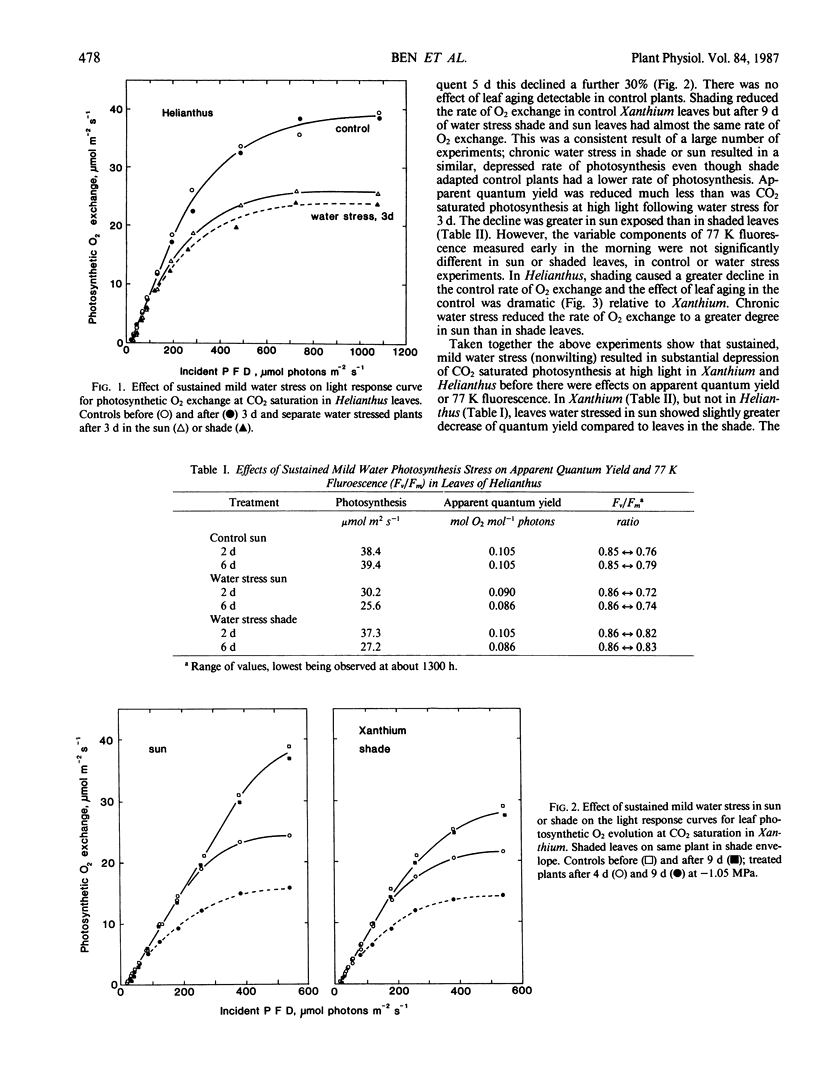
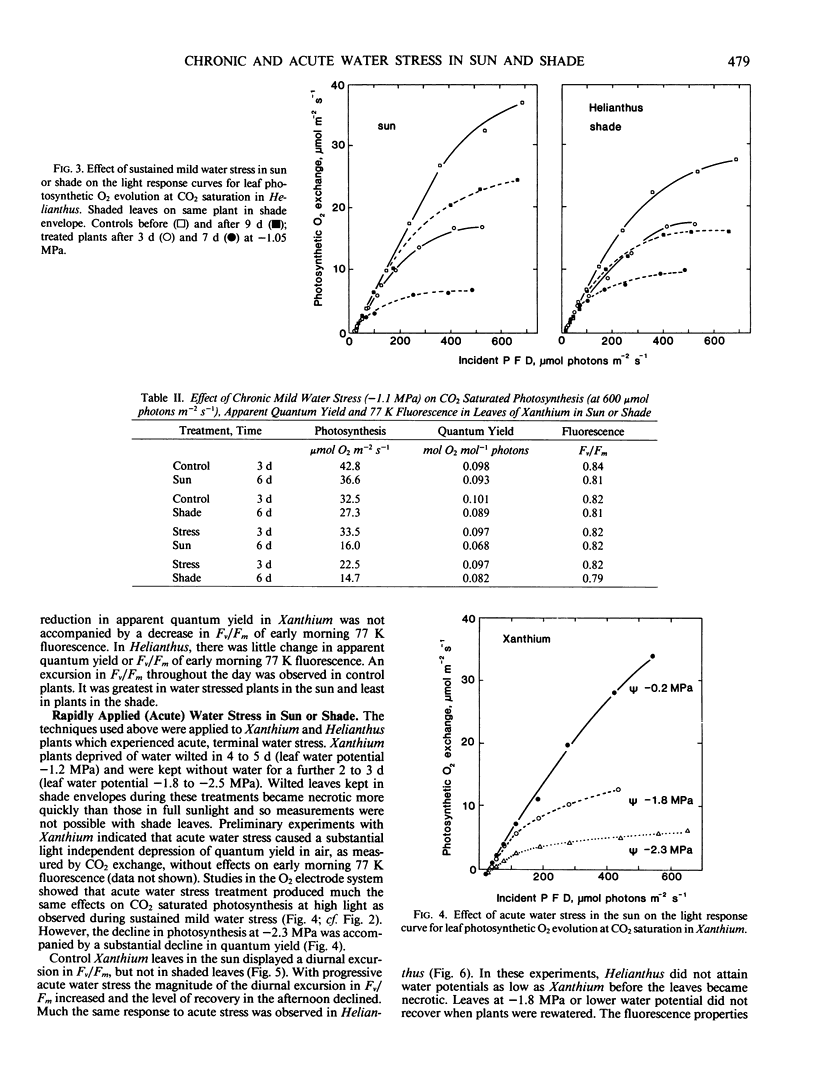
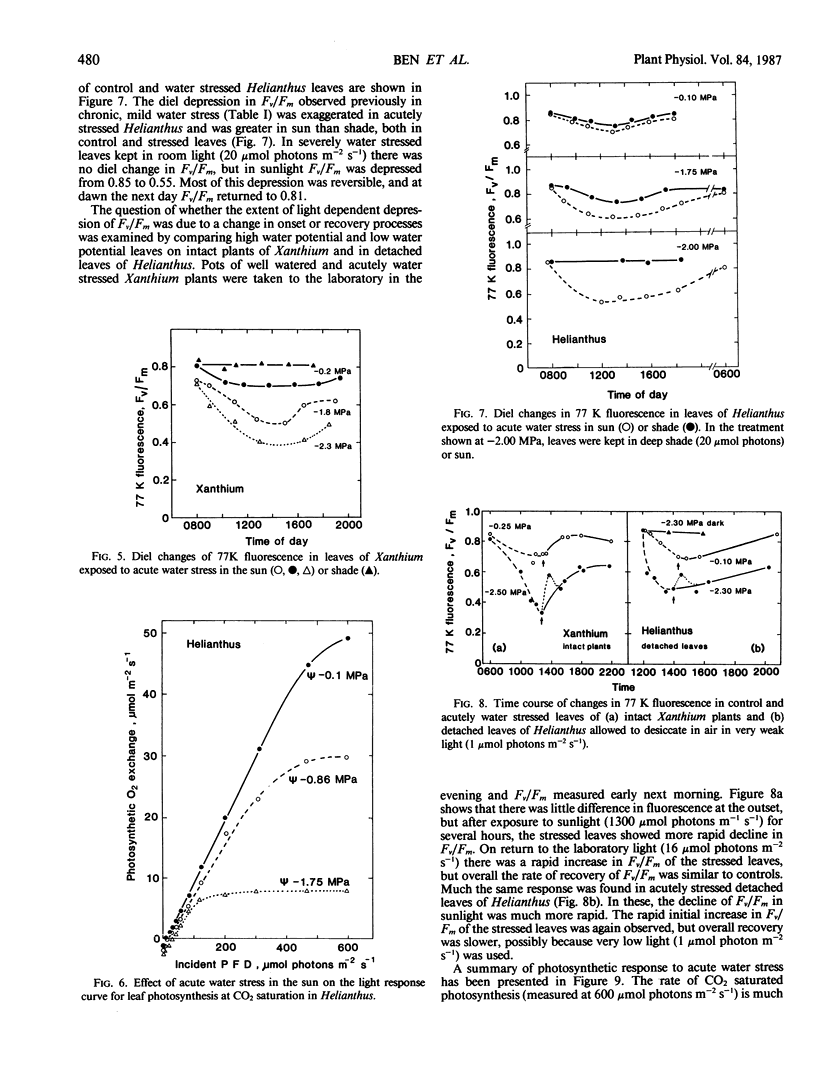
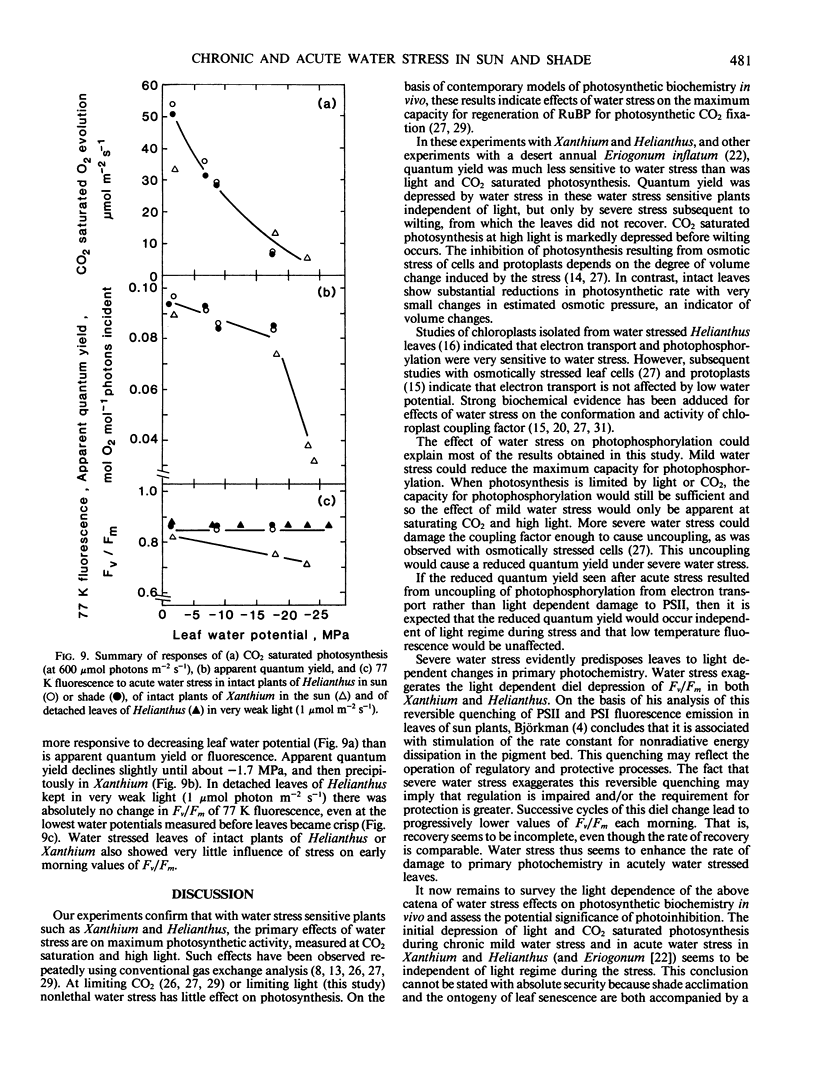
Abstract
We have examined the effects of mild, chronic water stress and acute water stress on two water stress sensitive plants, Xanthium strumarium and Helianthus annuus. Using a combination of the leaf disc O2 electrode to measure the light responses of photosynthesis and 77 K fluorescence to monitor damage to the primary photochemistry, we have found the following: (a) The CO2 saturated rate of photosynthesis at high light is the most water stress sensitive parameter measured. (b) The apparent quantum yield (moles O2 per mole photons) was slightly, if at all, affected by mild water stress (>−1.5 megapascals). (c) Severe water stress (<−1.5 megapascals) reduced the quantum yield of photosynthesis regardless of whether the stress was applied in sun or shade. The light independent reduction of quantum yield was not associated with a reduction in 77 K fluorescence (Fv/Fm) indicating that the quantum yield reduction was not the result of damage to primary photochemistry. (d) The diel fluctuation in 77 K fluorescence seen in sun-exposed control leaves was greatly exaggerated in water stressed leaves because of enhanced decline in 77 K fluorescence in the morning. The rate of recovery was similar in both control and water stressed leaves. Shaded leaves showed no change in 77 K fluorescence regardless of whether water stress was imposed or not. (e) The water stress sensitive plants used in these experiments did not recover from acute water stress severe enough to reduce the quantum yield or chronic water stress which lasted long enough that light dependent damage to primary photochemistry occurred.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams W. W., Nishida K., Osmond C. B. Quantum Yields of CAM Plants Measured by Photosynthetic O(2) Exchange. Plant Physiol. 1986 May;81(1):297–300. doi: 10.1104/pp.81.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S., Bowen B. L. Inhibition of oxygen evolution in chloroplasts isolated from leaves with low water potentials. Plant Physiol. 1970 May;45(5):612–615. doi: 10.1104/pp.45.5.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S. Nonstomatal inhibition of photosynthesis in sunflower at low leaf water potentials and high light intensities. Plant Physiol. 1971 Nov;48(5):532–536. doi: 10.1104/pp.48.5.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. J., Evans P. J. OCULAR CHANGES ASSOCIATED WITH NAEVUS FLAMMEUS. Br J Ophthalmol. 1939 Feb;23(2):95–105. doi: 10.1136/bjo.23.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck R. W., Boyer J. S. Chloroplast Response to Low Leaf Water Potentials: III. Differing Inhibition of Electron Transport and Photophosphorylation. Plant Physiol. 1974 Mar;53(3):474–479. doi: 10.1104/pp.53.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M., Butler W. L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim Biophys Acta. 1975 Jan 31;376(1):105–115. doi: 10.1016/0005-2728(75)90209-1. [DOI] [PubMed] [Google Scholar]
- Mohanty P., Boyer J. S. Chloroplast Response to Low Leaf Water Potentials: IV. Quantum Yield Is Reduced. Plant Physiol. 1976 May;57(5):704–709. doi: 10.1104/pp.57.5.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp R. E., Boyer J. S. Photosynthesis at low water potentials in sunflower: lack of photoinhibitory effects. Plant Physiol. 1986 Sep;82(1):90–95. doi: 10.1104/pp.82.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis H. M., Boyer J. S., Govindjee Conformation and activity of chloroplast coupling factor exposed to low chemical potential of water in cells. Biochim Biophys Acta. 1979 Nov 8;548(2):328–340. doi: 10.1016/0005-2728(79)90139-7. [DOI] [PubMed] [Google Scholar]


