Abstract
AZT (zidovudine, 3′-azido-3′-deoxythymidine), although metabolized primarily to AZT-glucuronide, is also metabolized to 3′-amino-3′-deoxythmidine (AMT) by reduction of the azide to an amine. The formation of the myelotoxic metabolite AMT has not been well characterized, but inhibition of AMT formation would be of therapeutic benefit. The aim of this study was to identify compounds that inhibit AMT formation. Using human liver microsomes under anaerobic conditions and [2-14C]AZT, Km values of AZT azido-reductase, estimated by radio-thin-layer chromatography, were 2.2 to 3.5 mM (n = 3). Oxygen completely inhibited this NADPH-dependent reduction. Thirteen of the 28 compounds tested inhibited the formation of AMT. In addition to the CYP3A4 inhibitors ketoconazole, fluconazole, indinavir, ritonavir, and saquinavir, metyrapone strongly inhibited AMT formation. An unexpected finding was the more-than-twofold increase in AMT formation in the presence of ethacrynic acid, dipyridamole, or indomethacin. Such activation of toxic metabolite formation would impair drug therapy.
AZT (zidovudine, 3′-azido-3′-deoxythymidine) is widely used for the management of AIDS and AIDS-related complexes in patients infected with human immunodeficiency virus (HIV). In the human body, AZT is primarily metabolized to AZT-glucuronide (GAZT), which is excreted in the urine (4, 14). Both AZT and GAZT undergo reduction of the azido group to an amino group, forming 3′-amino-3′-deoxythymidine (AMT) and AMT-glucuronide, respectively (8). AMT has been identified as an AZT metabolite in studies with human liver microsomes, gut bacteria, and rat hepatocytes, as well as in plasma of HIV-infected patients and of rhesus monkeys (8, 9, 15, 38).
The use of AZT is limited by its hematological toxicity. Since AMT is five- to sevenfold more toxic than the parent drug, this azido-reductase product plays a significant role in AZT-induced bone marrow suppression (8). Despite these toxic effects, AZT is used widely and indicated for prevention of maternal-fetal transmission of AIDS, which accounts for more than 80% of pediatric cases. Furthermore, lamivudine and the HIV protease inhibitors saquinavir, indinavir, and ritonavir, indicated for initial therapy, are more effective when given in combination with AZT (2, 7, 30).
The formation of AMT is of toxicological importance. Azido-reduction of AZT to AMT by human liver microsomes appears to be a complex process and the involvement of NADPH–cytochrome P-450 reductase and cytochrome P-450 isoforms, in particular, CYP3A, CYP2A6, and CYP2B1, has been suggested in this azido-reduction process (10, 11, 13, 26). The azido-reduction of AZT in human liver microsomes has been evaluated under both aerobic and anaerobic conditions. Under nitrogen, but not under air, AMT was formed, according to Placidi et al. (26), Rajaonarison et al. (28), Cretton et al. (10, 11), and Pan-Zhou et al. (25). In contrast, Eagling et al. (13) reported the azido-reduction of AZT under air, although this reduction was moderately enhanced under anaerobic condition or by the addition of flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN). It was also shown that NADH was as effective as NADPH in the microsomal azido-reduction of AZT under anaerobic condition, implying the involvement of NADH-cytochrome b5 reductase (25).
Because of the high prevalence of opportunistic infections and malignancies with AIDS, AZT is frequently prescribed in combination with antimicrobial agents, antipyretics, cytostatics, and immunomodulating drugs. Initially, the focus of drug interactions was the glucuronidation pathway. Interference with AZT glucuronidation has been shown with several drugs in vitro in human liver microsomes (27, 33) and in clinical studies with rifampin, valproic acid, and fluconazole (5, 6, 24, 29).
However, knowledge of interference with the reductive system which converts AZT’s azido group to an amino group (AMT) is limited. In vitro, AMT formation was inhibited significantly by 0.1 mM ketoconazole, which is a selective inhibitor of CYP3A at low, submicromolar concentrations (13). Formation of AMT significantly increased in liver microsomes of rats pretreated with phenobarbital, dexamethasone, and clofibrate, inducers of CYP2B, CYP3A, and CYP4A, respectively. The formation of AMT may also be enhanced through inhibition of AZT glucuronidation. Furthermore, the half-life of AMT in plasma is longer than that of AZT, and accumulation of this toxic metabolite is possible (34). Any change in the conversion of AZT to AMT would impact cytotoxicity; any decrease in AZT toxicity in therapy will have clinical benefits.
The goal of the present study was to identify compounds that inhibit the enzyme which mediates AZT azido-reduction to AMT. Such an inhibitor could be appropriate for use as an adjunct drug in AZT therapy to diminish or eliminate toxicity. In pursuit of this objective, a radio-thin-layer chromatography (TLC) method was developed enabling detection and measurement of AZT and its metabolites in human liver microsomes.
To obtain clinically relevant data, we used human liver microsomes to monitor the activity and inhibition properties of AZT azido-reductase. We screened 28 compounds, including the substrate and inhibitors of NADPH-dependent reductases and cytochrome P-450. Some compounds activated the azido-reductase, resulting in as much as two-fold increase in AMT formation. The activation by these compounds was further investigated, and two possible mechanisms are discussed.
MATERIALS AND METHODS
Materials.
[2-14C]AZT (specific activity, 55 mCi/mmol) was purchased from Moravek Biochemical (Brea, Calif.). AZT, AMT, GAZT, NADPH, NADH, FAD, FMN, and all of the other drugs tested for their effects on AZT reduction were obtained from Sigma Chemical Co. (St. Louis, Mo.). HIV protease inhibitors were supplied by the following pharmaceutical manufacturers: saquinavir (Invirase), from Roche UK; indinavir (Crixivan), Merck, West Point, Pa.; ritonavir (Norvir), Abbott, Chicago, Ill. Silica gel TLC plates with a preadsorbent spotting area were from J. T. Baker, Phillipsburg, N.J. (20 by 20 glass plates with 19 channels; catalog no. 7010-04.
Human liver preparations.
Human liver samples (from kidney donors) in the University of Toronto Liver Bank, stored at −70°C, were partially thawed, homogenized 1:1 in 1.15% KCl, and centrifuged at 9,000 × g for 20 min, and the supernatant was centrifuged at 100,000 × g for 60 min (35). The microsomal pellet was washed and resuspended in phosphate buffer (pH 7.4) and stored at −70°C until used. Protein concentrations were determined by the bicinchoninic acid method (Pierce Chemical Co., Rockford, Ill.) with bovine serum albumin as the standard.
Incubation and radio-TLC conditions.
The 0.5-ml standard incubation mixtures (in duplicate) contained a constant amount of [2-14C]AZT (0.3 μCi) and various concentrations of unlabelled AZT (0.1 to 30 mM), 5 mM MgCl2, 5 mM NADPH, and 100 μl of microsomes (about 2.7 mg of protein) in 10 mM Tris-HCl buffer (pH 7.5). The assay was performed in conical glass tubes and started by the addition of microsomes, followed by incubation at 37°C under anaerobic conditions (by continuously bubbling the mixture with nitrogen) for 30 min. The reaction was terminated by the addition of 100 μl of methanol and centrifugation at 1,000 × g for 5 min to sediment the protein. Reaction mixture aliquots (60 μl) and unlabelled AZT and AMT were applied to the preadsorbent spotting area (loading zone) of a TLC plate, which was developed (for 30 to 40 min) in a solvent system consisting of chloroform-methanol-water-aqueous ammonia (80:20:2:0.2). AZT, AMT, and GAZT separated well, and the band shapes were uniform and well resolved with Rf values of 0.85, 0.2, and 0.08. The TLC bands were visualized under UV light, and the areas corresponding to AZT and AMT were scraped into scintillation vials. After addition of 0.5 ml of methanol and 9 ml of scintillation cocktail, the radioactivity was measured with a Beckman liquid scintillation counter. Control incubations were performed in the absence of either NADPH or microsomes. Enzyme activity was determined by expressing the radioactivity in the AMT region as a percentage of the radioactivity in the AMT and AZT bands combined.
Cofactor requirements were tested under anaerobic conditions, and the control had 5 mM NADPH as described above. The various cofactors studies were FAD, FMN, FMN plus FAD, and NADH with or without NADPH, all at 5 mM.
Kinetic parameters and interindividual variability.
The Km and Vmax values of AMT formation in microsomes of three human livers were determined by the least-squares method in Lineweaver-Burke double-reciprocal plots. Microsomes prepared from seven human liver samples were examined for AZT azido-reductase activity. The incubation and assay conditions were as described above, except that the AZT concentration was 0.5 mM and the microsomal protein content was about 2.7 mg per incubation.
Inhibition and activation studies.
Screening for inhibitors of AZT azido-reductase in human liver microsomes was conducted by using acetaminophen, bromovinyluracil, caffeine, chloramphenicol, chlorzoxazone, coumarin, cyclosporin A, dipyridamole, ethacrynic acid, fluconazole, 5-fluorouracil, indinavir, indomethacin, ketoconazole, mephenytoin, methylpyrazole, metyrapone, naproxen, α-naphthoflavone (αNF), nitrazepam, nitroimidazole, phenobarbital, rifampin, ritonavir, saquinavir, sulfaphenazole, testosterone, and warfarin. The incubation conditions and radio-TLC assay procedure used were as described above, except that the AZT concentration was 0.1 mM and the test drug concentration was 1 mM. Both were added in methanol, the solvent was removed under a stream of nitrogen, and the residue was dissolved in 20 μl of 70% ethanol containing [2-14C]AZT. This amount of ethanol did not affect the AZT reductase activity. The inhibition data were expressed as AMT formation in the inhibition mixture relative to AMT formation in the control incubation (without the inhibitor).
RESULTS
A radio-TLC method was developed for separation, identification, and measurement of AZT, AMT, and GAZT in human liver microsomes by using channelled silica gel TLC plates. The incubation mixture was applied directly to a preadsorbent spotting area, eliminating the extraction and derivatization steps commonly required for high-pressure liquid chromatography methods (39). The recovery of radioactivity from incubation and radio-TLC analysis was quantitative (93 to 100%).
Table 1 summarizes the effects of various cofactors on AMT formation under the anaerobic condition achieved by continuously gassing the medium with nitrogen during the incubation period. Formation of AMT was NADPH dependent, and under air, no product was formed. NADH also catalyzed the azido-reduction, but its addition did not increase the rate of the NADPH-dependent reduction. AMT formation was not enhanced by addition of FAD or riboflavin, while it was inhibited weakly by FMN. Although the aerobic condition was not actively pursued, the microsome with FAD plus FMN catalyzed AMT formation as efficiently as did the anaerobic reaction (data not shown). This reaction under air was NADPH dependent with all cofactors at 5 mM.
TABLE 1.
Cofactor requirements (anaerobic) of AZT azido-reductase activity in human liver microsomes
| Cofactor(s) | Meana % of control activity ± SE | P value |
|---|---|---|
| NADPH | 100 ± 2 | |
| NADH | 75 ± 8 | NSb |
| NADPH + NADH | 102 ± 3 | NS |
| NADPH plus: | ||
| Riboflavin | 109 ± 2 | NS |
| FAD | 92 ± 6 | NS |
| FMN | 71 ± 3 | <0.01 |
| FAD + FMN | 70 ± 14 | <0.01 |
| FAD + FMN | 14 ± 1 | <0.005 |
| NADPH + air | No product |
Means of triplicate experiments are shown. Concentrations: AZT substrate, 0.5 mM; cofactor and flavin, 5 mM.
NS, not significantly different from control (t test).
To further characterize AMT formation, the incubation was carried out under various pH conditions. The formation of AMT increased as the pH of the incubation medium increased from 7.4 to 8.4, while the activity remained unchanged at pH 6.4. The azido-reductase activity was twofold greater at the alkaline pH, at which the azido group is nonprotonated, than at physiological pH, at which it is largely protonated.
Three human liver microsomes, designated K14, K19, and K21, were used to determine the kinetic parameters Km and Vmax of AMT formation; the Km values were 2.2, 2.3, and 3.5 mM, and the Vmax values were 0.90, 0.66, and 0.65 nmol/min/mg of protein, respectively.
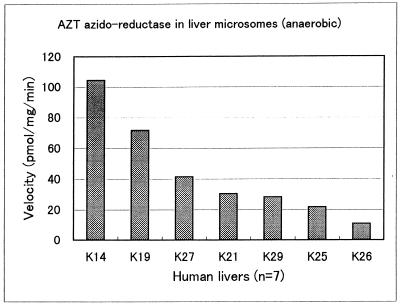
Figure 1 shows the interindividual variability of AMT formation in microsomal fractions from seven different human liver samples. The rate of AMT formation ranged from 9 to 110 pmol/min/mg of protein, showing 11-fold variability. Two livers, K14 and K19, were chosen for inhibition studies on the basis of their relatively high azido-reductase activity.
FIG. 1.
Interindividual variability of AZT azido-reductase activity in human liver microsomes (n = 7). Concentrations: AZT substrate, 0.5 mM; NADPH, 5 mM (anaerobic).
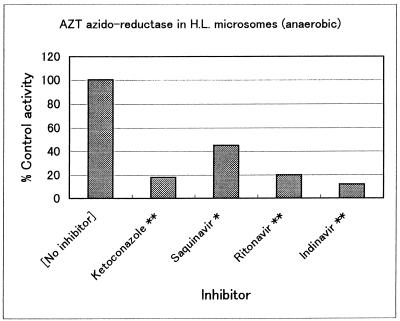
As shown in Table 2 and Fig. 2, AMT formation was inhibited to various degrees. An AZT concentration of 0.1 mM, notably lower than the Km, was selected and is in line with the literature (13, 26). The inhibitor concentration was 10-fold higher than the substrate concentration. About half of the 28 compounds tested exhibited no significant inhibition, while metyrapone, ketoconazole, and three HIV protease inhibitors, indinavir, ritonavir, and saquinavir, were potent inhibitors of AZT azido-reductase.
TABLE 2.
Inhibition of AZT azido-reductase activity (anaerobic) in human liver microsomes
| Inhibitor | % of control activitya | P value | Inhibitor for: | Subtrate for: |
|---|---|---|---|---|
| None | 100 | |||
| Acetaminophen | 129 | NSb | Glucuronidation | |
| Bromovinyluracil | 124 | NS | Dihydropyrimidine dehydrogenase | |
| Naproxen | 124 | NS | Steroid dehydrogenase | |
| Nitrazepam | 123 | NS | Nitroreductase | |
| Coumarin | 114 | NS | CYP2A6 | |
| Warfarin | 96 | NS | DT-diaphorase | |
| Mephenytoin | 87 | NS | CYP2C19 | |
| Phenobarbital | 87 | NS | Aldehyde reductase | |
| Chlorzoxazone | 86 | NS | CYP2E1 | |
| 5-Fluorouracil | 86 | NS | Dihydropyrimidine dehydrogenase | |
| Methylpyrazole | 85 | NS | Alcohol dehydrogenase | |
| Caffeine | 83 | NS | CYP1A2 | |
| Rifampin | 70 | <0.05 | Esterase | |
| Testosterone | 67 | <0.05 | CYP3A4 | |
| Sulfaphenazole | 63 | <0.05 | CYP2C9 | |
| Nitroimidazole | 62 | <0.05 | Nitroreductase | |
| α-NF | 60 | <0.05 | CYP1A1/2 | |
| Cyclosporin A | 60 | <0.05 | CYP3A4 | |
| Chloramphenicol | 52 | <0.01 | Nitroreductase | |
| Fluconazole | 33 | <0.01 | CYP3A4 | |
| Metyrapone | 17 | <0.001 | Carbonyl reductase |
Means of triplicate experiments are shown. Standard deviations were less than 9% of the means. Substrate concentration: 0.1 mM AZT. Inhibitor concentration: 1 mM.
NS, not significantly different from control (t test).
FIG. 2.
Effects of three HIV protease inhibitors and ketoconazole on AMT formation in human liver (H.L.) microsomes. Concentrations: AZT substrate, 0.1 mM; NADPH, 5 mM (anaerobic); inhibitor, 1.0 mM. *, P < 0.01; **, P < 0.001.
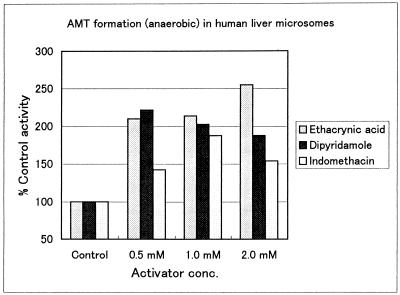
Activation of AMT formation was observed for three compounds, as shown in Fig. 3. At concentrations of 0.5 to 2.0 mM, ethacrynic acid and dipyridamole enhanced the azido-reduction by 80 to 160% (P < 0.001) and indomethacin did so by 40 to 80% (P < 0.01). In the presence of 2 mM ethacrynic acid, the Km of the azido-reductase decreased from 2.6 to 0.74 mM (P < 0.01) and the catalytic efficiency (Vmax/Km) increased by about threefold. Indomethacin at 2 mM influenced the kinetic constants of the azido-reductase to a lesser extent than did ethacrynic acid and increased the catalytic efficiency by 44%; the Km changed insignificantly, from 2.6 to 1.9 mM.
FIG. 3.
Activation of AZT azido-reductase by ethacrynic acid (P < 0.001), dipyridamole (P < 0.001), and indomethacin (P < 0.01) in human liver microsomes. Concentrations: AZT substrate, 0.1 mM; NADPH, 5 mM (anaerobic).
DISCUSSION
To find an effective inhibitor of AZT azido-reductase in human liver microsomes, 28 compounds, including substrates and inhibitors of NADPH-dependent reductases and P-450 enzymes, were screened (Table 2 and Fig. 2 and 3). We observed their impact, inhibition or activation, on AMT formation.
To establish the optimal incubation condition for maximal formation of AMT by human liver microsomes, various experiments were performed to establish conditions for linear reaction with regard to cofactor requirements, protein, pH, and time. To address conflicting reports in the literature regarding the oxygen sensitivity of the AZT azido-reductase, both aerobic and anaerobic conditions were initially used. When we carried out incubation under air in the presence of NADPH in human liver microsomes, no conversion of AZT to AMT was detected. Thereafter, the azido-reduction of AZT to AMT in human liver microsomes was evaluated only under the anaerobic condition. The sensitivity of azido-reductase to oxygen may be explained as follows (20). Electrochemical-reduction studies have shown that AZT azido-reduction is a two-electron process with removal of two nitrogen atoms and formation of a reactive nitrene intermediate which reacts with oxygen, yielding a molecule of superoxide anion radical, which, in turn, interacts with the nitrene intermediate, hence inhibiting the reaction.
Thirteen compounds significantly inhibited the formation of AMT to various degrees, from 30 to 88% (Table 2 and Fig. 2). The most marked inhibitory effects were observed with metyrapone, ketoconazole, and three HIV protease inhibitors, indinavir, ritonavir, and saquinavir (Fig. 2). Ketoconazole was reported as an inhibitor previously (13), and the data are comparable. Metyrapone inhibits many P-450-catalyzed reactions (12, 22). It is interesting that this compound, also a known inhibitor of carbonyl reductase (18), so potently inhibited, by 83%, AMT formation.
AZT is often coadministered with HIV protease inhibitors. The present in vitro results support the combination from a kinetic viewpoint, since the formation of toxic AMT may be reduced in vivo. Pharmacokinetic parameters in vivo, such as plasma half-life and clearance, can be calculated only when drug concentrations are markedly low in relation to the Km or saturable concentration of the enzyme (2). The AZT concentration used in this interaction study, 0.1 mM, is more than 20-fold lower than the Km value. The metabolic pharmacokinetic interaction study might show lower AMT levels after concurrent administration of indinavir or ritonavir.
The list of compounds in Table 2 includes those compounds that did not inhibit NADPH-dependent enzymes. Phenobarbital, methylpyrazole, and warfarin had no significant effect on AZT azido-reductase, even at a concentration 10 times that of AZT, indicating that their respective enzyme systems, aldehyde reductase, aldehyde dehydrogenase, and DT-diaphorase, do not contribute to the azido-reduction of AZT. Furthermore, dihyropyrimidine dehydrogenase, which metabolizes the uracil moiety, is not involved in AMT formation.
The anaerobic condition is relevant in hepatocytes, since portal vein blood (80% of the hepatic blood supply) has low oxygen tension and some hepatocytes, tolerant to a hypoxic state, within tissue receive very little oxygen.
Unexpectedly, a number of drugs enhanced the formation of AMT. The most potent activators were ethacrynic acid (a carbonyl reductase inhibitor), indomethacin (a steroid dehydrogenase inhibitor), and dipyridamole (a glucuronyl transferase substrate), all of which increased AMT formation by about twofold.
Nonsteroidal anti-inflammatory drugs are often used for relief of nonspecific fever and musculoskeletal pain in HIV patients. Indomethacin and naproxen inhibited GAZT formation in vivo and in human liver microsomes (1, 33). In our in vitro study, indomethacin activated the azido-reduction of AZT by 40 to 80%. Dipyridamole enhanced AMT formation by 80 to 120%, while this compound in vitro potentiated the antiretroviral activity of AZT (3).
Enzyme activation in vitro is well known for P-450 isoforms, in particular, CYP3A. Huang et al. (16) reported the activation of benzo[a]pyrene hydroxylation by αNF to be CYP isoform and substrate selective. Furthermore, αNF activated the acetaminophen-reactive metabolite formation in rat liver microsomes and losartan oxidation in human liver microsomes (23, 37). Recently, Irshaid et al. (19) showed that αNF, progesterone, testosterone, amiodarone, and lithocholic acid elevated the formation of an unidentified metabolite of dapsone in human liver microsomes. The other activators of CYP isoforms include caffeine, metyrapone, and testosterone (21, 23, 36). However, all of these activators of CYP3A inhibited AMT formation.
Several mechanisms have been suggested for the in vitro activation of CYP isoforms. Huang et al. (17) proposed that the activation by αNF could be explained, in part, by promotion of electron transfer from cytochrome P-450 reductase to P-450. Schwab et al. (31) suggested that flavones are allosteric effectors which can increase catalytic efficiency (Vmax/Km) by lowering the Km for the liver CYP3A enzyme and increasing Vmax. To better characterize the activation of AZT azido-reductase activity by ethacrynic acid and indomethacin, kinetic analyses were carried out to determine the change in the Km and Vmax of AZT azido-reductase. Our investigation indicates that activation by these compounds proceeds by two different mechanisms.
In the presence of ethacrynic acid, the Km of the azido-reductase decreased significantly, from 2.6 to 0.74 mM, and the catalytic efficiency (Vmax/Km) increased by about threefold. Ethacrynic acid decreased the Km of AZT azido-reductase, suggesting an allosteric mechanism for the activation. For activation of the CYP3A enzyme, Shou et al. (32) demonstrated that a substrate can bind to the active site of the enzyme and influence the orientation of another substrate, thereby changing the regioselectivity of the metabolism. Indomethacin influenced the kinetic constants of the azido-reductase to a lesser extent than did ethacrynic acid and increased the catalytic efficiency by 44%; the Km changed insignificantly, from 2.6 to 1.9 mM. With indomethacin, the electron transfer may be accelerated.
Complexities of AIDS and its associated infections necessitate the coadministration of several chemotherapeutic agents, and drug interaction is inevitable. The combination therapy of protease inhibitors with AZT may, in addition to increasing antiviral activity, have the beneficial effect of inhibiting AMT formation. On the other hand, any interaction which favors the azido-reduction of AZT to AMT could increase its hematological toxicity. It has been observed in clinical studies, as well as in vitro, that AZT glucuronidation can be inhibited, potentially shunting more AZT to the azido-reduction pathway. Our findings provide the first kinetic evidence that the AZT azido-reductase in the human liver can be activated by several drugs. It is not known if these activators can influence the in vivo azido-reduction of AZT. If it occurred, significantly elevated levels of AMT would result. Although in vitro data have limitations and application to a clinical situation is often very difficult, our results will be useful in designing a clinical study.
Complementary clinical studies are needed to determine whether persons exposed to activators of the azido-reductase pathway or inhibitors of AZT glucuronidation will be more susceptible to the hematological toxicity of AZT therapy.
In summary, of the 28 compounds tested for inhibition of AMT formation in human liver microsomes, 13 compounds showed significant inhibition and the most potent of these are HIV protease inhibitors.
ACKNOWLEDGMENTS
We thank Nancy Fischer and David Stewart for discussion and manuscript preparation.
We thank the Medical Research Council of Canada and Dainippon Pharmaceutical for financial support.
REFERENCES
- 1.Barry M, Howe J, Back D, Breckenridge A, Brettle R, Mitchell R, Beeching N J, Nye F J. The effects of indomethacin and naproxen on zidovudine pharmacokinetics. Br J Clin Pharmacol. 1993;36:82–85. doi: 10.1111/j.1365-2125.1993.tb05898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertz R J, Granneman G R. Use of in vitro and in vivo data to estimate the likelihood of metabolic pharmacokinetic interactions. Clin Pharmacokinet. 1997;32:210–258. doi: 10.2165/00003088-199732030-00004. [DOI] [PubMed] [Google Scholar]
- 3.Betageri G V, Szebeni J, Hung K, Patel S S, Wahl L M, Corcoran M, Weinstein J N. Effect of dipyridamole on transport and phosphorylation of thymidine and AZT in human monocyte/macrophages. Biochem Pharmacol. 1990;40:867–870. doi: 10.1016/0006-2952(90)90328-i. [DOI] [PubMed] [Google Scholar]
- 4.Blum, M. R., S. H. T. Liao, S. S. Good, and P. DeMiranda. 1988. Pharmacokinetics and bioavailability of zidovudine in humans. Am. J. Med. 85(Suppl. 2A):189–194. [PubMed]
- 5.Burger D M, Meenhorst P L, Koks C H W, Beijnen J H. Drug interactions with zidovudine. AIDS. 1993;7:445–460. doi: 10.1097/00002030-199304000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Burger D M, Meenhorst P L, Koks C H W, Beijnen J H. Pharmacokinetic interaction between rifampin and zidovudine. Antimicrob Agents Chemother. 1993;37:1426–1431. doi: 10.1128/aac.37.7.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collier A C, Coombs R W, Schoenfeld D A, Bassett R L, Timpone J, Baruch A, Jones M, Facey K, Whitacre C, McAuliffe V J, Friedman H M, Merigan T C, Reichman R C, Hooper C, Corey L. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine, and zalcitabine. AIDS Clinical Trials Group. N Engl J Med. 1996;334:1011–1017. doi: 10.1056/NEJM199604183341602. [DOI] [PubMed] [Google Scholar]
- 8.Cretton E M, Xie M Y, Bevan R J, Schinazi R F, Sommadossi J P. Catabolism of AZT in hepatocytes and liver microsomes with evidence of formation of 3′-amino-3′-deoxythymidine, a highly toxic catabolite for human bone marrow cells. Mol Pharmacol. 1991;39:258–266. [PubMed] [Google Scholar]
- 9.Cretton E M, Schinazi R F, McClure H M, Anderson D C, Sommadossi J P. Pharmacokinetics of AZT and its catabolites and interactions with probenecid in rhesus monkeys. Antimicrob Agents Chemother. 1991;35:801–807. doi: 10.1128/aac.35.5.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cretton E M, Pan-Zhou X R, Maurel P, Sommadossi J P. Reduction of AZT in human liver microsomes. Clin Pharmacol Ther. 1994;53:138. doi: 10.1038/clpt.1993.128. . (Abstract.) [DOI] [PubMed] [Google Scholar]
- 11.Cretton E M, Placidi L, Sommadossi J P. Conversion of AZT to its toxic metabolite AMT is mediated by cytochrome P450 and NADPH-cytochrome C reductase in liver microsomes. Clin Pharmacol Ther. 1994;53:189. . (Abstract.) [Google Scholar]
- 12.Denner K, Vogel R, Schmalix W, Doehmer J, Bernhardt R. Cloning and stable expression of the human mitochondrial cytochrome P450IIB1 cDNA in V79 Chinese hamster cells and their application for testing of potential inhibitors. Pharmacogenetics. 1995;5:89–96. doi: 10.1097/00008571-199504000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Eagling V A, Howe J L, Barry M J, Back D J. The metabolism of zidovudine by human liver microsomes in vitro: formation of 3′-amino-3′-deoxythymidine. Biochem Pharmacol. 1994;48:267–276. doi: 10.1016/0006-2952(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 14.Good S S, Koble S C, Crouch R, Rideout J L, de Miranda J P. Isolation and characterization of an ether glucuronide of zidovudine, a major metabolite in monkeys and humans. Drug Metab Dispos. 1990;18:231–236. [PubMed] [Google Scholar]
- 15.Howe J L, Back D J, Colbert J. Extrahepatic metabolism of zidovudine. Br J Clin Pharmacol. 1992;33:190–192. doi: 10.1111/j.1365-2125.1992.tb04024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang M T, Johnson E F, Muller-Eberhard U, Koop D R, Coon M J, Conney A H. Specificity in the activation and inhibition by flavonoids of benzo[α]pyrene hydroxylation by cytochrome P-450 isozymes from rabbit liver microsomes. J Biol Chem. 1981;256:10897–10901. [PubMed] [Google Scholar]
- 17.Huang M T, Chang R L, Fortner J G, Conney A H. Studies on the mechanism of activation of microsomal benzo[a]pyrene hydroxylation by flavonoids. J Biol Chem. 1981;256:6829–6836. [PubMed] [Google Scholar]
- 18.Inaba T, Kovacs J. Haloperidol reductase in human and guinea pig livers. Drug Metab Dispos. 1989;17:330–333. [PubMed] [Google Scholar]
- 19.Irshaid Y, Branch R A, Adedoyin A. Metabolic interactions of putative cytochrome P450 3A substrates with alternative pathways of dapsone metabolism in human liver microsomes. Drug Metab Dispos. 1995;24:164–167. [PubMed] [Google Scholar]
- 20.Kawczynski W, Czochralska B, Shugar D. Electrochemical reduction products of azido nucleosides, including zidovudine (AZT): mechanisms and relevance to their intracellular metabolism. Acta Biochim Pol. 1993;40:213–223. [PubMed] [Google Scholar]
- 21.Kerlan V, Dreano Y, Beaune P H, Floch H H, Berthou F. Nature of cytochromes P450 involved in the 2-/4-hydroxylations of estradiol in human liver microsomes. Biochem Pharmacol. 1992;44:1745–1756. doi: 10.1016/0006-2952(92)90068-t. [DOI] [PubMed] [Google Scholar]
- 22.Laaksonen M, Kaliste-Korhonen E, Karenlampi S, Hanninen O. P450 enzyme CYP2B catalyzes the detoxification of diisopropyl fluorophosphate. Chem-Biol Interact. 1995;94:197–213. doi: 10.1016/0009-2797(94)03334-5. [DOI] [PubMed] [Google Scholar]
- 23.Lee C A, Thummel K E, Nelson S D, Slattery J T. Inhibition and activation of acetaminophen reactive metabolite formation by caffeine: role of cytochromes P-450 1A1 and 3A2. Drug Metab Dispos. 1991;19:348–354. [PubMed] [Google Scholar]
- 24.Lertora J J L, Rege A B, Greenspan D L, Akula S, George W J, Hyslop N E, Agrawal K. Pharmacokinetic interaction between zidovudine and valproic acid in patients infected with human immunodeficiency virus. Clin Pharmacol Ther. 1994;56:272–278. doi: 10.1038/clpt.1994.137. [DOI] [PubMed] [Google Scholar]
- 25.Pan-Zhou X R, Cretton-Scott E, Zhou X J, Yang M X, Lasker J M, Sommadossi J P. Human liver microsomal cytochrome P450 and b5 act as reductases in the reductive metabolism of 3′-azido-3′-deoxythymidine to 3′-amino-3′-deoxythymidine. Clin Pharmacol Ther. 1996;59:168. . (Abstract.) [Google Scholar]
- 26.Placidi L, Cretton E M, Placidi M, Sommadossi J P. Reduction of AZT to 3′-amino-3′-deoxythymidine in human liver microsomes and its relationship to cytochrome P450. Clin Pharmacol Ther. 1993;54:168–176. doi: 10.1038/clpt.1993.128. [DOI] [PubMed] [Google Scholar]
- 27.Rajaonarison J F, Lacarelle B, Catalin J, Placidi M, Rahmani R. 3′-azido-3′-deoxythymidine drug interactions: screening for inhibitors in human liver microsomes. Drug Metab Dispos. 1992;20:578–584. [PubMed] [Google Scholar]
- 28.Rajaonarison J F, Lacarelle B, Durand A, Catalin J. In vitro metabolism of zidovudine in man. Therapie. 1993;48:341–343. [PubMed] [Google Scholar]
- 29.Sahai J, Gallicano K, Cameron D W. Effect of fluconazole on zidovudine pharmacokinetics in patients infected with human immunodeficiency virus. J Infect Dis. 1994;169:1103–1107. doi: 10.1093/infdis/169.5.1103. [DOI] [PubMed] [Google Scholar]
- 30.Sahai, J. 1996. Risks and synergies from drug interactions. AIDS 10(Suppl. 1):S21–S25. [PubMed]
- 31.Schwab G E, Raucy J L, Johnson E F. Modulation of rabbit and human hepatic cytochrome P-450-catalyzed steroid hydroxylations by α-naphthoflavone. Mol Pharmacol. 1988;33:493–499. [PubMed] [Google Scholar]
- 32.Shou M, Grogan J, Mancewicz J A, Krausz K W, Gonzalez F J, Gelboin H V, Korzekwa K R. Activation of CYP3A4: evidence for the simultaneous binding of two substrates in a cytochrome P450 active site. Biochemistry. 1995;33:6450–6455. doi: 10.1021/bi00187a009. [DOI] [PubMed] [Google Scholar]
- 33.Sim S M, Back D J, Breckenridge A M. The effect of various drugs on the glucuronidation of zidovudine by human liver microsomes. Br J Clin Pharmacol. 1991;32:17–21. doi: 10.1111/j.1365-2125.1991.tb05607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stagg M P, Cretton E M, Kidd L, Diasio R B, Sommadossi J P. Clinical pharmacokinetics of zidovudine and catabolites with formation of a toxic catabolite, 3′-amino-3′-deoxythymidine. Clin Pharmacol Ther. 1992;51:668–676. doi: 10.1038/clpt.1992.79. [DOI] [PubMed] [Google Scholar]
- 35.Tyndale R F, Inaba T, Gonzalez F J, Hardwick J P, Kalow W. Sparteine metabolism capacity in human liver: structural variants of human P450IID1 as assessed by immunochemistry. Pharmacol Toxicol. 1990;67:14–18. doi: 10.1111/j.1600-0773.1990.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 36.Waxman D J, Walsh C. Cytochrome P-450 isozyme 1 from phenobarbital-induced rat liver: purification, characterization, and interactions with metyrapone and cytochrome b5. Biochemistry. 1983;22:4846–4855. doi: 10.1021/bi00289a035. [DOI] [PubMed] [Google Scholar]
- 37.Yun C H, Lee H S, Rho J K, Jeong H G, Guengerich F P. Oxidations of the angiotensin receptor antagonist losartan in human liver microsomes, role of cytochrome P450 3A(4) in formation of the active metabolite EXP3174. Drug Metab Dispos. 1995;23:285–289. [PubMed] [Google Scholar]
- 38.Zhou X J, Sommadossi J P. Comparative pharmacokinetics of zidovudine and its toxic catabolite 3′-amino-3′-deoxythymidine in HIV-infected patients. AIDS Res Hum Retroviruses. 1996;12:229–233. doi: 10.1089/aid.1996.12.229. [DOI] [PubMed] [Google Scholar]
- 39.Zhou X J, Sommadossi J P. Quantitation of 3′-amino-3′-deoxythymidine, a toxic catabolite of 3′-azido-3′-deoxythymidine (zidovudine), in human plasma by HPLC using precolumn derivatization with fluorescamine and fluorescence detection. J Chromatogr B. 1994;656:389–396. doi: 10.1016/0378-4347(94)00118-9. [DOI] [PubMed] [Google Scholar]