Abstract
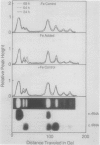
Hydroponically grown pea seedlings (Pisum sativum L., cv Alaska) were subjected to Fe stress for 10 to 16 days to produce mature chlorotic leaves. Greening was initiated by adding Fe to the nutrient solution. The levels of chlorophylls, chloroplast, and cytoplasmic rRNAs, and specific chloroplast- and nucleus-encoded mRNAs were all significantly lower in leaves developing during iron stress than in nonstressed leaves. In plants greening after addition of Fe, nuclear transcripts encoding chlorophyll a/b-binding protein and the small subunit of ribulose bisphosphate carboxylase/oxygenase increased about 5-fold in abundance following an 18 to 24 hour lag, as did the chloroplast-encoded transcript for the large subunit of the carboxylase/oxygenase. Chloroplast rRNA showed an increase over that in continually stressed control leaves only after a 40 hour lag. The chloroplast-encoded transcript encoding the QB-binding 32 kilodalton polypeptide of Photosystem II showed little change during greening. Chlorophyll itself increased gradually after a lag period of 24 hours, with an increase in chlorophyll a slightly preceding that of chlorophyll b. Kinetic considerations suggest that the changes observed represent a coordinate series of events initiated by readdition of Fe and occurring in parallel. Though accumulation of mRNA for light-harvesting, chlorophyll-a/b-binding protein might limit chlorophyll accumulation at the onset, subsequent changes in the mRNA do not parallel chlorophyll changes. All three of the mRNAs showing recovery on addition of Fe to Fe-stressed plants undergo sharp diurnal fluctuations in abundance. Such fluctuations are comparable to those in nonstressed controls (mRNA for light-harvesting protein) or considerably more pronounced (mRNAs for carboxylase large and small subunits). The carboxylase small subunit mRNA and that for light-harvesting chlorophyll-binding protein were measured under constant conditions of light and temperature. Though a rhythm in greening leaves was hard to detect, it was prominent in the Fe-sufficient controls, persisting undamped through three full cycles for both mRNAs, and hence is probably circadian.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Branton D., Jacobson L. Iron Transport in Pea Plants. Plant Physiol. 1962 Jul;37(4):539–545. doi: 10.1104/pp.37.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglie R., Bellemare G., Bartlett S. G., Chua N. H., Cashmore A. R. Cloned DNA sequences complementary to mRNAs encoding precursors to the small subunit of ribulose-1,5-bisphosphate carboxylase and a chlorophyll a/b binding polypeptide. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7304–7308. doi: 10.1073/pnas.78.12.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle J., Possingham J. V., Wells R., Leaver C. J., Loening U. E. The properties of chloroplast ribosomal-RNA. Symp Soc Exp Biol. 1970;24:303–325. [PubMed] [Google Scholar]
- Kaufman L. S., Briggs W. R., Thompson W. F. Phytochrome control of specific mRNA levels in developing pea buds : the presence of both very low fluence and low fluence responses. Plant Physiol. 1985 Jun;78(2):388–393. doi: 10.1104/pp.78.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio J. N., Abadía J., Terry N. Chlorophyll-Proteins and Electron Transport during Iron Nutrition-Mediated Chloroplast Development. Plant Physiol. 1985 Jun;78(2):296–299. doi: 10.1104/pp.78.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakrasi H. B., Goldenberg A., Sherman L. A. Membrane Development in the Cyanobacterium, Anacystis nidulans, during Recovery from Iron Starvation. Plant Physiol. 1985 Sep;79(1):290–295. doi: 10.1104/pp.79.1.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. D., Edwards H., Jorgensen R. A., Thompson W. F. Novel evolutionary variation in transcription and location of two chloroplast genes. Nucleic Acids Res. 1982 Nov 11;10(21):6819–6832. doi: 10.1093/nar/10.21.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C. A., Carell E. F. Control by Iron of Chlorophyll Formation and Growth in Euglena gracilis. Plant Physiol. 1964 Sep;39(5):862–868. doi: 10.1104/pp.39.5.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman D. M., Sherman L. A. Effect of iron deficiency and iron restoration on ultrastructure of Anacystis nidulans. J Bacteriol. 1983 Oct;156(1):393–401. doi: 10.1128/jb.156.1.393-401.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller S., Terry N. Limiting Factors in Photosynthesis: II. IRON STRESS DIMINISHES PHOTOCHEMICAL CAPACITY BY REDUCING THE NUMBER OF PHOTOSYNTHETIC UNITS. Plant Physiol. 1980 Jan;65(1):121–125. doi: 10.1104/pp.65.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry N. Limiting Factors in Photosynthesis: I. USE OF IRON STRESS TO CONTROL PHOTOCHEMICAL CAPACITY IN VIVO. Plant Physiol. 1980 Jan;65(1):114–120. doi: 10.1104/pp.65.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. C., Kaufman L. S., Thompson W. F. Developmental regulation of cytosine methylation in the nuclear ribosomal RNA genes of Pisum sativum. J Mol Biol. 1987 Jan 5;193(1):15–26. doi: 10.1016/0022-2836(87)90622-x. [DOI] [PubMed] [Google Scholar]