Abstract
The objective of the proposed study was to determine the distribution in plasma lipoprotein of free all-trans retinoic acid (ATRA) and liposomal ATRA (Atragen; composed of dimyristoyl phosphatidylcholine and soybean oil) following incubation in human, rat, and dog plasma. When ATRA and Atragen at concentrations of 1, 5, 10, and 25 μg/ml were incubated in human and rat plasma for 5, 60, and 180 min, the majority of the tretinoin was recovered in the lipoprotein-deficient plasma fraction. However, when ATRA and Atragen were incubated in dog plasma, the majority of the tretinoin (>40%) was recovered in the high-density lipoprotein (HDL) fraction. No differences in the plasma distribution between ATRA and Atragen were found. These data suggest that a significant percentage of tretinoin associates with plasma lipoproteins (primarily the HDL fraction) upon incubation in human, dog, and rat plasma. Differences between the lipoprotein lipid and protein profiles in human plasma and in dog and rat plasma influenced the plasma distribution of ATRA and Atragen. Differences in lipoprotein distribution between ATRA and Atragen were not observed, suggesting that the drug’s distribution in plasma is not influenced by its incorporation into these liposomes.
All-trans-retinoic acid (ATRA) has been proven effective against a variety of malignancies in isolated tissue culture systems and in human clinical trials (7, 13, 17, 21, 25). However, ATRA has a limited duration of action (17) and many patients relapse after a remission of short duration (7). In these patients, ATRA concentrations in plasma were found to be very low and the biological activity of ATRA appeared to be greatly impaired by the induction of retinoic acid binding protein (7) and increased drug catabolism by cytochrome P-450-mediated reactions (7, 25). A number of pharmacokinetic studies have shown that drug exposure in plasma declines substantially and rapidly when ATRA is administered in a long-term daily regimen (25). These observations have led to the hypothesis that the rapid development of acquired clinical resistance to ATRA may have a pharmacological basis and results from an inability to present an effective drug concentration to the leukemia cells during continuous treatment (25). The incorporation of ATRA into liposomes has allowed the preparation of a new intravenous ATRA formulation (7, 8, 17) that may significantly improve the potency and duration of ATRA’s activity in cases of leukemia and potentially other malignancies.
Previous studies have shown that liposomal ATRA (L-ATRA) effectively induces differentiation in human myeloid leukemia cell lines (HL-60, KG-1, and THP-1) and is as effective as free ATRA in inducing differentiation of cells from patients with acute promyelocytic leukemia (7). Parthasarathy and coworkers demonstrated that L-ATRA containing diphosphatidyl palmitoylcholine and stearylamine (9:1 [wt/wt]) had optimal drug incorporation, high stability, and minimal toxicity toward erythrocytes, while delivering a sufficiently high concentration of ATRA to inhibit the growth of a squamous-cell carcinoma cell line, MDA 886Ln (21). Mehta and coworkers reported that when L-ATRA was administered to rats over a prolonged period, the levels of the drug in the blood did not change over time (17). In vitro studies of isolated liver microsomes revealed that the catabolism of the drug was not altered in rats that were repeatedly given the L-ATRA formulation (17) whereas microsomes isolated from animals that were orally administered free ATRA the same number of times with the same doses showed a significant increase in the metabolism of the drug (17).
For most hydrophilic compounds, intravenous administration results in an instantaneous distribution of the compound within the blood. This is a consequence of homogeneous mixing of the compound with the aqueous components of the bloodstream (26). However, when compounds are hydrophobic in chemical nature or are incorporated into lipophilic carriers (liposomes), their distribution within the bloodstream may not be instantaneous. The interaction of these compounds with nonaqueous components of the bloodstream, including lipoproteins, appears to be an explanation for this observation.
Plasma lipoproteins are macromolecules of lipid and protein that transport polar and nonpolar lipids through the vascular and extravascular body fluids (10, 34). However, it is well known that plasma lipoprotein profiles vary considerably between different animal species (9). In addition, disease states can significantly influence plasma lipoprotein profiles, possibly resulting in altered therapeutic outcomes. Current research has shown that lipoprotein binding of drug compounds can significantly influence not only the pharmacological and pharmacokinetic properties of the drug but also the relative toxicity (1, 2, 4, 11, 14, 15, 18–20, 22, 24, 31, 38). An example of one such compound is amphotericin B (AmpB), a polyene antibiotic used in the treatment of systemic fungal infections.
There is growing evidence that increases in cholesterol concentrations in serum increase the renal toxicity of AmpB. Our laboratory has previously observed that AmpB is more nephrotoxic when it is administered to hypercholesterolemic insulin-dependent diabetic rats than when it is administered to nondiabetic rats (35). Koldin and coworkers demonstrated elevated AmpB-induced nephrotoxicity when AmpB bound to low-density lipoproteins (LDL) was administered to hypercholesterolemic rabbits (12). Lopez-Berestein observed that when AmpB was administered to patients with leukemia (16) and immunocompromised patients who exhibited lower cholesterol concentrations in serum, AmpB-induced renal toxicity decreased (23). Chabot and coworkers observed no measurable renal toxicity when AmpB was administered to cancer patients who exhibited hypocholesterolemia (5). We have further reported that patients with a higher percentage of AmpB bound to serum LDL are more susceptible to AmpB-induced kidney toxicity (32).
The studies presented in this manuscript determine the plasma lipoprotein distribution of free ATRA and L-ATRA (Atragen; Aronex Pharmaceuticals Inc.) following incubation in human, rat, and dog plasma and address the role of lipoprotein lipid and protein content in the distribution of ATRA to plasma lipoproteins.
MATERIALS AND METHODS
Chemicals, lipids, and plasma.
[3H]Atragen (specific activity, 49 μCi/mmol; lot no. 1051.85.1) was provided by Aronex Pharmaceuticals Inc. All-trans-[11,12(n)-3H]retinoic acid ([3H]ATRA; specific activity, 49 Ci/mmol; 5 mCi/ml in ethanol) was purchased from Amersham. Human plasma was provided by British Columbia Red Cross (blood samples are pooled from a variety of nondiseased human volunteers). Rat plasma was obtained from fasted Sprague-Dawley rats (250 to 300 g). Dog plasma was purchased from Harlan Bioproducts (Indianapolis, Ind.). Organic solvents (e.g., ethanol) were purchased from Fisher Canada. Ultracentrifugation supplies (centrifuge tubes and density gradient solutions, etc.) were purchased from Beckman Canada. Lipid and protein analysis kits were purchased from Sigma Chemical (St. Louis, Mo.). Affinity lipoprotein separation kits were purchased from Isolab Inc. (St. Louis, Mo.).
Atragen was manufactured in accordance with good manufacturing practices at BenVenue Laboratories as a lyophilized product containing tretinoin, dimyristoyl phosphatidylcholine (DMPC), and soybean oil. Following reconstitution with sterile saline, each vial of Atragen contains 2 mg of tretinoin per ml. This formulation was identical to that which was used in all preclinical and clinical studies.
[3H]Atragen was manufactured at Aronex Pharmaceuticals as a lyophilized product containing nonradiolabeled and all-trans-[11,12(n)-3H]retinoic acid, DMPC, and soybean oil. This formulation was identical to that of the nonradiolabeled Atragen used in clinical studies, except that it contained [3H]tretinoin. The purity of the tretinoin in the [3H]Atragen was 99.5% by high-performance liquid chromatography UV analysis and 91.1% by high-performance liquid chromatography radioactivity analysis (Aronex certificate of analysis).
[3H]ATRA (51 Ci/mmol) was manufactured by Amersham and was combined with nonradiolabeled tretinoin to make a final solution for injection containing 2 mg of tretinoin per ml and 11.13 μCi of [3H]ATRA per ml (51 Ci/mmol) in phosphate-buffered saline. [3H]ATRA contributed less than 1% to the total concentration of tretinoin (2 mg/ml) in the reconstituted [3H]ATRA.
Dose preparation.
For [3H]Atragen, 50 ml of 0.9% sterile saline for injection (USP) was added to a vial containing 100 mg of [3H]Atragen. The vial was shaken thoroughly to form the liposomal suspension and was used immediately. For [3H]ATRA, a stock solution of ATRA dissolved in ethanol (995 μl), spiked with 5 μl of the [3H]ATRA reconstituted in ethanol, was prepared. The final concentration of this solution was 12.5 mg/ml.
Harvesting of plasma from blood.
Blood collected from healthy human volunteers (screened by British Columbia Red Cross) was placed in drug-free glass test tubes which contained 0.05 M EDTA and was centrifuged by use of a tabletop centrifuge for 10 min at 2,000 rpm; the plasma was stored at −20°C until used in the study.
Lipoprotein separation.
The plasma was separated into its high-density lipoprotein (HDL), LDL, very low density lipoprotein (VLDL), and lipoprotein-deficient plasma (LPDP) fractions by step gradient ultracentrifugation with sodium bromide (29). Briefly, human (3.0-ml), rat (1.5-ml), or dog (1.5-ml) plasma samples were placed in centrifuge tubes and readjusted to 1.25 g/ml by the addition of sodium bromide. Once the sodium bromide was dissolved in the plasma, 2.8 ml of the sodium bromide solution with the highest density (density of 1.21 g/ml, which represents the HDL fraction) was layered onto the plasma solution. Then, 2.8 ml of the second sodium bromide solution (density of 1.063 g/ml, which represents the LDL fraction) was layered onto the sample, followed by 2.8 ml of the third sodium bromide solution (density of 1.006 g/ml, which represents the VLDL and chylomicron fraction). All sodium bromide solutions were kept at 4°C prior to the layering of the density gradient.
The sample-containing ultracentrifuge tubes were placed into individual titanium buckets (Beckman Canada Inc.), balanced, and capped. The buckets were then placed into their respective positions on a swinging-bucket rotor (SW 41 Ti; Beckman Canada Inc.) and centrifuged at 40,000 rpm (relative centrifugal field [× g] at rmax of 285,000) at a temperature of 15°C for 18 h in a high-speed ultracentrifuge (L8-80 M; Beckman Canada Inc.). After ultracentrifugation, the samples were carefully removed from the titanium buckets. Each density layer was removed with a Pasteur pipette, and the volume of each lipoprotein fraction was measured.
To ensure that the distribution of tretinoin found in each of these fractions was a result of its association with each lipoprotein or lipoprotein-deficient fraction and not a result of the density of the formulation, the densities of free ATRA reconstituted in methanol and of the [3H]Atragen formulation reconstituted in 0.9% sodium chloride (USP) following incubation for 1 h at 37°C in LPDP were determined by ultracentrifugation (28).
Determination of triglyceride, cholesterol, and protein concentrations in plasma lipoprotein.
Concentrations of total triglycerides (TG), cholesterol, and protein in the human, rat, and dog plasma used were determined by enzymatic assays purchased from Sigma Diagnostics (St. Louis, Mo.). Briefly, TG were first hydrolyzed by lipoprotein lipase to glycerol and free fatty acids. Glycerol was then phosphorylated by ATP, forming glycerol-1-phosphate and ADP in the reaction catalyzed by glycerol kinase. Glycerol-1-phosphate was then oxidized by glycerol phosphate oxidase to dihydrooxyacetone phosphate and hydrogen peroxide. A quinoneimine dye was produced by the peroxidase-catalyzed coupling of 4-aminoantipyrine and sodium N-ethyl-N-(3-sulfopropyl)m-anisidine with hydrogen peroxide. This dye shows a maximum absorbancy at 500 nm, and its intensity is directly proportional to the triglyceride concentration of the sample. Absorbancies of plasma and lipoprotein samples were determined and compared to an external calibration curve for TG (linear range of 10 to 300 mg/dl; r2 = 0.95).
In the determination of cholesterol concentrations, cholesterol esters were first hydrolyzed to cholesterol by cholesterol esterase. The cholesterol was then oxidized by cholesterol oxidase to cholest-4-en-3-one and hydrogen peroxide. A quinoneimine dye was produced by the peroxidase-catalyzed coupling of 4-aminoantipyrine and p-hydroxybenzenesulfonate with hydrogen peroxide. This dye shows a maximum absorbancy at 500 nm, and its intensity is directly proportional to the cholesterol concentration of the sample. Absorbancies of plasma and lipoprotein samples were determined and compared to an external calibration curve for cholesterol (linear range of 10 to 450 mg/dl; r2 = 0.96).
In the determination of protein concentrations, an alkaline cupric tartrate reagent complexes with the peptide bonds and forms a purple dye when the phenol reagent is added. This dye shows a maximum absorbancy at 750 nm, and its intensity is directly proportional to the protein concentration of the sample. Absorbancies of plasma and lipoprotein samples were determined and compared to an external calibration curve for protein (linear range of 5 to 160 mg/dl; r2 = 0.97).
Tretinoin quantification.
[3H]ATRA and [3H]Atragen were quantitated in each lipoprotein and LPDP fraction by radioactivity analysis. All samples were counted in a scintillation counter and analyzed against an external standard calibration curve for each lipoprotein and lipoprotein-deficient fraction to correct for any quenching.
Experimental design.
To assess the distribution of [3H]ATRA and [3H]Atragen within rat, dog, and human plasma, ATRA and [3H]Atragen (1, 5, 10, and 25 μg of tretinoin/ml of plasma) were incubated in rat, dog, and human plasma for 5, 60, and 180 min at 37°C. (It is important to note that 5 to 20 μg of tretinoin/ml of plasma is a concentration close to the peak levels in blood on days 1 and 15, respectively, that were observed in humans after administration of L-ATRA at concentrations of 90 to 175 mg/m2 [8]. The time required to reach peak levels following administration has been reported to be approximately 60 min [8]). Plasma samples were removed and assayed for drug in each of the lipoprotein and LPDP fractions. Control experiments were done; in these, ethanol and 0.9% sodium chloride without drug were incubated in plasma. Previous studies have demonstrated that ethanol at the incubation volume needed to delivery 100 μg of tretinoin per 1 ml of plasma does not alter the composition or concentration of plasma lipoproteins. Tretinoin is light sensitive; therefore, all the experiments were done under subdued light. All tubes containing tretinoin were protected from light at all times.
Statistical analysis.
Differences in the distribution of [3H]ATRA, [3H]Atragen and rat, dog, and human lipoprotein lipid and protein concentrations in plasma were determined by analysis of variance without repeated measures (INSTAT; Human Systems Dynamics). Critical differences were assessed by the Newman-Keuls and Tukey post hoc tests. A difference was considered significant if the probability of chance explaining the results was reduced to less than 5% (P < 0.05). All data were expressed as means ± standard deviations.
RESULTS
Analysis of human, dog, and rat plasma lipoprotein lipid and protein content.
The total cholesterol (esterified and unesterified), triglyceride, and protein concentrations within human, dog, and rat plasma lipoproteins are reported in Table 1. Differences in total and lipoprotein fraction concentrations of cholesterol, triglyceride-rich lipoproteins (TRL), which include VLDL and chylomicrons, HDL protein, and TRL triglyceride between human, dog, and rat plasma samples were observed. The lipoprotein compositions of human, dog, and rat plasma are reported in Table 2. Differences in TRL and HDL lipid and protein contents between human, dog, and rat plasma were observed.
TABLE 1.
Concentrations of cholesterol (esterified plus unesterified), triglyceride, and protein in plasma lipoproteins from three different speciesa
| Lipid or protein and plasma type | Concn (mg/dl) in:
|
Total lipoprotein concn (mg/dl) | ||
|---|---|---|---|---|
| TRLb | LDL | HDL | ||
| Cholesterol (esterified + unesterified) | ||||
| Human | 30.4 ± 3.7 | 50.5 ± 3.7 | 28.7 ± 3.2 | 109.7 ± 6.7 |
| Dog | 4.4 ± 1.4c | 35.8 ± 6.3c | 164.9 ± 20.9c | 205.1 ± 17.3c |
| Rat | 11.7 ± 1.3c,d | 21.1 ± 5.8c | 45.3 ± 7.4c,d | 78.2 ± 4.2c,d |
| Triglyceride | ||||
| Human | 30.3 ± 2.3 | 20.0 ± 1.6 | 29.4 ± 8.5 | 79.8 ± 10.6 |
| Dog | 13.5 ± 3.6c | 18.9 ± 6.9 | 26.1 ± 8.8 | 58.5 ± 17.8 |
| Rat | 27.7 ± 2.3d | 7.2 ± 1.1c,d | 20.2 ± 2.1 | 55.1 ± 1.7c |
| Protein | ||||
| Human | 9.5 ± 3.2 | 35.4 ± 4.0 | 350.6 ± 111.3 | 4,245.6 ± 314.3 |
| Dog | 3.5 ± 2.2c | 40.4 ± 15.6 | 729.0 ± 76.7c | 4,855.5 ± 241.5 |
| Rat | 4.6 ± 1.1c | 19.2 ± 10.8 | 106.0 ± 7.1c,d | 4,940.8 ± 203.2 |
Data are expressed as means ± standard deviations (n = 11 for human and dog plasma; n = 6 for rat plasma).
TRL include VLDL and chylomicrons.
P < 0.05 compared to value for human plasma.
P < 0.05 compared to value for dog plasma.
TABLE 2.
Lipoprotein composition of plasma from three different species
| Lipoprotein fraction and component ratio (wt/wt)a | Results for plasma fromb:
|
||
|---|---|---|---|
| Human | Dog | Rat | |
| TRL | |||
| TC/TP | 3.5 ± 1.2 | 1.5 ± 0.9c | 2.6 ± 0.4 |
| TG/TP | 3.5 ± 1.1 | 3.2 ± 1.7 | 6.2 ± 1.0c,d |
| TG/TC | 1.0 ± 0.1 | 3.0 ± 1.1c | 2.4 ± 0.1c,d |
| LDL | |||
| TC/TP | 1.4 ± 0.2 | 1.0 ± 0.2c | 0.2 ± 0.1c,d |
| TG/TP | 0.6 ± 0.1 | 0.5 ± 0.3 | 0.07 ± 0.01c,d |
| TG/TC | 0.4 ± 0.04 | 0.5 ± 0.2 | 0.35 ± 0.07 |
| HDL | |||
| TC/TP | 0.09 ± 0.03 | 0.23 ± 0.04c | 0.10 ± 0.002c |
| TG/TP | 0.09 ± 0.03 | 0.04 ± 0.01c | 0.004 ± 0.001c,d |
| TG/TC | 1.0 ± 0.3 | 0.2 ± 0.1c | 0.5 ± 0.03c,d |
TC, total cholesterol (esterified plus unesterified); TG, total triglycerides; TP, total protein.
Data are expressed as means ± standard deviations (n = 11 for human plasma; n = 10 for dog plasma; n = 6 for rat plasma).
P < 0.05 compared to value for human plasma.
P < 0.05 compared to value for dog plasma.
Effect of liposomal incorporation on ATRA distribution with lipoproteins.
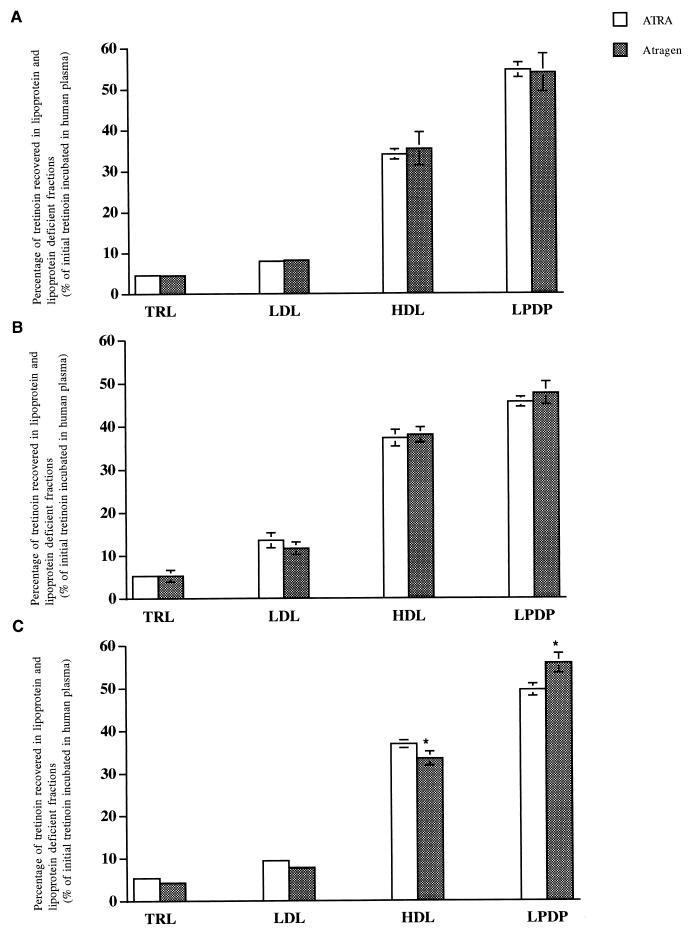
Incubation of ATRA (25 μg of tretinoin/ml) with human plasma for 5 min at 37°C resulted in 4.6% ± 0.2% of the initial tretinoin concentration in the TRL fraction, 8.0% ± 0.3% in the LDL fraction, 34.0% ± 1.3% in the HDL fraction, and 54.5% ± 1.8% in the LPDP fraction (Fig. 1A). The distribution of tretinoin following incubation of L-ATRA (Atragen) in human plasma for 5 min was 4.5% ± 0.2% in the TRL fraction, 8.2% ± 0.5% in the LDL fraction, 35.4% ± 4.0% in the HDL fraction, and 53.9% ± 4.6% in the LPDP fraction (Fig. 1A).
FIG. 1.
Distribution of free ATRA and Atragen (25 μg/ml) in human plasma following incubation for 5 (A), 60 (B), and 180 (C) min at 37°C. Data are expressed as percentages of total tretinoin distributed into TRL, LDL, HDL, and LPDP fractions and are reported as means ± standard deviations (error bars) (n = 6). ∗, P < 0.05 compared to value for ATRA.
Incubation of ATRA (25 μg of tretinoin/ml) with human plasma for 60 min at 37°C resulted in 5.2% ± 0.6% of the initial tretinoin concentration in the TRL fraction, 13.6% ± 1.7% in the LDL fraction, 37.2% ± 1.9% in the HDL fraction, and 45.6% ± 1.2% in the LPDP fraction (Fig. 1B). The distribution of tretinoin following incubation of L-ATRA (Atragen) in human plasma for 60 min was 5.2% ± 1.4% in the TRL fraction, 11.7% ± 1.5% in the LDL fraction, 38.0% ± 1.8% in the HDL fraction, and 47.6% ± 2.7% in the LPDP fraction (Fig. 1B).
Incubation of ATRA (25 μg of tretinoin/ml) with human plasma for 180 min at 37°C resulted in 5.4% ± 0.7% of the initial tretinoin concentration in the TRL fraction, 9.4% ± 0.7% in the LDL fraction, 36.8% ± 0.9% in the HDL fraction, and 49.6% ± 1.4% in the LPDP fraction (Fig. 1C). The distribution of tretinoin following incubation of L-ATRA (Atragen) in human plasma for 180 min was 4.3% ± 0.2% in the TRL fraction, 7.8% ± 0.6% in the LDL fraction, 33.4% ± 1.6% in the HDL fraction, and 55.8% ± 2.3% in the LPDP fraction (Fig. 1C). Similar results were observed following the incubation of 1, 5, and 10 μg of free ATRA or Atragen per ml (data not shown). The incubation of tretinoin-free liposomes at 5, 60, and 180 min concurrently with free ATRA did not alter ATRA distribution in human plasma (data not shown).
ATRA and Atragen distribution in human, dog, and rat plasma.
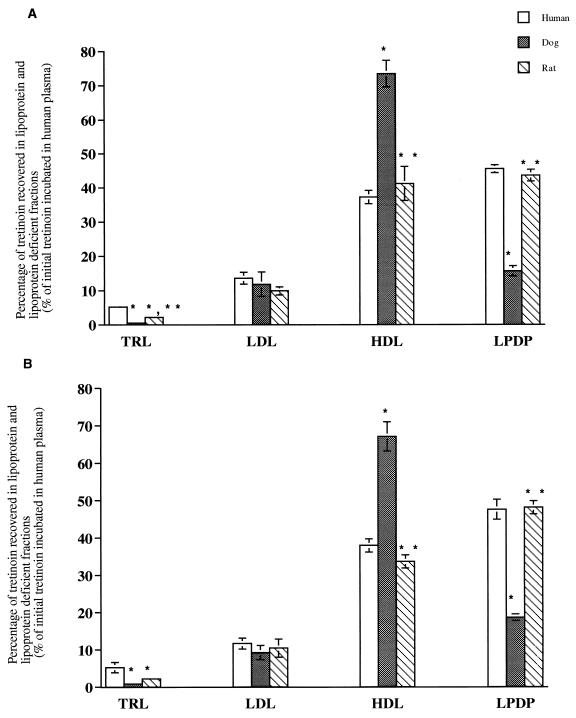
To determine the distribution of ATRA and Atragen in plasma lipoproteins, [3H]ATRA and [3H]Atragen (1, 5, 10, and 25 μg of tretinoin/ml) were incubated for 5, 60, and 180 min at 37°C in plasma samples from a nonfasted normolipidemic human, a nonfasted-dog, and a nonfasted CD 1 rat. Data for results with the 5-μg/ml concentration are presented in Table 3. Data for the 25-μg/ml concentration are presented in Fig. 2. Data for concentrations of 1 and 10 μg/ml are not shown. Incubation of ATRA in human, dog, and rat plasma resulted in a higher percentage of tretinoin recovered in dog and rat plasma HDL than in human plasma HDL (Fig. 2A and Table 3). A significantly lower percentage of tretinoin was recovered in the LPDP fractions of dog and rat plasma than in the same fraction of human plasma (Fig. 2A and Table 3). A significantly higher percentage of tretinoin was recovered in human and rat plasma TRL than in dog plasma TRL. These findings were similar regardless of the incubation time or drug concentration used. Furthermore, plasma distributions of tretinoin were similar following 5 to 180 min of incubation for all plasma types tested.
TABLE 3.
Distribution of ATRA and Atragen at 5 μg of tretinoin/ml within human, dog, and rat plasma following incubation for 5, 60, and 180 min at 37°Ca
| Drug and plasma type | Incubation time (min) | % of drugb recovered from:
|
Total % of drug recovered | |||
|---|---|---|---|---|---|---|
| TRL fraction | LDL fraction | HDL fraction | LPDP fraction | |||
| ATRA | ||||||
| Human | 5 | 4.3 ± 0.3 | 10.0 ± 0.9 | 25.0 ± 0.3 | 56.7 ± 1.8 | 95.9 ± 2.1 |
| 60 | 4.5 ± 0.4 | 10.4 ± 0.8 | 28.0 ± 1.8 | 56.1 ± 3.1 | 99.0 ± 5.9 | |
| 180 | 5.0 ± 0.5 | 10.8 ± 0.2 | 28.0 ± 1.8 | 54.8 ± 2.4 | 98.6 ± 1.2 | |
| Dog | 5 | 0.6 ± 0.1c | 6.1 ± 2.3 | 63.9 ± 0.9c | 32.1 ± 1.6c | 102.7 ± 2.6 |
| 60 | 0.5 ± 0.2c | 4.6 ± 1.0c | 60.2 ± 7.9c | 36.4 ± 2.5c | 101.7 ± 9.5 | |
| 180 | 0.5 ± 0.1c | 3.4 ± 0.3c | 53.2 ± 0.7c | 38.9 ± 2.7c | 95.9 ± 3.1 | |
| Rat | 5 | 3.7 ± 2.3 | 12.3 ± 3.4d | 41.0 ± 3.9c,d | 40.5 ± 1.3c,d | 97.5 ± 1.3 |
| 60 | 2.1 ± 0.2c,d | 8.3 ± 1.0d | 37.9 ± 0.8d | 45.9 ± 3.3d | 94.2 ± 2.7 | |
| 180 | 1.6 ± 0.3d | 9.2 ± 1.1d | 38.9 ± 3.5c,d | 49.4 ± 4.5d | 99.1 ± 3.1 | |
| Atragen | ||||||
| Human | 5 | 4.7 ± 0.4 | 10.1 ± 0.2 | 25.2 ± 0.9 | 58.5 ± 0.6 | 98.5 ± 0.9 |
| 60 | 4.6 ± 0.5 | 9.6 ± 0.2 | 28.5 ± 0.5 | 60.1 ± 3.5 | 102.8 ± 3.8 | |
| 180 | 4.6 ± 0.2 | 10.1 ± 0.5 | 26.1 ± 1.0 | 55.3 ± 2.0 | 96.3 ± 1.6 | |
| Dog | 5 | 1.0 ± 0.1c | 6.0 ± 0.8c | 57.0 ± 3.0c | 35.5 ± 2.5c | 99.5 ± 3.1 |
| 60 | 0.9 ± 0.1c | 6.0 ± 0.4c | 51.4 ± 4.1c | 42.0 ± 1.9c | 100.4 ± 2.6 | |
| 180 | 0.9 ± 0.2c | 4.1 ± 1.3c | 48.5 ± 2.0c | 44.7 ± 1.0c | 98.2 ± 1.8 | |
| Rat | 5 | 3.5 ± 0.04c,d | 11.8 ± 0.9c,d | 37.1 ± 2.2c,d | 45.2 ± 1.2c,d | 97.6 ± 2.2 |
| 60 | 2.4 ± 0.4c,d | 9.1 ± 1.4d | 35.0 ± 3.9d | 50.8 ± 8.0 | 97.3 ± 13.6 | |
| 180 | 1.6 ± 0.01c,d | 9.9 ± 2.2d | 34.8 ± 0.9c,d | 50.7 ± 3.7 | 97.1 ± 5.3 | |
Following incubation, plasma samples were assayed by radioactivity for drug in each of the lipoprotein and LPDP fractions. Plasma was separated into its lipoprotein and lipoprotein-deficient fractions by density gradient ultracentrifugation.
Data are expressed as means ± standard errors of the means (n = 3). Percentages shown are percentages of initial tretinoin amount.
P < 0.05 compared to human plasma distribution value.
P < 0.05 compared to dog plasma distribution value.
FIG. 2.
Distribution of free ATRA (A) and Atragen (B) (25 μg/ml) in human, dog, and rat plasma following incubation for 60 min at 37°C. Data are expressed as percentages of total tretinoin distributed into TRL, LDL, HDL, and LPDP fractions and are reported as means ± standard deviations (error bars) (n = 6). ∗, P < 0.05 compared to value for human plasma; ∗∗, P < 0.05 compared to value for dog plasma.
Incubation of Atragen in human, dog, and rat plasma resulted in a higher percentage of tretinoin recovered in dog and rat plasma HDL than in human plasma HDL at concentrations of 1 μg/ml (data not shown), 5 μg/ml (Table 3), and 10 μg/ml (data not shown) but not at a concentration of 25 μg/ml (Fig. 2B). The percentage of tretinoin recovered in the LPDP fractions of dogs and rat plasma samples was significantly lower than that recovered from human plasma (Fig. 2B and Table 3). A significantly higher percentage of tretinoin was recovered in human and rat plasma TRL than in dog plasma TRL. These findings were similar regardless of which incubation time or drug concentration was used. Furthermore, plasma distributions of tretinoin were similar following 5 to 180 min of incubation for all plasmas tested.
Based on the correlations calculated between the amounts of tretinoin recovered within the TRL, HDL, and LDL plasma fractions following incubation of free ATRA (25 μg/ml) for 60 min at 37°C and the amounts of cholesterol (esterified and unesterified), triglyceride, and protein within these fractions for human, dog, and rat plasma, the following relationships were observed. As the amounts of cholesterol, triglyceride, and protein in the TRL fraction from dog plasma (lowest), rat plasma, and human plasma (highest) increase, the amount of tretinoin recovered within this fraction increases proportionally (Table 4). As the amounts of cholesterol and triglyceride in the LDL fraction from rat plasma (lowest), dog plasma, and human plasma (highest) increase, the amount of tretinoin recovered within this fraction proportionally increases (Table 4). As HDL cholesterol from human plasma (lowest), rat plasma, and dog plasma (highest) increases, the amount of tretinoin recovered within this fraction proportionally increases (Table 4). When correlations between the amounts of tretinoin recovered in each lipoprotein fraction and the lipoprotein compositions were determined, the following relationships were observed. As the ratio of total cholesterol to total protein in the TRL fraction increased, the amount of tretinoin recovered in this fraction increased, while as the TG/total cholesterol ratio increased, the amount of tretinoin recovered decreased (Table 4). When the total cholesterol/total protein ratio increased, the amount of tretinoin recovered in the HDL fraction proportionally increased (Table 4).
TABLE 4.
Correlation between the amount of tretinoin recovered in each lipoprotein fraction and lipid and protein concentrations and compositions in plasma lipoprotein from humans, dogs, and rats following incubation with [3H]ATRA and [3H]Atragena
| Drug and lipoprotein component or ratioa | Correlation with amt of tretinoin recovered fromb:
|
||
|---|---|---|---|
| TRL fraction | LDL fraction | HDL fraction | |
| [3H]ATRA | |||
| TC | 0.99d | 0.91d | 0.95d |
| TG | 0.98d | 0.79d | 0.24e |
| TP | 0.88d | 0.04e | −0.49e |
| TC/TP | 0.87d | 0.59e | 0.95d |
| TG/TP | −0.12e | 0.51e | −0.15e |
| TG/TC | −0.88d | 0.20e | −0.60e |
| [3H]Atragen | |||
| TC | 0.92d | 0.91d | 0.92d |
| TG | 0.90d | 0.71d | 0.53e |
| TP | 0.86d | −0.22e | −0.57e |
| TC/TP | 0.29e | 0.68e | 0.98d |
| TG/TP | −0.44e | 0.35e | 0.14e |
| TG/TC | −0.64e | −0.10e | −0.47e |
[3H]ATRA or [3H]Atragen was incubated at a concentration of 25 μg of tretinoin/ml in either human, dog, or rat plasma for 60 min at 37°C.
TC, total cholesterol; TG, total triglycerides; TP, total protein.
Data are given as Pearson correlation coefficients (r) between amount of tretinoin associated with lipoproteins and lipid and protein concentrations and compositions in plasma lipoproteins.
Significant at P < 0.05.
Not significant.
Based on the correlations calculated between the amounts of tretinoin recovered within the TRL, HDL, and LDL plasma fractions following incubation of Atragen (25 μg of tretinoin/ml) for 60 min at 37°C and the amounts of cholesterol (esterified and unesterified), triglyceride, and protein within these fractions for plasma from all three species, the following relationships were observed. As cholesterol, triglyceride, and protein in the TRL fraction from dog plasma (lowest), rat plasma, and human plasma (highest) increase, the amount of tretinoin recovered within this fraction proportionally increases (Table 4). As cholesterol and triglyceride in the LDL fraction from rat plasma (lowest), dog plasma, and human plasma (highest) increase, the amount of tretinoin recovered within this fraction proportionally increases (Table 4). As cholesterol in the HDL fractions from human plasma (lowest), rat plasma, and dog plasma (highest) increases, the amount of tretinoin recovered within this fraction proportionally increases (Table 4). When correlations between the amount of tretinoin recovered in each lipoprotein fraction and the lipoprotein composition were determined, the following relationship was observed. As the total cholesterol/total protein ratio increased in the HDL fraction, the amount of tretinoin recovered in HDL proportionally increased (Table 4). Correlations calculated by using free ATRA and Atragen concentrations of 1, 5, and 10 μg/ml resulted in similar findings (data not shown).
DISCUSSION
The purpose of these studies was to determine the lipoprotein distribution of free ATRA and L-ATRA (Atragen) following incubation in human, rat, and dog plasma and to address the role of lipoprotein lipid and protein content in the distribution of tretinoin to plasma lipoproteins. We observed that the incorporation of tretinoin into liposomes did not alter the distribution of the drug in plasma. Increases in lipid and protein concentrations in the TRL fraction from one species to another increased the amount of tretinoin recovered in this fraction following the incubation of free ATRA and Atragen in human, dog, and rat plasma. Increases in LDL lipid and HDL cholesterol concentrations from one species to another increased the amount of tretinoin recovered in those fractions following the incubation of free ATRA and Atragen in human, dog, and rat plasma.
We have previously observed that AmpB predominantly associates with HDL in human serum and that the amount of AmpB associated with HDL increases when AmpB is incorporated into liposomes containing DMPC and dimyristoyl phosphatidylglycerol (DMPG) (31, 38). When annamycin (Ann), an anticancer anthracycline analog, and nystatin (Nys) were incorporated into liposomes with the same phospholipid composition, the majority of Ann and Nys was recovered in the HDL fraction (30, 33, 36). Since HDL and LDL are found not in an equimolar ratio in human plasma but at LDL/HDL ratios ranging from 4:1 to 6:1 (37), these data suggest that a mechanism(s) besides random probability or mass lipoprotein lipid levels must drive these drug-liposome complexes towards HDL rather than LDL. One such mechanism appears to be related to liposome composition. We have observed that the DMPG component of liposomal AmpB and Ann distributes predominantly into HDL because of its interaction with the protein components (apolipoproteins AI and AII) of HDL (38).
However, in the present study, no differences in plasma lipoprotein distribution of tretinoin were observed following the incubation of free ATRA or L-ATRA (Atragen) in human plasma (Fig. 1 and Table 3). These findings suggest that a liposome-lipid acts as a solubilizing agent and does not influence the distribution of tretinoin in plasma. Furthermore, L-ATRA (Atragen), unlike liposomal AmpB, Ann, and Nys, does not contain DMPG in its formulation. Thus, the increased distribution of AmpB, Ann, and Nys into the HDL fraction observed when these drugs were incorporated into liposomes containing DMPG was not reported when tretinoin was incorporated into liposomes. In addition, we have previously observed that increases in the HDL protein concentration in humans increased the amount of free Nys recovered in this fraction (3). Similarly, it is possible that the greater recovery of tretinoin in human HDL than in TRL and LDL (Fig. 1 and Table 3) may be due to a higher protein content within HDL than within TRL and LDL (Table 1).
Previous studies with AmpB have suggested that an alteration in lipid concentrations in plasma modify this drug’s pharmacological behavior. Chavanet and coworkers have demonstrated that an increase in the triglyceride concentration in plasma leads to a reduction in AmpB toxicity (6). These findings suggested that triglycerides, or their main vehicle in serum, chylomicrons, as well as LDL and VLDL were involved in the protective effect against AmpB toxicity. Souza and coworkers have further shown that a triglyceride-rich emulsion that behaves in vivo like chylomicrons was able to reduce the in vivo and in vitro toxicity of AmpB (27). Our laboratory has recently shown enhanced AmpB-induced kidney toxicity within patients who exhibited elevated LDL cholesterol concentrations in serum (32).
In the present study, we have observed differences in the distribution of tretinoin in plasma when free ATRA and Atragen were incubated in human plasma compared to that in dog or rat plasma (Fig. 2 and Table 3). It appears that these differences can be attributed to differences in the species lipoprotein lipid and protein concentration (Table 1) and composition (Table 2) profiles. In particular, increases in HDL cholesterol found in dog plasma resulted in the recovery of more tretinoin from this fraction than from the same fraction from human or rat plasma (Fig. 2 and Table 3). Furthermore, increases in TRL lipid and protein and LDL lipid concentrations from plasma from the different species resulted in more tretinoin recovered in these fractions (Table 4). These findings suggest that tretinoin distribution in lipoprotein may be partially regulated by lipoprotein cholesterol, triglyceride, and to a lesser extent protein concentrations in plasma.
In addition, we observed that increasing the total cholesterol/total protein ratio within the TRL and HDL fractions, decreasing the TG/total cholesterol ratio within the TRL fraction for free ATRA (Table 4), and increasing the total cholesterol/total protein ratio within the HDL fraction only for Atragen (Table 4) resulted in the recovery of more tretinoin from these fractions. These findings suggest that in addition to lipid, protein mass, and lipoprotein composition, the type of lipoprotein in which these changes occur is another possible factor in determining to which lipoprotein tretinoin binds.
In conclusion, we have determined that the plasma distribution of tretinoin does not change when the drug is incorporated into liposomes composed of DMPC and soybean oil. Furthermore, the distribution of tretinoin among plasma lipoproteins of different species is defined by the relative levels of individual lipoproteins as well as their lipid and protein composition, possibly an important consideration when evaluating the pharmacokinetics, toxicity, and activity of these compounds following administration to different animal species.
ACKNOWLEDGMENT
This work was funded by a grant from Aronex Pharmaceuticals Inc. (to K.M.W.).
REFERENCES
- 1.Aten R F, Kolodecik T R, Behrman H R. Ovarian vitamin E accumulation: evidence for a role of lipoproteins. Endocrinology. 1994;135:533–539. doi: 10.1210/endo.135.2.8033800. [DOI] [PubMed] [Google Scholar]
- 2.Brajtburg J, Elberg S, Medoff G. Interaction of plasma proteins and lipoproteins with amphotericin B. J Infect Dis. 1984;149:986–992. doi: 10.1093/infdis/149.6.986. [DOI] [PubMed] [Google Scholar]
- 3.Cassidy S M, Strobel F W, Wasan K M. Plasma lipoprotein distribution of liposomal nystatin is influenced by protein content of high- density lipoproteins. 1998. Antimicrob. Agents Chemother., in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cenni B, Meyer J, Brandt R, Betschart B. The antimalarial drug halofantrine is bound mainly to low and high density lipoproteins in human serum. Br J Clin Pharmacol. 1995;39:519–526. doi: 10.1111/j.1365-2125.1995.tb04489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chabot G G, Pazdur R, Valeriote F A, Baker L H. Pharmacokinetics and toxicity of continuous infusion of amphotericin B in cancer patients. J Pharm Sci. 1989;78:307–310. doi: 10.1002/jps.2600780409. [DOI] [PubMed] [Google Scholar]
- 6.Chavanet P, Joly V, Rigaud D, Bolard J, Carbon C, Yeni P. Influence of diet on experimental toxicity of amphotericin B deoxycholate. Antimicrob Agents Chemother. 1994;38:963–968. doi: 10.1128/aac.38.5.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drach J, Lopez-Berestein G, McQueen T, Andreeff M, Mehta K. Induction of differentiation in myeloid leukemia cell lines and acute promyelocytic leukemia cells by liposomal all-trans-retinoic acid. Cancer Res. 1993;53:2100–2104. [PubMed] [Google Scholar]
- 8.Estey E, Thall P F, Mehta K, Rosenblum M, Brewer T, Jr, Simmons V, Cabanillas F, Kurzrock R, Lopez-Berestein G. Alterations in tretinoin pharmacokinetics following administration of liposomal all-trans retinoic acid. Blood. 1996;87:3650–3654. [PubMed] [Google Scholar]
- 9.Ha Y C, Barter P J. Differences in plasma cholesteryl ester transfer activity in sixteen vertebrate species. Comp Biochem Physiol. 1982;71:265–269. doi: 10.1016/0305-0491(82)90252-8. [DOI] [PubMed] [Google Scholar]
- 10.Harmony J A K, Aleson A L, McCarthy B M. Chapter 15. In: Scanu A M, Spector A A, editors. Biochemistry and biology of plasma lipoproteins. New York, N.Y: Marcel Dekker, Inc.; 1986. pp. 403–430. [Google Scholar]
- 11.Humberstone A J, Porter C J H, Edwards G A, Charman W N. Distribution of Halofantrine between plasma lipoprotein fractions after IV administration to pre- and post-prandial beagle dogs. Pharm Res. 1995;12:S–356. [Google Scholar]
- 12.Koldin M H, Kobayashi G S, Brajtburg J, Medoff G. Effects of elevation of serum cholesterol and administration of amphotericin B complexed to lipoproteins on amphotericin B-induced toxicity to rabbits. Antimicrob Agents Chemother. 1985;28:144–145. doi: 10.1128/aac.28.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazzarino M, Regazzi M B, Corso A. Clinical relevance of all-trans retinoic acid pharmacokinetics and its modulation in acute promyelocytic leukemia. Leuk Lymphoma. 1996;23:539–543. doi: 10.3109/10428199609054862. [DOI] [PubMed] [Google Scholar]
- 14.Lemaire M, Pardridge W M. Influence of blood components on the tissue uptake indices of cyclosporin in rats. J Pharmacol Exp Ther. 1988;244:740–743. [PubMed] [Google Scholar]
- 15.Lemaire M, Tillement J P. Role of lipoproteins and erythrocytes in the in vitro binding and distribution of cyclosporin A in the blood. J Pharm Pharmacol. 1982;34:715–718. doi: 10.1111/j.2042-7158.1982.tb06206.x. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Berestein G. Liposomes as carriers of antifungal drugs. Ann N Y Acad Sci. 1988;544:590–597. doi: 10.1111/j.1749-6632.1988.tb40459.x. [DOI] [PubMed] [Google Scholar]
- 17.Mehta K, Sadeghi T, McQueen T, Lopez-Berestein G. Liposome encapsulation circumvents the hepatic clearance mechanisms of all-trans-retinoic acid. Leuk Res. 1994;18:587–596. doi: 10.1016/0145-2126(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 18.Milton K A, Edwards G, Ward S A, Orme M L E, Breckenridge A M. Pharmacokinetics of halofantrine in man: effects of food and dose size. Br J Clin Pharmacol. 1989;28:71–77. doi: 10.1111/j.1365-2125.1989.tb03507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nemunaitis J, Deeg H J, Yee G C. High cyclosporin levels after bone marrow transplantation associated with hypertriglyceridemia. Lancet. 1986;i:744–745. doi: 10.1016/s0140-6736(86)90254-0. [DOI] [PubMed] [Google Scholar]
- 20.Northcote R J, Todd I C, Ballantyne D. Beta blockers and lipoproteins: a review of current knowledge. Scott Med J. 1986;31:220–228. doi: 10.1177/003693308603100402. [DOI] [PubMed] [Google Scholar]
- 21.Parthasarathy R, Sacks P G, Harris D, Brock H, Mehta K. Interaction of liposome-associated all-trans-retinoic acid with squamous carcinoma cells. Cancer Chemother Pharmacol. 1994;34:527–534. doi: 10.1007/BF00685666. [DOI] [PubMed] [Google Scholar]
- 22.Pike E, Skuterud B, Kierulf P, Lunde P K. Significance of lipoproteins in serum binding variations of amitriptyline, nortriptyline, and quinidine. Clin Pharmacol Ther. 1982;32:599–606. doi: 10.1038/clpt.1982.209. [DOI] [PubMed] [Google Scholar]
- 23.Pontain D R, Sun D, Brim J D, et al. Inhibition of HIV replication by liposomal amphotericin B. Antivir Res. 1989;13:119–125. doi: 10.1016/0166-3542(89)90023-5. [DOI] [PubMed] [Google Scholar]
- 24.Rajaram O V, Fatterpaker P, Sreenivasan A. Involvement of binding lipoproteins in the absorption and transport of alpha-tocopherol in the rat. Biochem J. 1974;140:509–516. doi: 10.1042/bj1400509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regazzi M B, Iacona I, Gervasutti C, Lazzarino M, Toma S. Clinical pharmacokinetics of tretinoin. Clin Pharmacokinet. 1997;32:382–402. doi: 10.2165/00003088-199732050-00004. [DOI] [PubMed] [Google Scholar]
- 26.Rowland M. Plasma protein binding and therapeutic monitoring. Ther Drug Monit. 1980;2:29–37. doi: 10.1097/00007691-198001000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Souza L C, Maranhao R C, Schrier S, Campa A. In vitro and in vivo studies of the decrease of amphotericin B toxicity upon association with a triglyceride-rich emulsion. J Antimicrob Agents. 1993;32:123–132. doi: 10.1093/jac/32.1.123. [DOI] [PubMed] [Google Scholar]
- 28.Wasan, K. M., S. M. Cassidy, M. Ramaswamy, A. Kennedy, F. W. Strobel, and S. P. Ng. A comparison of step-gradient and sequential density ultracentrifugation and the use of lipoprotein deficient plasma controls in determining the plasma lipoprotein distribution of hydrophobic compounds. Submitted for publication. [DOI] [PubMed]
- 29.Wasan K M, Pritchard P H, Ramaswamy M, Wong W, Donnachie E M, Brunner L J. Differences in lipoprotein lipid concentration and composition modify the plasma distribution of cyclosporine. Pharm Res. 1997;14:1614–1621. doi: 10.1023/a:1012190620854. [DOI] [PubMed] [Google Scholar]
- 30.Wasan K M, Ramaswamy M, Cassidy S M, Kazemi M, Strobel F W, Thies R L. Physical characteristics and lipoprotein distribution of liposomal nystatin in human plasma. Antimicrob Agents Chemother. 1997;41:1871–1875. doi: 10.1128/aac.41.9.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wasan K M, Lopez-Berestein G. Diversity of lipid-based polyene formulations and their behavior in biological systems. Eur J Clin Microbiol Infect Dis. 1997;16:81–92. doi: 10.1007/BF01575125. [DOI] [PubMed] [Google Scholar]
- 32.Wasan K M, Conklin J S. Enhanced amphotericin B nephrotoxicity in intensive care patients with elevated levels of low-density lipoprotein cholesterol. Clin Infect Dis. 1997;24:78–80. doi: 10.1093/clinids/24.1.78. [DOI] [PubMed] [Google Scholar]
- 33.Wasan K M, Morton R E. Differences in lipoprotein concentration and composition modify the plasma distribution of free and liposomal annamycin. Pharm Res. 1996;13:462–468. doi: 10.1023/a:1016065114515. [DOI] [PubMed] [Google Scholar]
- 34.Wasan K M. Modifications in plasma lipoprotein concentration and lipid composition regulate the biological activity of hydrophobic drugs. J Pharmacol Toxicol Methods. 1996;36:1–11. doi: 10.1016/1056-8719(96)00054-8. [DOI] [PubMed] [Google Scholar]
- 35.Wasan K M, Conklin J S. Evaluation of renal toxicity and antifungal activity of free and liposomal amphotericin B following a single intravenous dose to diabetic rats with systemic candidiasis. Antimicrob Agents Chemother. 1996;40:1806–1810. doi: 10.1128/aac.40.8.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wasan K M, Perez-Soler R. Distribution of free and liposomal annamycin within human plasma is regulated by plasma triglyceride concentrations but not by lipid transfer protein. J Pharm Sci. 1995;84:1094–1100. doi: 10.1002/jps.2600840912. [DOI] [PubMed] [Google Scholar]
- 37.Wasan K M, Lopez-Berestein G. Targeted liposomes in fungi: modifying the therapeutic index of amphotericin B by its incorporation into negatively charged liposomes. J Liposome Res. 1995;5:883–903. [Google Scholar]
- 38.Wasan K M, Brazeau G A, Keyhani A, Hayman A C, Lopez-Berestein G. Role of liposome composition and temperature in distribution of amphotericin B in serum lipoproteins. Antimicrob Agents Chemother. 1993;37:246–250. doi: 10.1128/aac.37.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]