Abstract
Interactions between biofilm cells of Pseudomonas aeruginosa and levofloxacin were studied. P. aeruginosa incubated for 6 days with Teflon sheets formed a biofilm on its surface. Against the biofilm bacteria, levofloxacin at an MIC determined by the standard method for the strain was highly bactericidal whereas gentamicin, ceftazidime, and ciprofloxacin showed no significant killing activity. Levofloxacin, ciprofloxacin, and gentamicin, but not ceftazidime, exhibited killing activity against nongrowing cells of the strain incubated in phosphate buffer. In addition, levofloxacin, ciprofloxacin, and ceftazidime, but not gentamicin, showed the ability to penetrate an agar containing alginate. These findings may explain the efficacy of levofloxacin and the ineffectiveness of gentamicin and ceftazidime against biofilm bacteria; however, the cause of the ineffectiveness of ciprofloxacin still remains to be determined. In experimental pneumonia in guinea pigs, in which the biofilm mode of growth of the strain was observed in the lung, only levofloxacin exhibited substantial therapeutic efficacy. These findings suggest the significant role of levofloxacin in therapy of biofilm bacterium-associated infectious diseases.
Although Pseudomonas aeruginosa is an opportunistic pathogen, it has emerged as a dominant pulmonary pathogen with biofilm-forming capability, resulting in progressive and intractable chronic pulmonary infections especially in patients with cystic fibrosis (23). These infections cannot be completely cured when treated with antibiotics to which the bacteria were highly susceptible in vitro (1, 15). There are two main reasons why biofilm bacteria are hard to eradicate by common antibiotic therapy (5). One is that alginate, which is the main constituent of Pseudomonas biofilms, acts as a barrier to protect the infecting cells from the humoral and cellular host defense systems (8, 13, 31) as well as from the action of antibiotics (4, 6, 9, 10). Another is that the biofilm bacteria are slow- or nongrowing (29). The concentrations of antibiotics needed to kill bacteria in the sessile phase are often much higher than those required for bacteria in the planktonic phase (16, 33).
Fluoroquinolones are highly potent, broad-spectrum agents that penetrate bacterial cell envelopes and inhibit DNA gyrase activity, rapidly killing susceptible organisms (3, 26). It has been reported that fluoroquinolones also have bactericidal activities toward nongrowing cells of P. aeruginosa (30) and that these drugs are able to eradicate preformed biofilms in vitro (34). However, the antibacterial potency of fluoroquinolones against the bacteria in the biofilm mode of growth is still controversial because the potency might depend largely on experimental conditions, such as age of biofilm, concentration of drug, and duration of exposure to the drug.
In this study, using a relatively low concentration, namely, the MIC for a test strain, we investigated the bactericidal activity of levofloxacin, one of the potent fluoroquinolones, against P. aeruginosa in the biofilm mode of growth in vitro. The in vitro potency of levofloxacin was further confirmed by the therapeutic efficacy of the drug against experimental pneumonia in guinea pigs, for which development of biofilm-associated pulmonary lesions was reported (12).
MATERIALS AND METHODS
Animals.
Female Hartley guinea pigs weighing 250 to 300 g, purchased from Charles River Japan Inc. (Kanagawa, Japan), were used.
Bacterial strain.
P. aeruginosa 2126, a mucoid clinical isolate was used for in vitro and in vivo experiments. The strain produces elastase, protease, and exotoxin A. MICs of levofloxacin, ciprofloxacin, ceftazidime, and gentamicin for P. aeruginosa 2126, determined by the standard agar dilution method (17a), were 0.78, 0.20, 3.13, and 1.56 μg/ml, respectively. Escherichia coli KP (gift from K. Shimizu, Tokyo University), E. coli MK3804C (gift from Merck Co.), and Staphylococcus aureus FDA 209-P were used for bioassay.
Antibacterial agents.
Levofloxacin and ciprofloxacin were synthesized at Daiichi Pharmaceutical Co., Ltd. (Tokyo, Japan). Ceftazidime and gentamicin were purchased from Tanabe Seiyaku (Tokyo, Japan) and Shering Plough (Osaka, Japan), respectively.
In vitro preparation of bacterial biofilm.
The method used for bacterial-biofilm formation was described previously by Ohgaki (20). Briefly, the bacteria preincubated in tryptic soy broth (Eiken, Tokyo, Japan) for 18 h at 37°C were washed thrice with saline, resuspended in the same solution at 106 CFU/ml, and subsequently incubated with Teflon sheets (1 by 1 by 0.3 cm) for 6 days at 37°C. The bacteria recovered from the sheets and from the saline were designated “biofilm-producing sessile cells” and “floating cells,” respectively.
Electron-microscopic study.
The bacterial biofilm on the Teflon sheets was fixed by the method described previously by Kellenberger et al. (14). The Teflon sheets were dehydrated in graded concentrations of ethanol, transferred to isoamyl acetate, dried at the critical point of carbon dioxide, and covered with platinum palladium. They were observed with a scanning electron microscope (S800; Hitachi Ltd.).
Bactericidal activity of antibacterials against biofilm-forming sessile cells and floating cells.
To test the bactericidal activity of antibiotics against the sessile cells, the Teflon pieces incubated with the organism as described above were taken out, washed gently with saline, and transferred to saline containing a given antibiotic. At time intervals of 3, 6, and 24 h during incubation at 37°C, the Teflon pieces were transferred to fresh saline and stirred vigorously with a vortex mixture for 2 min. The suspensions were diluted and plated on heart infusion agar (HIA) (Eiken) plates, and viable cells were counted after incubation for 24 h at 37°C. As for the floating cells, 0.1 ml of the saline the Teflon piece had been soaked in was transferred to the fresh tube and saline containing the desired antibiotic was added. The number of surviving bacteria was determined in the same way as for the sessile cells.
Bactericidal activity of antibacterials against nongrowing bacteria.
The bactericidal activities of the antibiotics against nongrowing bacteria were determined by the method of Tanaka et al. (30). Overnight cultures in tryptic soy broth were diluted with fresh heart infusion broth (Nissui Seiyaku, Tokyo, Japan) to about 105 CFU/ml and were then incubated for a further 2 to 3 h with shaking at 37°C. The logarithmically growing bacteria were centrifuged at 5,000 × g for 15 min at 23°C and washed twice with phosphate-buffered saline (pH 7.4). The last pellet was resuspended with phosphate-buffered saline (pH 7.4) and readjusted to the desired inoculum size. The bacterial suspension was then incubated for 1 h at 37°C to achieve the nongrowing condition. Different concentrations of antibiotics were added to the test tubes. A 0.1-ml aliquot of the culture was removed 3, 6, and 24 h after the start of exposure. The suspensions were diluted and plated on HIA plates, and bacterial colonies were counted after incubation for 24 h at 37°C.
Extraction of exopolysaccharide.
The procedure for production and extraction of exopolysaccharide was described by Govan et al. (7). After incubation of the bacteria on HIA for 24 h at 37°C and for 24 h at 21°C, the surface growth was collected into saline and vortexed vigorously until uniformly dispersed. After removal of the whole bacteria, alginate was precipitated by ethanol, collected, washed twice with 95% ethanol and once with absolute alcohol, and dried.
Sandwich cup method for determination of the permeability of the alginate layer to antibiotics.
The alginate was dissolved at 1 or 2% (wt/vol) in phosphate buffer (pH 7.0) containing 1% Noble agar. This alginate-containing buffer was poured onto a membrane filter (0.4 μm) in a culture insert (Millicell CM; Millipore) and used in the conventional cup method. Five hundred microliters of 50 μg of antibacterial solution per ml was poured onto the alginate layer, and the concentration of the drug that passed through the filter was measured by bioassay. E. coli KP was used for levofloxacin and ciprofloxacin, E. coli MK3804C was used for ceftazidime, and S. aureus FDA 209-P was used for gentamicin. The limit of detection of levofloxacin, ciprofloxacin, and ceftazidime was 0.39 μg/ml, and that of gentamicin was 0.78 μg/ml.
Experimental chemotherapy against pseudomonal pneumonia.
Pneumonia due to P. aeruginosa 2126 was induced in guinea pigs as previously described (21). Immediately or 2 days after infection, the animals were administered an antibacterial for 3 consecutive days. According to the optimal regimen of each drug in experimental chemotherapy, levofloxacin and ciprofloxacin were administered orally thrice a day at a 4-h interval (22), whereas gentamicin was administered subcutaneously once a day (22, 24). The animals were sacrificed by exsanguination under ether anesthesia 18 h after the last treatment, and the lungs were assessed for bacterial number by the pour plate method.
RESULTS
In vitro biofilm mode of growth of bacteria on the Teflon surface.
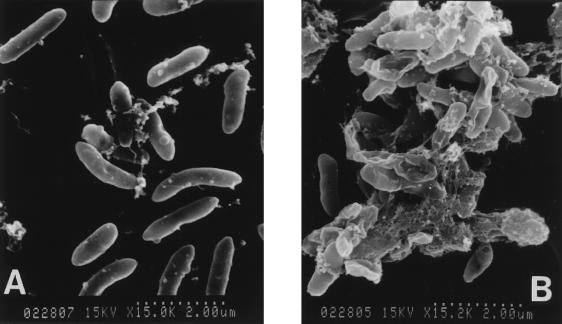
Figure 1 shows scanning electron micrographs of sessile cells on the surface of two pieces of Teflon that had been incubated with P. aeruginosa 2126 for 1 or 6 days. The bacteria on the Teflon incubated for 6 days were covered with thick membranous and fibrous structure and cohered to each other through this fibrous structure unlike bacteria incubated for 1 day.
FIG. 1.
Scanning electron micrographs of P. aeruginosa 2126 on the surface of Teflon sheets incubated for 1 day (A) or 6 days (B).
Bactericidal activities of antibacterials against biofilm-forming sessile cells and floating cells.
Table 1 shows the bactericidal actions of levofloxacin, ciprofloxacin, gentamicin, and ceftazidime at their MICs, determined by the standard method, on sessile cells of P. aeruginosa 2126. Levofloxacin decreased the number of viable cells from about 106 to 103 CFU/ml within 24 h of incubation, whereas the other drugs including ciprofloxacin were hardly effective. With respect to floating cells, levofloxacin, ciprofloxacin, and gentamicin decreased the number of viable cells within 24 h of incubation, but ceftazidime had hardly any effect (Table 2).
TABLE 1.
Bactericidal activities of levofloxacin, ciprofloxacin, gentamicin, and ceftazidime against P. aeruginosa in biofilms
| Treatment (n = 3) | Bacterial count (log10 CFU/ml) ata:
|
||
|---|---|---|---|
| 3 h | 6 h | 24 h | |
| None (control) | 5.43 ± 0.08 | 5.24 ± 0.22 | 5.17 ± 0.41 |
| Levofloxacin | 5.58 ± 0.17 | 4.87 ± 0.28 | 2.69 ± 0.73** |
| Ciprofloxacin | 5.16 ± 0.12 | 5.01 ± 0.20 | 4.72 ± 0.28 |
| Gentamicin | 5.30 ± 0.24 | 5.10 ± 0.19 | 4.70 ± 0.15 |
| Ceftazidime | 5.42 ± 0.16 | 5.35 ± 0.17 | 4.99 ± 0.18 |
At 0 h, the bacterial count was 5.75 ± 0.10 log10 CFU/ml. Values are means ± standard deviations. **, significantly different from the corresponding value for the control.
TABLE 2.
Bactericidal activities of levofloxacin, ciprofloxacin, gentamicin, and ceftazidime against floating-form P. aeruginosa
| Treatment (n = 3) | Bacterial count (log10 CFU/ml) ata:
|
||
|---|---|---|---|
| 3 h | 6 h | 24 h | |
| None (control) | 5.81 ± 0.25 | 6.03 ± 0.12 | 6.46 ± 0.24 |
| Levofloxacin | 6.02 ± 0.13 | 5.62 ± 0.36 | 3.15 ± 0.25** |
| Ciprofloxacin | 4.72 ± 0.18** | 4.62 ± 0.16** | 4.19 ± 0.37** |
| Gentamicin | 5.23 ± 0.33 | 4.67 ± 0.31** | 3.76 ± 0.40** |
| Ceftazidime | 5.45 ± 0.27 | 5.29 ± 0.16** | 5.35 ± 0.46 |
At 0 h, the bacterial count was 5.53 ± 0.14 log10 CFU/ml. Values are means ± standard deviations. **, significantly different from the corresponding value for the control.
Bactericidal effects of antibacterials on nongrowing bacteria.
Table 3 shows bactericidal actions of levofloxacin, ciprofloxacin, gentamicin, and ceftazidime on nongrowing cells. The number of viable cells rapidly decreased from about 106 to 103 CFU/ml within 6 h even by treatment with just the MIC of levofloxacin. At 16× MIC, levofloxacin completely killed the cells within 6 h. At 16× MIC, ciprofloxacin also killed the cells within 24 h. With respect to gentamicin, no decrease in the number of viable cells was observed at MIC, but cells treated with 4× or 16× MIC were killed progressively within 24 h. In contrast, the cells were not killed within 24 h by ceftazidime at doses up to 16× MIC.
TABLE 3.
Bactericidal activities of levofloxacin, ciprofloxacin, gentamicin, and ceftazidime against nongrowing P. aeruginosa
| Drug and concn | Bacterial count (log10 CFU/ml) ata:
|
||
|---|---|---|---|
| 3 h | 6 h | 24 h | |
| None (control) | 5.84 | 5.78 | 5.30 |
| Levofloxacin | |||
| MIC | 3.57 | 2.79 | 3.10 |
| 4× MIC | 2.08 | 1.60 | 1.78 |
| 16× MIC | 1.30 | <1.00 | <1.00 |
| Ciprofloxacin | |||
| MIC | 4.07 | 3.47 | 2.14 |
| 4× MIC | 3.27 | 2.48 | 1.48 |
| 16× MIC | 2.23 | 1.78 | <1.00 |
| Gentamicin | |||
| MIC | 5.62 | 5.18 | 4.60 |
| 4× MIC | 5.66 | 4.84 | 3.19 |
| 16× times MIC | 5.51 | 4.62 | <1.00 |
| Ceftazidime | |||
| MIC | 5.72 | 5.47 | 4.86 |
| 4× MIC | 5.80 | 5.41 | 4.43 |
| 16× times MIC | 5.55 | 5.32 | 4.65 |
At 0 h, the bacterial count was 6.15 log10 CFU/ml.
Permeation of antibacterials through the alginate layer.
Table 4 shows the rate of drug diffusion through the agar layer containing 1 or 2% alginate. The concentration for permeation of drugs through the agar layer without alginate was assigned a value of 100%. When alginate was added at 1% to the agar, the diffusion rates of levofloxacin, ciprofloxacin, and ceftazidime were decreased but remained over 60%, though that of gentamicin was under the detection limit. With agar containing 2% alginate, the diffusion rates of levofloxacin, ciprofloxacin, and ceftazidime further decreased. The diffusion rate of ciprofloxacin was the lowest excluding gentamicin.
TABLE 4.
Rates of diffusion through alginate-containing Noble agar
| Drug | Diffusion rate (%)a
|
|
|---|---|---|
| 1% alginate | 2% alginate | |
| Levofloxacin | 60.43 ± 2.29 | 37.07 ± 0.31 |
| Ciprofloxacin | 60.30 ± 7.02 | 24.07 ± 4.45 |
| Gentamicin | <14.9 | <14.9 |
| Ceftazidime | 80.83 ± 5.69 | 75.47 ± 0.06 |
Relative to the rate without alginate. Values are means ± standard deviations for three replicate observations, except for gentamicin.
Experimental chemotherapy.
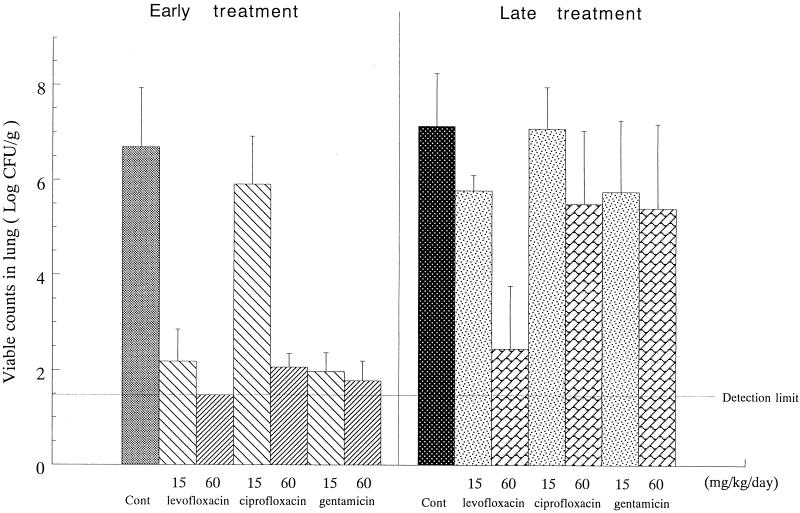
When treatment with antibacterials was commenced immediately after infection, before biofilm formation in vivo, all antibacterials tested eliminated almost completely the bacteria from the lungs irrespective of the dose employed, except for ciprofloxacin, which had no substantial effect at the low dose, 15 mg/kg of body weight (Fig. 2). In contrast, only levofloxacin, at a high dose of 60 mg/kg, exhibited a significant therapeutic efficacy when the therapy was delayed to day 2 of infection.
FIG. 2.
Therapeutic effects of levofloxacin, ciprofloxacin, and gentamicin on experimental pseudomonal pneumonia in cortisone-treated guinea pigs. The numbers of P. aeruginosa bacteria were determined in lungs of animals receiving oral doses of levofloxacin or ciprofloxacin three times a day, or a subcutaneous dose of gentamicin once a day, for 3 consecutive days starting on day 0 (early treatment) or day 2 (late treatment) of infection and sacrificed at 18 h after the last dosing (day 3 or 5 of infection). The data are geometric means with standard deviations. Cont, control.
DISCUSSION
Against the biofilm-forming cells, levofloxacin showed a strong bactericidal activity; however, ciprofloxacin, ceftazidime, and gentamicin were hardly effective. There are two main reasons that the latter antibacterial agents are not as effective on biofilm-forming cells as they are on planktonic cells. One is that the biofilm-forming cells are slow- or nongrowing (29). Another is a reduction in antibacterial penetration through the biofilm layer, because alginate, the main constituent of the biofilm, plays a barrier role (6, 9, 10). The bactericidal activity of levofloxacin against nongrowing cells of P. aeruginosa was as strong as that of ciprofloxacin but superior to that of gentamicin. Ceftazidime hardly showed bactericidal activity against nongrowing cells. However, the diffusion rate of levofloxacin through the alginate layer was not as high as that of ceftazidime but slightly higher than that of ciprofloxacin. The diffusion rate of gentamicin was below the limit of detection. These results suggest that levofloxacin was the most effective agent against the biofilm bacteria because it was not limited for either of the above reasons. On the other hand, gentamicin and ceftazidime were not so effective against the biofilm bacteria, since they were limited for one or the other reason. With regard to the role of alginate (exopolysaccharide) as a penetration barrier to aminoglycosides, Nichols et al. (18, 19) came to a conclusion different from ours. They reported that binding of tobramycin to the exopolysaccharide of P. aeruginosa, and resulting inhibition of diffusion of the antibiotic, did not significantly increase the time of penetration through biofilms. However, there is a difference in conditions between the two studies. They used very thin (0.1-mm) biofilm for studying diffusion, while our alginate-containing agar layer was more than 2 mm thick. The concentration of alginate in vivo is not known, so it is probable that the different balances with concentrations of antibiotics and the condition of biofilm easily affect diffusion. Meanwhile, Shigeta et al. (27) recently reported data that supports our conclusion. They used biofilm-forming bacteria adhered on the membrane of a cell culture insert for studying diffusion and confirmed that the penetration of gentamicin was strongly inhibited by biofilm.
Ciprofloxacin, a quinolone antibiotic like levofloxacin, hardly had any bactericidal activity against the biofilm bacteria in vitro. In this respect, our results are contrary to the report that biofilm exhibited less recalcitrance toward ciprofloxacin than toward levofloxacin (32). However, a relatively high concentration of drug was used in that study and the length of the drug exposure period was short. These differences in experimental conditions make it difficult to compare directly our results with such findings. In contrast, there is another report that supports our conclusion that ciprofloxacin at its MIC was not effective against biofilm bacteria (28). Others also have confirmed that a high concentration of ciprofloxacin was necessary for eradication of a preformed biofilm, although the drug could reduce the adhesion and survival of P. aeruginosa even at a subinhibitory concentration (25, 34). Anyhow, our in vitro experiments on bactericidal activity against nongrowing bacteria and on the rate of permeation through the alginate did not reveal any remarkable differences between levofloxacin and ciprofloxacin. Actually, a biofilm is composed of various exopolysaccharides, though the main component is alginate. When P. aeruginosa forms a biofilm on a Teflon sheet, the biofilm probably becomes more tenacious by incorporating some bacterial ingredients with the alginate. Moreover, the biofilm would be more tenacious in the in vivo situation because fibrin, inflammatory exudates, components of phagocytes, and erythrocytes are incorporated into the polysaccharide matrix (2). So, it is possible that the alginate layer used for our permeability test might represent the simple or young stage of the biofilm, and this may make it difficult to find a remarkable difference in penetration between levofloxacin and ciprofloxacin. In contrast, Shigeta et al. (27) confirmed the difference in the rates of penetration through the biofilms between levofloxacin and ciprofloxacin. Anyway, there might also be some unknown factors that make the biofilm resistant to ciprofloxacin at its MIC.
The data of our in vivo experiments supported our in vitro results. Early treatment with levofloxacin or gentamicin could eradicate the bacteria even at the low dose of 15 mg/kg, but 60 mg of ciprofloxacin per kg was required to eradicate the bacteria in the lungs of guinea pigs with experimental pneumonia. On the other hand, in late treatment, 60 mg of levofloxacin per kg was needed to completely eradicate the bacteria; however, the same dose of gentamicin or ciprofloxacin could not eliminate the bacteria. We earlier demonstrated that ceftazidime does not exhibit any therapeutic efficacy against this experimental pneumonia even at the very high dose of 180 mg/kg (12). At the time of late treatment, i.e., day 2 of infection, the pulmonary lesions are characterized by the formation of granulomas that surround spherical grains consisting of an outer shell and inner bacterial colonies (12). The outer shell consists of material that is positive for ruthenium red staining, suggesting that the material could include bacterial glycocalyx (12). The loss of efficacy of gentamicin in the late treatment may be attributable to the formation of a biofilm in the lungs. This finding is consistent with the in vitro data showing that gentamicin hardly penetrated the alginate layer. In the case of levofloxacin, the efficacy at 15 mg/kg surely decreased in late treatment; however, 60 mg of levofloxacin per kg was still effective. These data indicate that levofloxacin was slightly affected by the biofilm. When we focused on ciprofloxacin, only 60 mg/kg was effective in early treatment. This lower chemotherapeutic efficacy in early treatment may be due to pharmacokinetics, i.e., the oral absorbability and the distribution of ciprofloxacin to the lungs were shown to be not as good as those of levofloxacin (11, 12). Ciprofloxacin was hardly effective even at 60 mg/kg in late treatment. It’s possible that ciprofloxacin is more influenced by biofilm formation than levofloxacin; however, we cannot rule out the influence of the poor pharmacokinetics of ciprofloxacin on its in vivo efficacy.
Although the experimental conditions were restricted, the potency of levofloxacin to kill biofilm bacteria might have clinical significance because the activity was observed even at the MIC for the test strain. However, clarification of the basis for the finding that activities against biofilm bacteria can be different among quinolone antibacterials is needed for a better understanding of the role of fluoroquinolones in therapy of biofilm-associated infections.
ACKNOWLEDGMENT
We thank Mayumi Tanaka for valuable suggestions.
REFERENCES
- 1.Anwar H, Dasgupta M, Lam K, Costerton J W. Tobramycin resistance of mucoid Pseudomonas aeruginosa biofilm grown under iron limitation. J Biomed Mater Res. 1989;25:865–874. doi: 10.1093/jac/24.5.647. [DOI] [PubMed] [Google Scholar]
- 2.Buret A, Ward K H, Olspn M E, Costerton J W. An in vivo model to study the pathobiology of infectious biofilms on biomaterial surfaces. J Antimicrob Chemother. 1991;24:647–655. doi: 10.1002/jbm.820250706. [DOI] [PubMed] [Google Scholar]
- 3.Chu D T W, Fernandes P B. Structure-activity relationships of the fluoroquinolones. Antimicrob Agents Chemother. 1989;33:131–135. doi: 10.1128/aac.33.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chuard C, Lucet J C, Rohner P, Herrmann M, Auckenthaler R, Waldyogel F A, Lew D P. Resistance of Staphylococcus aureus recovered from infected foreign body in vivo to killing by antimicrobials. J Infect Dis. 1991;163:1369–1373. [PubMed] [Google Scholar]
- 5.Costerton J W, Cheng K J. The bacterial glycocalyx in nature and disease. Annu Rev Microbiol. 1981;35:299–324. doi: 10.1146/annurev.mi.35.100181.001503. [DOI] [PubMed] [Google Scholar]
- 6.Evans D J, Alison D G, Brown M R W, Gilbert P. Susceptibility of Pseudomonas aeruginosa and Escherichia coli biofilms toward ciprofloxacin: effect of specific growth rate. J Antimicrob Chemother. 1991;27:177–184. doi: 10.1093/jac/27.2.177. [DOI] [PubMed] [Google Scholar]
- 7.Govan J R W, Fyfe J A M. Mucoid Pseudomonas aeruginosa and cystic fibrosis: resistance of the mucoid form to carbenicillin, flucloxacillin and tobramycin and the isolation of mucoid variants in vitro. J Antimicrob Chemother. 1978;4:233–240. doi: 10.1093/jac/4.3.233. [DOI] [PubMed] [Google Scholar]
- 8.Gray E D, Peter G, Verstegen M, Regelmann W E. Effect of extracellular slime substance from Staphylococcus epidermidis on the human cellular immune response. Lancet. 1984;i:365–367. doi: 10.1016/s0140-6736(84)90413-6. [DOI] [PubMed] [Google Scholar]
- 9.Gristina A G, Hobgood C D, Webb L X, Myrvik Q N. Adhesive colonization of biomaterials and antibiotic resistance. Biomaterials. 1987;8:423–426. doi: 10.1016/0142-9612(87)90077-9. [DOI] [PubMed] [Google Scholar]
- 10.Hoyle B D, Costerton J W. Bacterial resistance to antibiotics: the role of biofilms. Prog Drug Res. 1991;37:91–105. doi: 10.1007/978-3-0348-7139-6_2. [DOI] [PubMed] [Google Scholar]
- 11.Ishida Y, Otani T, Ohashi M, Someya K, Yoshida K, Hayakawa I, Osada Y. Program and abstracts of the 33rd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1993. DU-6859a, a new quinolone: therapeutic efficacy on experimental pneumonia due to Pseudomonas aeruginosa and Streptococcus pneumoniae, abstr. 998; p. 302. [Google Scholar]
- 12.Ishida Y. Experimental pneumonia with Pseudomonas aeruginosa in immunosuppressed guinea pigs as a model for biofilm-associated infection. J Jpn Assoc Infect Dis. 1995;69:572–581. doi: 10.11150/kansenshogakuzasshi1970.69.572. [DOI] [PubMed] [Google Scholar]
- 13.Johnson G M, Lee D A, Regelmann W E, Gray E D, Peters G, Quie P G. Interference with granulocyte function by Staphylococcus epidermidis slime. Infect Immun. 1986;54:13–20. doi: 10.1128/iai.54.1.13-20.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kellenberger E, Ryter A, Sechaud J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol Mol Gen Genet. 1958;4:671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi H. Relationship with host condition and pulmonary infection. J Jpn Soc Intern Med. 1991;80:663–668. . (In Japanese.) [Google Scholar]
- 16.LeChevallier M W, Cawthon C D, Lee R G. Inactivation of biofilm bacteria. Appl Environ Microbiol. 1988;54:2492–2499. doi: 10.1128/aem.54.10.2492-2499.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore R D, Lietman P S, Smith C R. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987;155:93–99. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- 17a.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 2nd ed. M7-A2. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1990. [Google Scholar]
- 18.Nichols W W, Evans M J, Slack M P E, Walmsley H L. The penetration of antibiotics into aggregates of mucoid and nonmucoid Pseudomonas aeruginosa. J Gen Microbiol. 1989;135:1291–1303. doi: 10.1099/00221287-135-5-1291. [DOI] [PubMed] [Google Scholar]
- 19.Nichols W W, Dorrington S M, Slack M P E, Walmsley H L. Inhibition of tobramycin diffusion by binding to alginate. Antimicrob Agents Chemother. 1988;32:518–523. doi: 10.1128/aac.32.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohgaki N. Bacterial biofilm in chronic airway infection. J Jpn Assoc Infect Dis. 1994;68:138–151. doi: 10.11150/kansenshogakuzasshi1970.68.138. [DOI] [PubMed] [Google Scholar]
- 21.Otani T, Katami K, Une T, Osada Y, Ogawa H. Nonbacteremic Pseudomonas aeruginosa pneumonia in immunosuppressed guinea pigs. Microbiol Immunol. 1982;26:67–76. doi: 10.1111/j.1348-0421.1982.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 22.Otani T, Nakajima R, Hashimoto S, Iigo Y, Ishida Y, Une T, Osada Y. Chemotherapeutic efficacy of ofloxacin against experimental pneumonia with Pseudomonas aeruginosa in guinea pigs. Arzneim Forsch. 1989;39:694–697. [PubMed] [Google Scholar]
- 23.Pier G B. Pulmonary disease associated with Pseudomonas aeruginosa in cystic fibrosis: current status of the host-bacterium interaction. J Infect Dis. 1985;151:575–580. doi: 10.1093/infdis/151.4.575. [DOI] [PubMed] [Google Scholar]
- 24.Prins J M, Buller H R, Kuijper E J, Tange R A, Speelman P. Once versus thrice daily gentamicin in patients with serious infections. Lancet. 1993;341:335–339. doi: 10.1016/0140-6736(93)90137-6. [DOI] [PubMed] [Google Scholar]
- 25.Reid G, Sharma S, Advikolanu K, Tieszer C, Martin R A, Bruce A W. Effects of ciprofloxacin, norfloxacin, and ofloxacin on in vitro adhesion and survival of Pseudomonas aeruginosa AK1 on urinary catheters. Antimicrob Agents Chemother. 1994;38:1490–1495. doi: 10.1128/aac.38.7.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosen T. The fluoroquinolone antibacterial agents. Prog Med Chem. 1990;27:235–295. doi: 10.1016/s0079-6468(08)70293-5. [DOI] [PubMed] [Google Scholar]
- 27.Shigeta M, Tanaka G, Komatsuzawa H, Sugai M, Suginaka H, Usui T. Permeation of antimicrobial agents through Pseudomonas aeruginosa biofilms: a simple method. Chemotherapy (Basel) 1997;43:340–345. doi: 10.1159/000239587. [DOI] [PubMed] [Google Scholar]
- 28.Soboh F, Khoury A E, Zamboni A C, Davidson D, Mittelman M W. Effects of ciprofloxacin and protamine sulfate combinations against catheter-associated Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 1995;39:1281–1286. doi: 10.1128/aac.39.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart P S. Biofilm accumulation model that predicts antibiotic resistance of Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 1994;38:1052–1058. doi: 10.1128/aac.38.5.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka M, Otsuki M, Nishino T. Bactericidal activities of ofloxacin and its optically active isomer (DR-3355) on non-growing cells of Escherichia coli and Pseudomonas aeruginosa. Chemotherapy (Basel) 1992;38:21–27. doi: 10.1159/000238938. [DOI] [PubMed] [Google Scholar]
- 31.Vaudaux P E, Zulian G, Huggler E, Waldvogel F A. Attachment of Staphylococcus aureus to polymethyl-methacrylate increases its resistance to phagocytosis in foreign body infection. Infect Immun. 1985;50:472–477. doi: 10.1128/iai.50.2.472-477.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vrany J D, Stewart P S, Suci P A. Comparison of recalcitrance to ciprofloxacin and levofloxacin exhibited by Pseudomonas aeruginosa biofilms displaying rapid-transport characteristics. Antimicrob Agents Chemother. 1997;41:1352–1358. doi: 10.1128/aac.41.6.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright J B, Ruseska I, Athar M A, Corbett S, Costerton J W. Legionella pneumophila grows adherent to surfaces in vitro and in situ. Infect Control Hosp Epidemiol. 1989;10:408–415. doi: 10.1086/646062. [DOI] [PubMed] [Google Scholar]
- 34.Yassien M, Khardori N, Ahmedy A, Toama M. Modulation of biofilms of Pseudomonas aeruginosa by quinolones. Antimicrob Agents Chemother. 1995;39:2262–2268. doi: 10.1128/aac.39.10.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]