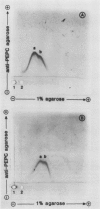
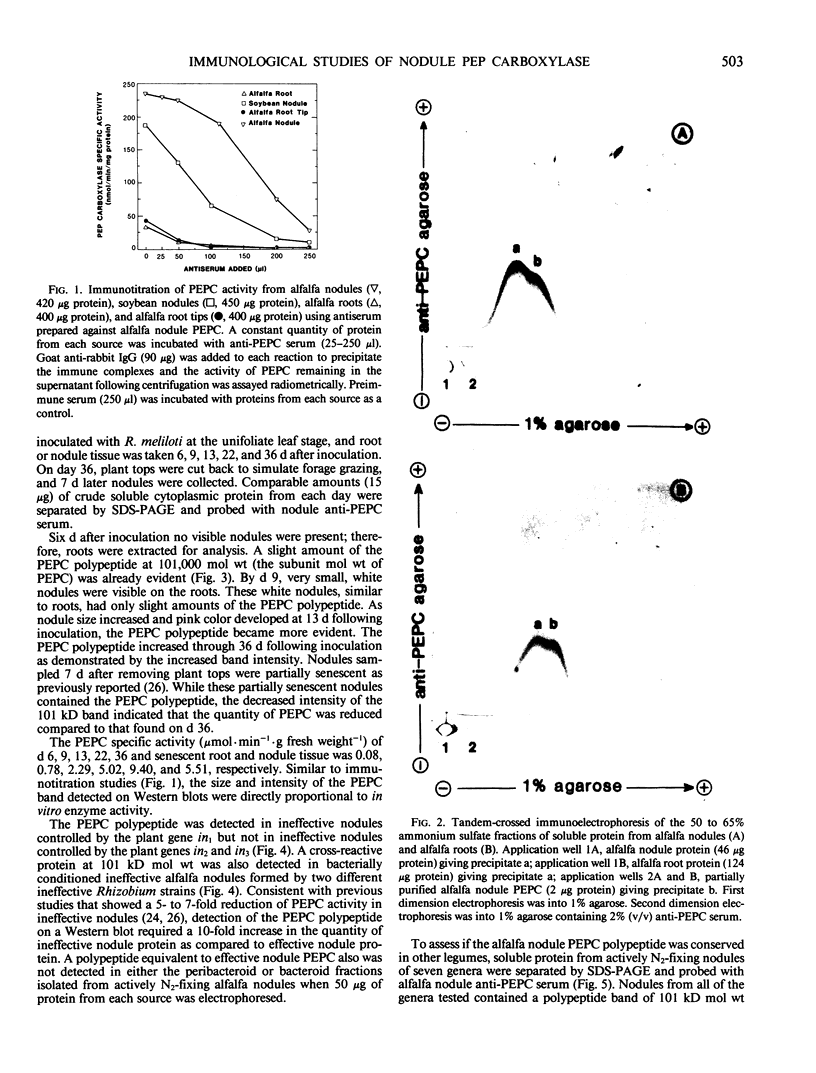
Abstract
Antiserum was prepared in rabbits against purified alfalfa (Medicago sativa L.) nodule phosphoenolpyruvate carboxylase (PEPC). Immunotitration assays revealed that the antiserum recognized the enzyme from alfalfa nodules, uninoculated alfalfa roots, and from soybean nodules. Tandem-crossed immunoelectrophoresis showed that the PEPC protein from alfalfa roots and nodules was immunologically indistinguishable. The 101 kilodalton polypeptide subunit of alfalfa nodule PEPC was identified on Western blots. The PEPC polypeptide was detected in low quantities in young alfalfa roots and nodules but was present at increased levels in mature nodules. Senescent nodules appeared to contain a reduced amount of the PEPC polypeptide. PEPC was also detected by western blot in some plant- and bacterially-conditioned ineffective alfalfa nodules but was not detected in bacteroids isolated from effective nodules. Alfalfa nodule PEPC is constitutively expressed in low levels in roots. In nodules, expression of PEPC polypeptide increases several-fold, resulting in increased PEPC activity. Antiserum prepared against the C4 PEPC from maize leaves recognized the PEPC enzyme in all legume nodules and roots tested, while the antiserum prepared against alfalfa nodule PEPC also recognized the leaf PEPC of several C4 plant species. Neither antiserum reacted strongly with any C3 leaf proteins. The molecular weight of the PEPC polypeptide from C4 leaves and legume nodules appears to be similar.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergmann H., Preddie E., Verma D. P. Nodulin-35: a subunit of specific uricase (uricase II) induced and localized in the uninfected cells of soybean nodules. EMBO J. 1983;2(12):2333–2339. doi: 10.1002/j.1460-2075.1983.tb01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisseling T., Been C., Klugkist J., Kammen A., Nadler K. Nodule-specific host proteins in effective and ineffective root nodules of Pisum sativum. EMBO J. 1983;2(6):961–966. doi: 10.1002/j.1460-2075.1983.tb01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groat R. G., Vance C. P. Root Nodule Enzymes of Ammonia Assimilation in Alfalfa (Medicago sativa L.) : DEVELOPMENTAL PATTERNS AND RESPONSE TO APPLIED NITROGEN. Plant Physiol. 1981 Jun;67(6):1198–1203. doi: 10.1104/pp.67.6.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groat R. G., Vance C. P. Root and Nodule Enzymes of Ammonia Assimilation in Two Plant-Conditioned Symbiotically Ineffective Genotypes of Alfalfa (Medicago sativa L.). Plant Physiol. 1982 Mar;69(3):614–618. doi: 10.1104/pp.69.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock K., Tsang V. C. India ink staining of proteins on nitrocellulose paper. Anal Biochem. 1983 Aug;133(1):157–162. doi: 10.1016/0003-2697(83)90237-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lang-Unnasch N., Ausubel F. M. Nodule-specific polypeptides from effective alfalfa root nodules and from ineffective nodules lacking nitrogenase. Plant Physiol. 1985 Apr;77(4):833–839. doi: 10.1104/pp.77.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legocki R. P., Verma D. P. Identification of "nodule-specific" host proteins (nodoulins) involved in the development of rhizobium-legume symbiosis. Cell. 1980 May;20(1):153–163. doi: 10.1016/0092-8674(80)90243-3. [DOI] [PubMed] [Google Scholar]
- Nutman P. S. Genetics of symbiosis and nitrogen fixation in legumes. Proc R Soc Lond B Biol Sci. 1969 Apr 1;172(1029):417–437. doi: 10.1098/rspb.1969.0030. [DOI] [PubMed] [Google Scholar]
- Peterson J. B., Evans H. J. Phosphoenolpyruvate carboxylase from soybean nodule cytosol. Evidence for isoenzymes and kinetics of the most active component. Biochim Biophys Acta. 1979 Apr 12;567(2):445–452. doi: 10.1016/0005-2744(79)90130-x. [DOI] [PubMed] [Google Scholar]
- Ting I. P., Osmond C. B. Multiple forms of plant phosphoenolpyruvate carboxylase associated with different metabolic pathways. Plant Physiol. 1973 Mar;51(3):448–453. doi: 10.1104/pp.51.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C. P., Boylan K. L., Maxwell C. A., Heichel G. H., Hardman L. L. Transport and Partitioning of CO(2) Fixed by Root Nodules of Ureide and Amide Producing Legumes. Plant Physiol. 1985 Aug;78(4):774–778. doi: 10.1104/pp.78.4.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C. P. Rhizobium infection and nodulation: a beneficial plant disease? Annu Rev Microbiol. 1983;37:399–424. doi: 10.1146/annurev.mi.37.100183.002151. [DOI] [PubMed] [Google Scholar]
- Vance C. P., Stade S. Alfalfa Root Nodule Carbon Dioxide Fixation : II. Partial Purification and Characterization of Root Nodule Phosphoenolpyruvate Carboxylase. Plant Physiol. 1984 May;75(1):261–264. doi: 10.1104/pp.75.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C. P., Stade S., Maxwell C. A. Alfalfa root nodule carbon dioxide fixation : I. Association with nitrogen fixation and incorporation into amino acids. Plant Physiol. 1983 Jun;72(2):469–473. doi: 10.1104/pp.72.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]