of IBD are ulcerative colitis (UC) and Crohn’s disease (CD). Consequently, these diseases cause diarrhea, abdominal pain, intestinal ulcers, fatigue, weight loss, and rectal bleeding. IBD has evolved into a more common pathology in people of all ages, with an estimated 6.8 million individuals being affected globally. The first peak age of onset of IBD was reported to be 30-40 years and the second peak at 60-70 years [4,6,7]. IBS-like manifestations are frequently observed in patients before the diagnosis of IBD [8].
Endoscopy and histopathological assessment continue to be the benchmark for identifying and evaluating bowel inflammation. It does, however, have the drawbacks of being invasive in nature, time-consuming, and poorly accepted by patients. The most commonly used laboratory inflammatory factors, such as erythrocyte sedimentation rate and C-reactive protein, were found to have poor specificity or sensitivity and are inadequately correlated with disease activity [8]. Using fecal calprotectin (FCP) as a diagnostic procedure to differentiate OGID from FGID might mitigate the need for invasive techniques such as a colonoscopy [2]. FCP is a cytosolic protein that has an affinity to bind with calcium which was noticed in the neutrophils and macrophages of the patients. FCP has antiproliferative and antimicrobial characteristics that constitute approximately 60% of the total protein in the cytosol fraction. Fecal markers may have an increased degree of specificity for OGID as feces come into close proximity with the mucosa of the colon [2,9].
FCP is also thought to be a biological marker of intestinal inflammation because it is associated with the infiltration of neutrophils of the intestinal mucosa. It is also resistant to degradation caused by enzymes during digestion and can be stored at ambient temperature for a period of seven days. A readily available quantitative enzyme-linked immunosorbent assay (ELISA) can be used for assessing FCP levels [10]. Additionally, alterations in FCP levels serve as a good marker of healing of the mucous membrane or recurrent episodes of inflammation. As a result, FCP can be utilized for evaluating IBD patients as well as to detect those who are vulnerable to relapse [4].
This review aims to assess the diagnostic utility of FCP as a fecal marker for identifying OGID in patients undertaking colonoscopy for gastrointestinal discomfort or active progression in IBD through monitoring its concentration.
Review
Methodology
Primary Outcome
To evaluate the diagnostic utility of FCP as a fecal marker for identifying OGID in patients undergoing colonoscopy for gastrointestinal discomfort or active progression of IBD through the assessment of its concentration.
Secondary Outcome
To determine the sensitivity and specificity of FCP in distinguishing OGID from non-OGID conditions in patients with gastrointestinal discomfort or active progression of IBD.
The study protocol adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The structured question for the review was “Is the FCP testing an exceptionally useful means of differentiating OGID from FGID for those with lower gastrointestinal symptoms?”
Information Sources and Search Strategy
Scholarly studies published in the English language between January 2013 and December 2022 evaluating the clinical efficacy of FCP testing for differentiating OGID against FGID were identified using PubMed, Cochrane, and Scopus databases. The following keywords were applied singly or in combination: (“Crohn’s disease,” OR “Ulcerative colitis,” OR “Inflammatory bowel disease,” OR “Functional gastrointestinal disorder,” OR “Irritable colon”) AND (“IBS,” OR “IBD”) AND (“fecal calprotectin,” OR “Primary care provider,” OR “Secondary care”) AND (“Endoscopy” OR “Colonoscopy”). We also searched the references of the identified articles for additional studies.
Eligibility Criteria
Population: Individuals of all age groups with lower gastrointestinal symptoms. Patients with symptoms such as positive occult blood tests in the feces, explicit rectal bleeding, iron deficiency anemia, abdominal masses, colon cancer, or a family history of bowel cancer were excluded.
Intervention: FCP was investigated as a diagnostic biomarker either in the primary, secondary, or tertiary care settings. Data were collected either prospectively or retrospectively using stool samples and were regarded as the index test.
Comparator: FCP testing was contrasted with a reference test, such as colonoscopy, or endoscopy.
Outcome measures: FCP was assessed using the standard ELISA method.
Study design: The clinical diagnostic studies that evaluated FCP in the context of OGID, IBD, UC, or CD were included. Animal studies, preclinical studies, case reports, case series, systematic reviews, and meta-analyses were excluded.
Selection of Studies, Data Collection, and Data Extraction Process
Studies that met the inclusion criteria were selected for full-text evaluation after being scrutinized based on their titles or abstracts. Two independent authors evaluated the studies that had been chosen. Whenever there was a disagreement, a third author was approached.
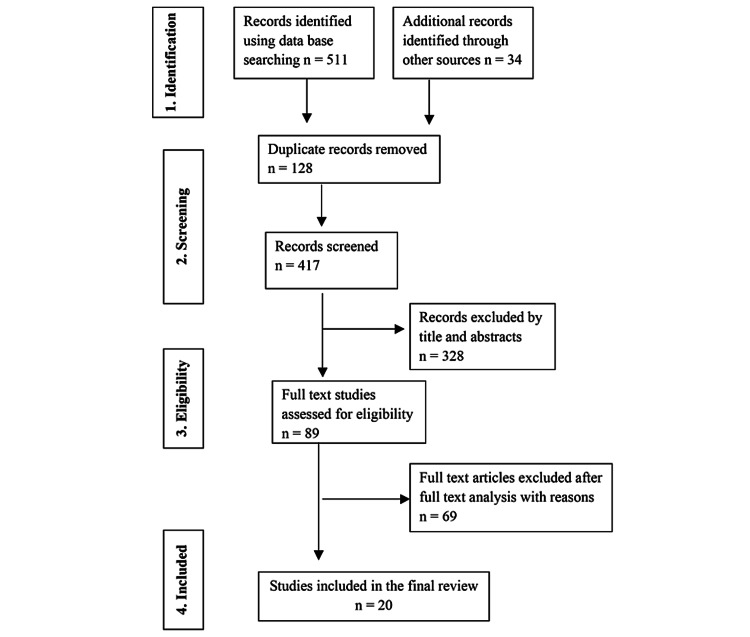
Figure 1 depicts the search and selection strategy for the review. In a predefined table, author(s), year of publication, location of the study, sample size, mean age and gender of the sample population, outcome measures, reference or standard tests used, cut-off values, sensitivity, and specificity obtained from the receiver operating characteristic (ROC) curve were collected. If there was a discrepancy in the data gathered, the corresponding authors of each article were approached. A meta-analysis was not feasible due to the multiple facets of the reviewed studies regarding methodological quality, setting, the population of interest, and measurements of outcomes.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-analyses flowchart of the included studies.
Quality Assessment of Individual Studies
All included studies for review were subjected to the revised Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool for assessing the methodological quality by two independent authors [11]. QUADAS-2 evaluates diagnostic accuracy studies in four distinct areas, namely, patient selection, index test, reference standard, and flow and timing. The risk of bias (RoB) and the applicability of the study outcomes were assessed for each domain. When there was a low RoB in six or more of the seven subdomains, a study was assigned to have high quality. When there was high risk or unclear risk in a minimum of four subdomains, a study was ranked as low quality. All other studies were judged as moderate quality. Disagreements were resolved through deliberation with a third reviewer.
Results
Study Selection
The PRISMA flowchart was used to guide the article review and data extraction process (Figure 1). An initial literature search yielded 511 articles through an electronic search and 34 studies from a manual search. Duplicates were removed, rendering 417 records. After reading the abstracts and titles, 328 studies were eliminated. Out of 89 articles that were eligible for full-text screening, 69 were rejected because they either failed to establish adequate information or did not evaluate the effectiveness of FCP testing. Hence, the qualitative synthesis consisted of 20 articles [12-31].
The efficient use of FCP testing for differentiating IBD from IBS was investigated in 15 studies [12,14,16,18-20,22,23,25-31]. Two of the included studies assessed the diagnostic ability of FCP testing to distinguish OGID from FGID [21,24] and one study distinguished patients with CD [15]. Jha et al. [17] reported on its efficacy in discerning UC from IBS, and Schoepfer et al. [13] compared its clinical utility in those with UC to that of healthy individuals. Table 1 summarizes the studies, sample sizes, demographic details of the sample population, and assessment tools. The majority of the studies reviewed used retrospective study designs [12,13,16,18,19,22-24,27-29,31] In eight studies, the prospective study design was applied [14,15,17,20,21,25,26,30].
Table 1. Summary of demographic characteristics of the reviewed studies.
NS = not specified; CD = Crohn’s disease; UC = ulcerative colitis; IBD = inflammatory bowel disease; IBS = irritable bowel syndrome; YFCCP = York faecal calprotectin care pathway
| Author, year | Country | Study design and setting | Sample size and sample population | Mean age (years) | Gender M/F (M:F) |
| Wang et al., 2013 [12] | China | Retrospective tertiary care | 260 adults | Study group: 46.3 ± 22.5 Control group: 40.9 ± 27.3 | Study group: 72/138 Control group: 25/25 |
| Schoepfer et al., 2013 [13] | Switzerland | Retrospective tertiary care | 280 adults | Study group: 41 ± 13 Control group: 37 ± 9 | Study group: 90 females Control group: 39 females |
| Pavlidis et al., 2013 [24] | UK | Retrospective, primary care | 962 adults | 33 ± 7 | 2:3 |
| Chang et al., 2014 [25] | Taiwan | Prospective, secondary care | 104 adults | 20–70 | NS |
| Kolho et al., 2014 [26] | Finland | Prospective tertiary care | 110 pediatric patients 27 controls | 1.3–18 | 70 males |
| Caviglia et al., 2014 [27] | Sweden | Retrospective, secondary care | 66 adults | 42 (18–78) | 20:46 |
| Kennedy et al., 2015 [28] | UK | Retrospective tertiary care | 895 adults | Median: 29.8 (24.2–39.7) | 51.6% females |
| Kalantari et al., 2015 [29] | Iran | Retrospective tertiary care | 88 adults | 43.2 ± 15.2 years | 50:38 |
| Dhaliwal et al., 2015 [30] | UK | Prospective, secondary care | 311 adults | NS | 1:1.8 |
| Banarjee et al., 2015 [31] | UK | Retrospective, primary care | 119 adults | 46 | 55:64 |
| Turvil et al., 2016 [14] | UK | Prospective, primary care | 262 adults | 36.8 ± 10.9 | 70% females |
| Shitrit et al., 2017 [15] | Israel | Prospective, secondary care | 68 adults | CD: 34 Non-CD: 46 | CD: 65% males Non-CD: 51% males |
| Moein et al., 2017 [16] | Iran | Retrospective, secondary care | 30 adults | 31 ± 7 | 16/14 |
| Jha et al., 2018 [17] | India | Prospective study, tertiary care | 106 adults | UC: 14–60 IBS: 21–60 | UC: 2:1 IBS: 4:1 |
| Sharbatdaran et al., 2018 [18] | Iran | Retrospective tertiary care | 90 adults | 34.69 ± 10.42 | 43.3% males and 56.7% females |
| Conroy et al., 2019 [19] | UK | Retrospective, primary care | 410 adults | 16–91 median age 42 | 162 males |
| Turvil et al., 2018 [20] | UK | Prospective, primary care (YFCCP) | 1,005 adults | 38 | NS |
| Walker et al., 2018 [21] | UK | Prospective, primary care | 789 adults | 18–46 | 54% females |
| Turvill et al., 2020 [22] | UK | Retrospective YFCCP, Primary care | 7,304 adults | 18–60 years | NS |
| Chowdhury et al., 2021 [23] | Bangladesh | Retrospective, tertiary care | 90 adults | IBD: 32.24 ± 9.76 IBS: 33.80 ± 9.70 | IBD: 28:17 IBS: 30:15 |
Table 2 shows the diagnostic kit used for FCP testing, as well as the cut-off values, sensitivity, specificity, and area under the curve (AUC) of ROC curve analysis for predicting FCP diagnostic significance and overall study quality.
Table 2. Summary of receiver operating characteristic curve analysis.
NS = not specified; CI = confidence interval; CD = Crohn’s disease; UC = ulcerative colitis; IBD = inflammatory bowel disease; IBS = irritable bowel syndrome; OGID = organic gastrointestinal disease; FGID = functional gastrointestinal disease; YFCCP = York faecal calprotectin care pathway
| Author, year | Disease | Kit used | Reference standard | Cut-off value | Sensitivity | Specificity | AUC | Study quality |
| Wang et al., 2013 [12] | IBD vs non-IBD | ELISA BÜHLMANN Laboratories | Upper or lower endoscopy | 45.40 µg/g | 0.944 | 0.643 | 0.949 | High |
| Schoepfer et al., 2013 [13] | UC vs. healthy controls | ELISA PhiCal Test | Endoscopy based on the Modified Baron Score and the Lichtiger Clinical Activity Index | 57 µg/g | 91 | 90 | 0.939 (95% CI = 0.898–0.965) | High |
| Pavlidis et al., 2013 [24] | OGID vs. NOGID | BÜHLMANN, Calprotectin ELISA, EK-CAL | Endoscopy | 50 mg/g | 82% (95% CI = 73–89) | 77% (95% CI = 74–80) | 0.89 (0.85–0.93) | High |
| Chang et al., 2014 [25] | IBD vs. IBS | ELISA Quantum Blue LF‑CAL | Endoscopy with biopsies and radiological criteria | 50 mg/g | 62% | 95% | 0.931 ± 0.029 | High |
| Kolho et al., 2014 [26] | IBD and non-IBD | PhiCal ELISA | Upper and lower endoscopy | 59.5 μg/g | 81.8% (95% CI = 73.3–88.5) | 96.3 % (95 % CI = 81.0–99.9) | 0.944 (95 % CI = 0.907–0.981) | High |
| Caviglia et al., 2014 [27] | IBS vs. IBD | ELISA using polyclonal antibody | Colonoscopy with microscopic examination | 150 mg/g | 87.5% | 90.5% | 0.931 | High |
| Kennedy et al., 2015 [28] | IBD vs. IBS | ELISA | Upper or lower endoscopy (Lennard-Jones criteria for diagnosis of IBD and the Montreal criteria to classify clinical phenotype) | 100 μg/g | 96% | 87% | NS | Moderate |
| Kalantari et al., 2015 [29] | IBS vs. IBD | ELISA based on monoclonal antibodies | Colonoscopy | 164 µg/g | 57 (CI = 41%–71.6%) | 75 (CI = 59.7%–56.8%) | 0.67 | High |
| Dhaliwal et al., 2015 [30] | IBD vs. IBS | BÜHLMANN, PhiCal v1 and PhiCal v2 | Endoscopic, histological, and/or radiological confirmation | 50 µg/g | 88% | 78% | 0.84 (CI = 0.78–0.90) | High |
| Banarjee et al., 2015 [31] | IBD vs. IBS | Immunodiagnostik mono-clonal antibody-based ELISA | Colonoscopy with histological examination | 50 µg/g | 100% | 60% | NS | Moderate |
| Turvil et al., 2016 [14] | IBD vs. IBS | ELISA BÜHLMANN | Colonoscopy | 50 µg/g | NS | NS | 0.86 (95% CI = 0.77–0.95) | Moderate |
| Shitrit et al., 2017 [15] | CD vs. non-CD | ELISA IBD SCAN | Capsule endoscopy | 95 mg/kg | 77% | 73% | 0.767 | High |
| Moein et al., 2017 [16] | IBD vs. non-IBD | EK- CAL ELISA (BÜHLMANN) | Colonoscopy with histological examination | 78.4 µg/g | 100% | 100% | 1 | Moderate |
| Jha et al., 2018 [17] | UC vs. IBS | Phadia 100 Calprotectin | Colonoscopy based on Mayo score | 188 µg/g | 98.5% | 96.6% | 0.999 | High |
| Sharbatdaran et al., 2018 [18] | IBD vs. IBS | ELISA Buhlmann Laboratories Kit | Colonoscopy with histopathological examination | 127.65 µg/g | 73% | 89% | 0.83 (95% CI = 0.74–0.91) | High |
| Conroy et al., 2018 [19] | IBD vs. IBS | ELISA (Immundiagnostik) | Colonoscopy | 50 µg/g | 72.7% | 64.9% | 0.69 | High |
| Turvil et al., 2018 [20] | IBD vs. IBS | EK-CAL Calprotectin ELISA (BÜHLMANN) | Endoscopy | 100µg/g | 0.94 (0.85–0.98) | 0.92 (0.90–0.94) | NS | Moderate |
| Walker et al., 2018 [21] | OGID vs. FGID | ELISA (Immundiagnostik) | Colonoscopy | 100 µg/g | 64% | 90.1% | 0.93 (95% CI = 0.88–0.98) | Low |
| Turvill et al., 2020 [22] | YFCCP vs. non- YFCCP | ELISA | Colonoscopy | 100 µg/g | 90.6% (CI = 86–94) | 57.6% (54–61) | NS | Low |
| Chowdhury et al., 2021 [23] | IBD vs. IBS | BÜHLMANN Quantum Blue Reader ELISA | Endoscopy with histological and radiological findings | 50 µg/g | 91.1% | 86.7% | 0.959 (95% CI = 0.909–1.0) | High |
Characteristics of Selected Studies
Three of the eight prospective studies were conducted in primary care centers [14,20,21], three in secondary healthcare centers [15,25,30], and two in tertiary healthcare facilities [17,26]. Similarly, four of the retrospective studies reviewed used samples from primary care settings [19,22,24,31], two from secondary care settings [16,27], and six from tertiary healthcare settings [12,13,18,23,28,29]. Nine reviewed studies were undertaken in the United Kingdom [14,19-22,24,28,30,31], three in Iran [16,18,29], and one each in China [12], Switzerland [13], India [17], Bangladesh [23], Taiwan [25], Finland [26], Sweden [27], and Israel [15].
Except for a study by Kolho et al. [26], which was conducted among children with an average age of 1.3 to 18 years who had chronic lower gastrointestinal symptoms indicating either OGID or FGID, the rest of the studies were conducted among adults 18 years and older. Turvill et al. [22] audited colonoscopy activity to assess the diagnostic precision and influence of the York FCP care pathway among 7,304 adults aged 18 to 60 years.
In the majority of investigations, endoscopy was used as the gold standard. Schoepfer et al. used the Modified Baron Score to assess endoscopically the extent of the disease. This was then correlated with clinical activity measured by the Lichtiger Index and levels of different biological indicators such as C-reactive protein, hemoglobin, platelets, leukocytes, and FCP [13]. Kennedy et al. utilized the Lennard-Jones criteria for IBD diagnosis and the Montreal criteria for the classification of clinical traits [28]. Using the Montreal classification, Jha et al. determined the degree of UC severity during its active phase. The disease activity was classified using Mayo endoscopic subscores [17].
The EK-CAL kit (Bühlmann Laboratories) with a monoclonal antibody against calprotectin was the most commonly used ELISA kit [12,14,16-21,23-25,29-31]. Dhaliwal et al. contrasted three ELISA kits to assess FCP in 311 patients with changed bowel habits: Buhlmann, PhiCal v1, and PhiCal v2 [30]. A polyclonal antibody against FCP (Phical) was used in five other studies [13,15,26-28]. Two studies addressed the economic viability of FCP evaluation [22,28]
RoB Within Studies Using QUADAS-2 Grading
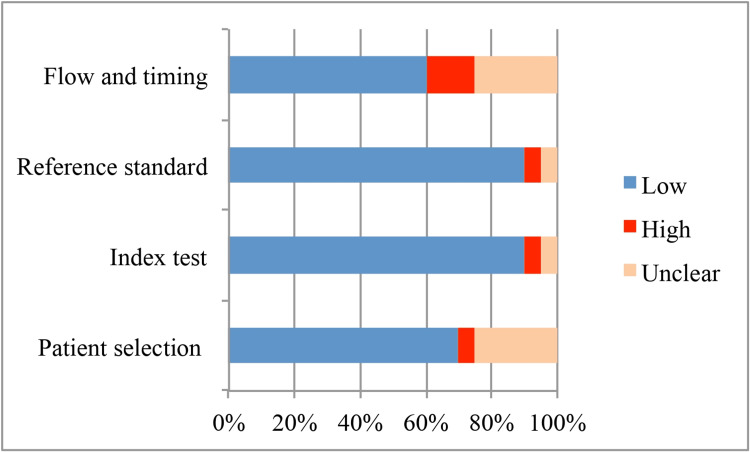
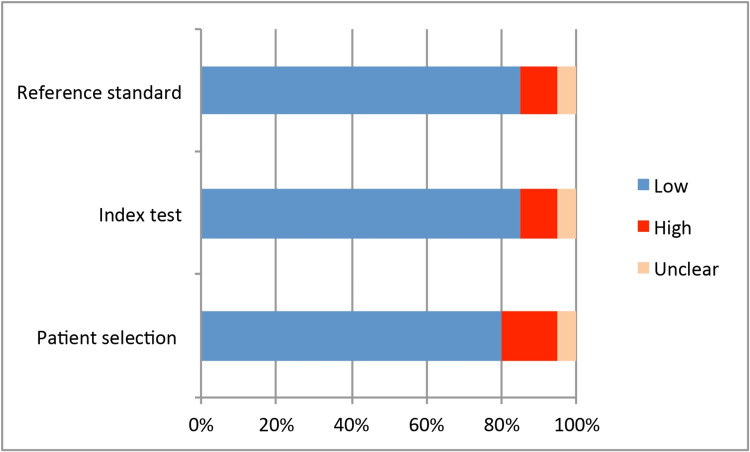
Figure 2 and Figure 3 depict the QUADAS-2 quality assessment of RoB and concerns about the applicability of the reviewed studies, respectively. Overall study quality was high for 13 (65%) studies [12,13,15,17-19,23-27,29,30], moderate for five (25%) studies [14,16,20,28,31], and low for two (10%) studies [21,22]. The RoB of the subdomains of patient selection, flow, and timing were the most common. In six studies, patient selection failed to reflect the intended target population in terms of RoB (5% of high risk, 25% of unclear), and concerns about the applicability of patient selection (10% of high risk, 5% of unclear). The RoB and applicability of the reference standard that verified the final diagnosis were at high risk in 5% and 10% of studies, respectively, and unclear in 5% of studies.
Figure 2. Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) assessment tool of Risk of Bias (RoB).
Figure 3. Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) assessment tool for concerns regarding applicability.
Cut-Off Value and Diagnostic Accuracy of FCP
Despite the fact that results were reported with a variety of cut-off values, almost one-third of the reviewed studies used 50 μg/g (seven studies) [14,19,23-25,30,31], and four studies utilized 100 μg/g [20-22,28] as a cut-off. Moein et al. reported a sensitivity and specificity estimation of 100% with a threshold value of 78.4 μg/g [16]. Schoepfer et al. found an AUC of 0.939 at a cut-off of 57 μg/g, with a sensitivity of 91% and a specificity of 90% on 280 adults [13].
Kolho et al. [26] found an AUC of 0.944 for FCP at a cut-off of 59.5 μg/g for the assessment of pediatric IBD, with a sensitivity and specificity of 81.8% and 96.3%, respectively. In a study of UC patients, FCP was found to exhibit an AUC of 0.999, and a sensitivity and specificity of 98.5% and 96.6%, respectively, at a threshold of 188 μg/g [17]. At a cut-off of 100 μg/g, an AUC of 0.93 was estimated for FCP to differentiate OGID from FGID, with 64% sensitivity and 90.1% specificity [21]. Turvill et al. found sensitivity and specificity of 90.6% and 57.6% in YFCCP versus non-YFCCP patients at a cut-off of 100 μg/g [22].
In a recent study of 90 patients, at a cut-off of 127.65 μg/g, an AUC of 0.83 with a sensitivity of 98% and a specificity of 96% was reported [18]. Wang et al. reported that FCP with a cut-off of 45.4 μg/g can identify patients with IBD from those without IBD (94.4% sensitivity, 64.3% specificity), with an AUC of 0.949 [12]. Caviglia et al. [27] documented a higher sensitivity (87.5%) and specificity (90.5%) in differentiating between IBS and IBD at a cut-off of 150 μg/g. Some studies, nevertheless, found significantly lower values. Accordingly, Kalantari et al. disclosed a sensitivity of 57% and a specificity of 75% at a cut-off of 164 μg/g in 44 patients with UC [29]. In another study, with an AUC of 0.69, FCP was found to have lower sensitivity (72.7%) and specificity (64.9%) rates [19]. Furthermore, sensitivity and specificity of 77% and 73%, respectively, were indicated at a cut-off of 95 μg/kg in determining capsule endoscopy observations for CD diagnosis [15]. Considering these findings, it appears that FCP lacks optimal sensitivity and specificity for the evaluation of IBD. Interestingly, it appears that FCP can be beneficial in ruling out IBD in those with IBS-like symptoms and lowering the frequency of colonoscopy.
Discussion
According to previous systemic reviews and meta-analyses, FCP was clinically beneficial for differentiating OGID from FGID and eliminating redundant endoscopies [3,32]. It has been proven to be a complementary technique that reflects the IBD activity and serves as a biological indicator of OGID. It has also been proposed as a low-cost evaluation tool for establishing prompt colonoscopy referrals in primary care settings [10].
There is no reference standard in the detection of IBD that is either 100% sensitive or specific. The review includes endoscopy of both the upper and lower gastrointestinal tracts, as well as histopathological examinations. Fecal sampling should optimally be performed fairly ahead of endoscopy before bowel preparation. A one-month delay was not considered to be detrimental because mucosal inflammation is not likely to heal spontaneously during this time [32]. The comprehension of FCP results in children should be accomplished cautiously as its specificity is more in adults as opposed to children [33]. Nonetheless, the National Institute for Clinical Excellence has supported calprotectin-based referral pathways for application in primary care as FCP is estimated to enhance the diagnostic utility of IBD and minimize superfluous secondary care expenditures for the investigation and management of FGID [21].
Carroccio et al. suggested that the higher cut-off of 100 µg/g indicated a higher positive predictive value with a lower negative predictive value and sensitivity compared to the lower cut-off of 50 µg/g. The assay was found to be more reliable in children than in adults [34]. A value below 50 μg/g is regarded as normal if sensitivity is deemed critical to avoid missing any cases of IBD. Some adults with IBS have elevated FCP and may be frequently referred for endoscopy. In theory, an exceptionally sensitive test can result in false-positive endoscopies for people with IBS, whereas a lesser sensitive strategy could result in missing some individuals with IBD, with potentially serious repercussions. Clinical intuition and observation should be used in clinical settings, resulting in a reduced number of false-positive colonoscopies [35].
Several commercially available products for FCP qualitative examination known as rapid calprotectin are also readily accessible wherein positive results varied between 0 and 300 µg/g. These kits are typically developed in accordance with the ELISA technique and certain types have measurements ranging from 6.5 to 2,100 µg/g [6]. Monoclonal and polyclonal antibodies can be procured in ELISA kits. Polyclonal antibodies are used in the Calpro and PhiCal ELISA assays, while monoclonal antibodies are used in the Calprotectin fCAL ELISA (Bühlmann Laboratories) [4,36]. For elevated FCP values, the PhiCal v2 ELISA kit has a greater maximum threshold of detection, limiting the total number of dilution steps. This is a crucial factor when monitoring IBD, but it is of lesser significance when differentiating between IBS and OGID. It has an incubation duration that is shorter and less challenging to apply than the PhiCal v1 [30].
Furthermore, many automated analyses such as chemiluminescence immunoassays (CLIA), fluoroenzyme immunoassays, and particle-enhanced turbidimetric immunoassays are now available. CLIA can observe values ranging from 5 to 8,000 µg/g. One of the most challenging situations in the laboratory assessment for FCP is determining the maximum permissible level in people who are otherwise healthy. There is substantial consensus among competent adults on 50 µg/g as the upper limit. As the standard range for FCP in healthy individuals, a prior study suggested a value of 112 µg/g in healthy individuals over 60 years old as opposed to 186 µg/g in children two to nine years old [6].
ELISA techniques for identifying fecal biological markers are laborious and expensive. Calprotectin point-of-care tests have been established to aid in the non-invasive strategy for distinguishing the inflammation of the gut from FGID in primary care settings among individuals with chronic abdominal discomfort [37]. According to the Centre for Economic Based Practice, FCP is more economically feasible than other alternate approaches [30].
When compared to endoscopy and contrast radiography, the application of Rome criteria, intestinal permeability, and FCP tests serve as a non-intrusive and effective method of screening individuals with OGID. Their holistic application may assist the professionals in determining the need for extensive examinations or potentially avert cases with clinical manifestations suggestive of a likelihood of IBS [1].
Limitations
Though numerous investigations have demonstrated the utility of FCP as a biological marker, there are certain drawbacks to consider. It has recently been demonstrated that after six consecutive days, FCP concentration could drop by about 35%. A further constraint to consider is the influence of certain drugs and systemic conditions on FCP levels. Non-steroidal anti-inflammatory drugs seem to elevate the FCP concentrations. Indomethacin and naproxen could raise FCP concentrations by more than two-fold. Proton pump inhibitors emerge to be capable of potentially enhancing FCP levels. Higher levels of FCP levels cannot be ascribed primarily to IBD. Cancer of the colon, infectious diarrhea, bacterial colonization of the small intestine, celiac/diverticular diseases, lactose intolerance, pancreatitis, ankylosing spondylitis, gastroesophageal reflux disease, and rheumatological disease are the most notable diseases in this context. As a result, comprehending the results of the FCP should be used prudently [6,38-40].
FCP is considered the most sensitive indicator for differentiating IBD from IBSc, with a sensitivity of 97% at a cut-off of 50 µg/g and 92% at a cut-off of 100 µg/g. Individuals with a negative result could be observed on a regular basis rather than having an endoscopic examination immediately following the diagnosis unless it is extremely critical. If patients refuse to undergo a stool examination, the most specific marker is anti-neutrophil cytoplasmic antibodies with a specificity of 0.971 [41]. FCP has a cumulative sensitivity and specificity of 0.93 and 0.96, respectively, as well as a considerable negative predictive value of 0.96-0.98 [28,30,32]. Knowing the advancement of IBD at diagnosis by employing several distinctive but clinically significant parameters will aid in the personalization of treatment strategies. This, in turn, will aid in improving therapeutic results over the course of therapy and may help them toward tailored treatment in IBD [5].
Conclusions
This review outlined the diagnostic accuracy of non-invasive FCP assessment for OGID in various clinical scenarios and individuals of various ages. FCP is used as a tool for screening in healthcare settings to determine the need for further comprehensive investigations. It serves to monitor the disease activity, thereby reducing the redundant use of invasive techniques.
Acknowledgments
We would like to express our sincere gratitude to Dr. Fawaz Pullishery for his invaluable contributions to this systematic review. Abdulaziz S. Asiri was actively involved in the conception and design of the study, providing valuable insights and expert opinions on the research topic. Saad S. Algarni, Anood Q. Althubaiti, and Mohammed A. Alzubaidi were involved in data collection, literature search, and analysis. Jamal A. Alghamdi and Ghazi A. Almalki were engaged in critically reviewing and synthesizing the findings, contributing to formulating evidence-based conclusions. Additionally, all authors participated in drafting and revising the manuscript, adhering to the guidelines for systematic reviews and ensuring clarity and coherence in the final publication.
The authors have declared that no competing interests exist.
References
- 1.Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Tibble JA, Sigthorsson G, Foster R, Forgacs I, Bjarnason I. Gastroenterology. 2002;123:450–460. doi: 10.1053/gast.2002.34755. [DOI] [PubMed] [Google Scholar]
- 2.Validation and clinical significance of a new calprotectin rapid test for the diagnosis of gastrointestinal diseases. Damms A, Bischoff SC. Int J Colorectal Dis. 2008;23:985–992. doi: 10.1007/s00384-008-0506-0. [DOI] [PubMed] [Google Scholar]
- 3.Faecal calprotectin testing for identifying patients with organic gastrointestinal disease: systematic review and meta-analysis. An YK, Prince D, Gardiner F, et al. Med J Aust. 2019;211:461–467. doi: 10.5694/mja2.50384. [DOI] [PubMed] [Google Scholar]
- 4.Faecal calprotectin. Pathirana WG, Chubb SP, Gillett MJ, Vasikaran SD. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6370282/ Clin Biochem Rev. 2018;39:77–90. [PMC free article] [PubMed] [Google Scholar]
- 5.Precision medicine in inflammatory bowel disease: concept, progress and challenges. Borg-Bartolo SP, Boyapati RK, Satsangi J, Kalla R. F1000Res. 2020;9:1–15. doi: 10.12688/f1000research.20928.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calprotectin in inflammatory bowel disease. Khaki-Khatibi F, Qujeq D, Kashifard M, Moein S, Maniati M, Vaghari-Tabari M. Clin Chim Acta. 2020;510:556–565. doi: 10.1016/j.cca.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The elderly IBD patient in the modern era: changing paradigms in risk stratification and therapeutic management. Hong SJ, Katz S. Therap Adv Gastroenterol. 2021;14:17562848211023399. doi: 10.1177/17562848211023399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Role of fecal calprotectin in gastrointestinal disorders. Montalto M, Gallo A, Santoro L, D'Onofrio F, Landolfi R, Gasbarrini A. https://www.europeanreview.org/article/4453. Eur Rev Med Pharmacol Sci. 2013;17:1569–1582. [PubMed] [Google Scholar]
- 9.Diagnostic value of fecal calprotectin and serum MMP-9 in diagnosing disease activity of ulcerative colitis. Ghweil A, Khodeary A, Aziz SP. Open J Gastroenterol. 2018;8:234–244. [Google Scholar]
- 10.Diagnostic accuracy of fecal calprotectin in predicting significant gastrointestinal diseases. Kan YM, Chu SY, Loo CK. JGH Open. 2021;5:647–652. doi: 10.1002/jgh3.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Whiting PF, Rutjes AW, Westwood ME, et al. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 12.Faecal calprotectin concentrations in gastrointestinal diseases. Wang S, Wang Z, Shi H, et al. J Int Med Res. 2013;41:1357–1361. doi: 10.1177/0300060513488499. [DOI] [PubMed] [Google Scholar]
- 13.Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the Lichtiger Index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Schoepfer AM, Beglinger C, Straumann A, et al. Inflamm Bowel Dis. 2013;19:332–341. doi: 10.1097/MIB.0b013e3182810066. [DOI] [PubMed] [Google Scholar]
- 14.Evaluation of a faecal calprotectin care pathway for use in primary care. Turvill J, O'Connell S, Brooks A, et al. Prim Health Care Res Dev. 2016;17:428–436. doi: 10.1017/S1463423616000049. [DOI] [PubMed] [Google Scholar]
- 15.A prospective study of fecal calprotectin and lactoferrin as predictors of small bowel Crohn's disease in patients undergoing capsule endoscopy. Bar-Gil Shitrit A, Koslowsky B, Livovsky DM, et al. Scand J Gastroenterol. 2017;52:328–333. doi: 10.1080/00365521.2016.1253769. [DOI] [PubMed] [Google Scholar]
- 16.Diagnostic accuracy of fecal calprotectin in assessing the severity of inflammatory bowel disease: from laboratory to clinic. Moein S, Qujeq D, Vaghari Tabari M, Kashifard M, Hajian-Tilaki K. Caspian J Intern Med. 2017;8:178–182. doi: 10.22088/cjim.8.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Optimal cut-off value of fecal calprotectin for the evaluation of ulcerative colitis: an unsolved issue? Jha AK, Chaudhary M, Dayal VM, et al. JGH Open. 2018;2:207–213. doi: 10.1002/jgh3.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fecal calprotectin Level in patients with IBD and noninflammatory disease of colon: a study in Babol, Northern, Iran. Sharbatdaran M, Holaku A, Kashifard M, Bijani A, Firozjahi A, Hosseini A, Siadati S. Caspian J Intern Med. 2018;9:60–64. [PMC free article] [PubMed] [Google Scholar]
- 19.Unrestricted faecal calprotectin testing performs poorly in the diagnosis of inflammatory bowel disease in patients in primary care. Conroy S, Hale MF, Cross SS, Swallow K, Sidhu RH, Sargur R, Lobo AJ. J Clin Pathol. 2018;71:316–322. doi: 10.1136/jclinpath-2017-204506. [DOI] [PubMed] [Google Scholar]
- 20.Evaluation of the clinical and cost-effectiveness of the York Faecal Calprotectin Care Pathway. Turvill J, Turnock D, Holmes H, Jones A, Mclaughlan E, Hilton V, Marriott S. Frontline Gastroenterol. 2018;9:285–294. doi: 10.1136/flgastro-2018-100962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faecal calprotectin effectively excludes inflammatory bowel disease in 789 symptomatic young adults with/without alarm symptoms: a prospective UK primary care cohort study. Walker GJ, Moore L, Heerasing N, et al. Aliment Pharmacol Ther. 2018;47:1103–1116. doi: 10.1111/apt.14563. [DOI] [PubMed] [Google Scholar]
- 22.Audit of the impact of the York faecal calprotectin care pathway on colonoscopy activity. Turvill J, Turnock D. Frontline Gastroenterol. 2020;11:285–289. doi: 10.1136/flgastro-2019-101315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faecal calprotectin in differentiating inflammatory bowel disease (IBD) from irritable bowel syndrome (IBS) Chowdhury M, Ghosh C, Miah M, et al. Bangladesh Med J. 2021;50:15–22. [Google Scholar]
- 24.Diagnostic accuracy and clinical application of faecal calprotectin in adult patients presenting with gastrointestinal symptoms in primary care. Pavlidis P, Chedgy FJ, Tibble JA. Scand J Gastroenterol. 2013;48:1048–1054. doi: 10.3109/00365521.2013.816771. [DOI] [PubMed] [Google Scholar]
- 25.Faecal calprotectin as a novel biomarker for differentiating between inflammatory bowel disease and irritable bowel syndrome. Chang MH, Chou JW, Chen SM, Tsai MC, Sun YS, Lin CC, Lin CP. Mol Med Rep. 2014;10:522–526. doi: 10.3892/mmr.2014.2180. [DOI] [PubMed] [Google Scholar]
- 26.Fecal calprotectin, MMP-9, and human beta-defensin-2 levels in pediatric inflammatory bowel disease. Kolho KL, Sipponen T, Valtonen E, Savilahti E. Int J Colorectal Dis. 2014;29:43–50. doi: 10.1007/s00384-013-1775-9. [DOI] [PubMed] [Google Scholar]
- 27.Fecal calprotectin is an effective diagnostic tool that differentiates inflammatory from functional intestinal disorders. Caviglia GP, Pantaleoni S, Touscoz GA, et al. Scand J Gastroenterol. 2014;49:1419–1424. doi: 10.3109/00365521.2014.934913. [DOI] [PubMed] [Google Scholar]
- 28.Clinical utility and diagnostic accuracy of faecal calprotectin for IBD at first presentation to gastroenterology services in adults aged 16-50 years. Kennedy NA, Clark A, Walkden A, et al. J Crohns Colitis. 2015;9:41–49. doi: 10.1016/j.crohns.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fecal calprotectin is a useful marker to diagnose ulcerative colitis from irritable bowel syndrome. Kalantari H, Taheri A, Yaran M. Adv Biomed Res. 2015;4:85. doi: 10.4103/2277-9175.156647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Utility of faecal calprotectin in inflammatory bowel disease (IBD): what cut-offs should we apply? Dhaliwal A, Zeino Z, Tomkins C, et al. Frontline Gastroenterol. 2015;6:14–19. doi: 10.1136/flgastro-2013-100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faecal calprotectin for differentiating between irritable bowel syndrome and inflammatory bowel disease: a useful screen in daily gastroenterology practice. Banerjee A, Srinivas M, Eyre R, Ellis R, Waugh N, Bardhan KD, Basumani P. Frontline Gastroenterol. 2015;6:20–26. doi: 10.1136/flgastro-2013-100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. van Rheenen PF, Van de Vijver E, Fidler V. BMJ. 2010;341:0. doi: 10.1136/bmj.c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clinical usefulness of the faecal calprotectin test in suspected paediatric inflammatory bowel disease. Akobeng AK. Acta Paediatr. 2018;107:2019–2023. doi: 10.1111/apa.14374. [DOI] [PubMed] [Google Scholar]
- 34.Diagnostic accuracy of fecal calprotectin assay in distinguishing organic causes of chronic diarrhea from irritable bowel syndrome: a prospective study in adults and children. Carroccio A, Iacono G, Cottone M, et al. Clin Chem. 2003;49:861–867. doi: 10.1373/49.6.861. [DOI] [PubMed] [Google Scholar]
- 35.Faecal calprotectin testing for differentiating amongst inflammatory and non-inflammatory bowel diseases: systematic review and economic evaluation. Waugh N, Cummins E, Royle P, et al. Health Technol Assess. 2013;17:xv-xix, 1-211. doi: 10.3310/hta17550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fecal calprotectin levels predict colorectal inflammation among patients with chronic diarrhea referred for colonoscopy. Limburg PJ, Ahlquist DA, Sandborn WJ, Mahoney DW, Devens ME, Harrington JJ, Zinsmeister AR. Am J Gastroenterol. 2000;95:2831–2837. doi: 10.1111/j.1572-0241.2000.03194.x. [DOI] [PubMed] [Google Scholar]
- 37.Diagnostic performance of rapid tests for detection of fecal calprotectin and lactoferrin and their ability to discriminate inflammatory from irritable bowel syndrome. Otten CM, Kok L, Witteman BJ, Baumgarten R, Kampman E, Moons KG, de Wit NJ. Clin Chem Lab Med. 2008;46:1275–1280. doi: 10.1515/CCLM.2008.246. [DOI] [PubMed] [Google Scholar]
- 38.The role of faecal calprotectin in diagnosis and staging of colorectal neoplasia: a systematic review and meta-analysis. Ross FA, Park JH, Mansouri D, Combet E, Horgan PG, McMillan DC, Roxburgh CS. BMC Gastroenterol. 2022;22:176. doi: 10.1186/s12876-022-02220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faecal calprotectin in the diagnosis of inflammatory bowel disease. Burri E, Beglinger C. Biochem Med (Zagreb) 2011;21:245–253. doi: 10.11613/bm.2011.034. [DOI] [PubMed] [Google Scholar]
- 40.Elevated faecal calprotectin in patients with a normal colonoscopy: does it matter in clinical practice? A retrospective observational study. Hovstadius H, Lundgren D, Karling P. Inflamm Intest Dis. 2021;6:101–108. doi: 10.1159/000513473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diagnostic utility of non-invasive tests for inflammatory bowel disease: an umbrella review. Shi JT, Zhang Y, She Y, Goyal H, Wu ZQ, Xu HG. Front Med (Lausanne) 2022;9:920732. doi: 10.3389/fmed.2022.920732. [DOI] [PMC free article] [PubMed] [Google Scholar]