Abstract
CD19-targeted chimeric receptor antigen (CAR)-T cell therapy has shown remarkable clinical efficacy in the treatment of relapsed or refractory (R/R) B-cell malignancies. However, 30%–60% of patients eventually relapsed, with the CD19-negative relapse being an important hurdle to sustained remission. CD22 expression is independent of CD19 expression in malignant B cells. Consequently, CD22 is a potential alternative target for CD19 CAR-T cell-resistant patients. CD22-targeted therapies, mainly including the antibody–drug conjugates (ADCs) and CAR-T cells, have come into wide clinical use with acceptable toxicities and promising efficacy. In this review, we explore the molecular and physiological characteristics of CD22, development of CD22 ADCs and CAR-T cells, and the available clinical data on CD22 ADCs and CAR-T cell therapies. Furthermore, we propose some perspectives for overcoming tumor escape and enhancing the efficacy of CD22-targeted therapies.
Keywords: CD22, CD22 CAR-T cell therapy, CD22 antibody–drug conjugate, Dual-targeting CAR-T cell, Combination therapies
Background
Chimeric antigen receptor (CAR)-T cell therapy has attracted much attention as a cellular immunotherapy. Although CD19 CAR-T cell therapy has achieved promising efficacy in clinical settings [1–5], 30%–60% of patients eventually relapsed with a poor prognosis [6–14]. One mechanism of relapse is the downregulation or loss of CD19 on the tumor cell surface [15–17]. In B-cell acute lymphoblastic leukemia (B-ALL), CD19-negative relapse accounts for up to 83% of relapse cases [6–11, 18]. In B-cell non-Hodgkin lymphoma (NHL), antigen loss also occurs in one–third of patients experiencing treatment failure after CD19 CAR-T cell therapy [15, 19].
CD22 is restrictively expressed in both normal and malignant B cells, which makes it a potential alternative target to CD19 [20, 21]. CD22 has been identified in the blasts of > 90% of B-ALL cases [22, 23]. Studies on the applicability of targeting CD22 have mainly focused on antibody–drug conjugate (ADC) and CAR-T cell therapy. Two CD22 ADCs, inotuzumab ozogamicin and moxetumomab pasudotox-tdfk, have been approved by the Food and Drug Administration for the treatment of relapsed or refractory (R/R) B-ALL and hairy-cell leukemia, respectively. While inotuzumab ozogamicin is increasingly used in therapeutic settings, moxetumomab pasudotox-tdfk was withdrawn from the market due to the low clinical uptake and complexity of the drug use. CD22 CAR-T cell therapies prove to be effective in treating R/R B-ALL [20, 21, 24–31]. However, treatment failure is inevitable in CD22-targeted immunotherapies, further narrowing down the avaliable treatment options. Comprehensive knowledge of CD22 molecule will help us to understand the mechanisms of resistance and propose corresponding strategies. This review focuses on the development, the available clinical data and the strategies to improve the efficacy of CD22-targeted therapies, with the aim of providing a perspective on future directions in CD22-targeted immunotherapies.
CD22 structure, function, and expression
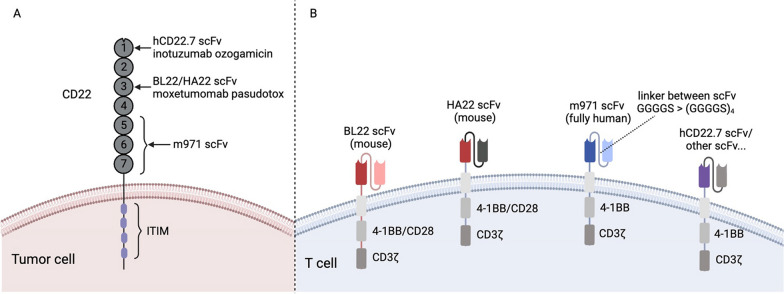
CD22, also known as sialic acid-binding Ig-like lectin 2, belongs to the siglec family and immunoglobulin superfamily. It consists of seven extracellular IgG-like domains and a 141-amino acid-long cytoplasmic tail [32, 33] (Fig. 1A). CD22 can bind to α 2,6-linked sialic acid residues of surface molecules (such as CD22 itself, CD45 and IgM) on B cells in a “cis” configuration. It can also bind to ligands on other cells as an adhesion molecule in a “trans” configuration [34, 35]. Cis-ligation negatively tunes B-cell receptor signaling mainly in a SH2 domain-containing tyrosine phosphatases 1-dependent manner. Trans-ligation modulates the migration and B-cell receptor signaling threshold of B cells [33, 34]. CD22 is restrictively expressed in the B-cell lineage, particularly in malignant B cells [23, 34, 36–40]. As an endocytic receptor, ligation of CD22 with its ligands triggers rapid internalization, which enables the application of CD22 ADCs [41].
Fig. 1.
CD22 and CD22 CAR structure. A CD22 molecule and the recognized domain of different CD22 scFv and ADCs. B CD22 CAR structures in preclinical and clinical use. ITIM immunoreceptor tyrosine-based inhibitory motif. The image was created using BioRender (Biorender, Toronto, ON, Canada)
CD22 ADC structures and preclinical results
ADC is composed of an antibody, a chemical linker, and a covalently attached cytotoxic agent (i.e., the payload). The ADC undergoes endocytosis after the antibody binds to the specific antigen on the tumor cell surface, and then releases the payload from the lysosomes. Choosing an optimal antibody is the first step in developing CD22 ADCs. The payload also plays a pivotal role in triggering the crosslinking or breakage of DNA, or inhibiting the tubulin activity, thus leading to cell cycle arrest and tumor cell apoptosis. The linker determines the stability of ADCs and also controls the release of payloads; it is designed to prevent unnecessary clustering of ADCs, which impairs the anti-tumor activity [42, 43]. The structures of CD22 ADCs/recombinant immunotoxins are summarized in Table 1.
Table 1.
CD22 ADC/recombinant immunotoxin structures
| ADC/recombinant immunotoxin | Anti-CD22 antibody | Cytotoxic payload | Linker | Disease |
|---|---|---|---|---|
| BL22 | RFB4 dsFv | Pseudomonas exotoxin A (PE38) | mc-VC-PABC (enzyme cleavable) | R/R HCL |
| Moxetumomab pasudotox/HA22 | RFB4 dsFv (SSY-THW) | Pseudomonas exotoxin A (PE38) | mc-VC-PABC (enzyme cleavable) | R/R B-cell HCL |
| Pinatuzumab vedotin | Hu10F4 antibody | Monomethyl auristatin E | mc-VC-PABC (enzyme cleavable) | R/R B-cell NHL |
| Anti-CD22-NMS249 | Hu10F4 antibody | PNU-159682 | mc-VC-PABC (enzyme cleavable) | R/R B-cell NHL |
| Anti-CD22-(LC:K149C)-SN36248 | Hu10F4 antibody | SN36248 × 2 | maleimide linker (uncleavable) | B-cell NHL |
| Inotuzumab ozogamicin | G544 antibody | Calicheamicin (Calich-DMH) | hydrazone (acid-labile linker) | R/R B-ALL |
dsFv disulfide-stabilized Fv fragment, R/R relapsed or refractory, HCL hairy cell leukemia, NHL non-Hodgkin lymphoma, ALL acute lymphoplastic leukemia
BL22 contains a disulfide-stabilized anti-CD22 fragment variable derived from a murine RFB4 antibody and a 38 kDa truncated form of pseudomonas exotoxin A [44]. HA22 is a refined form of BL22 that shows a higher affinity to CD22. In HA22, SSY residues in the hot spot region of complementarity-determining region 3 of heavy chain of BL22 were mutated to THW residues [45, 46]. HA22 showed improved in vivo and in vitro anti-tumor activity compared to BL22 [45]. Unlike moxetumomab pasudotox deriving from HA22, inotuzumab ozogamicin comprises the humanized anti-CD22 monoclonal IgG4 antibody G544 and a chemically linked DNA-damaging payload Calich-DMH. It recognizes the IgG-like domain 1 of CD22 and exerts a potent cytotoxic effect on tumor cells, leading to an obvious tumor mass regression in two lymphoma-xenograft bearing models [47, 48]. Pinatuzumab vedotin incorporates the humanized anti-CD22 monoclonal IgG1 antibody Hu10F4 and a microtubule inhibitor monomethyl auristatin E. Pinatuzumab vedotin demonstrated better anti-tumor activity than the standard rituximab plus CHOP regimen in a xenograft model bearing Ramos cells and also showed anti-lymphoma effects in vitro [49]. CD22-NMS249, with the same antigen-binding region of pinatuzumab vedotin and a more potent anthracycline analogue PNU-159682, displayed better cytotoxicity than pinatuzumab vedotin [50]. Anti-CD22-(LC:K149C)-SN36248 uses the same antibody of pinatuzumab vedotin and two SN36248 molecules as payload. The K149C conjugation site in the light chain promotes the in vivo conjugation stability of the ADC. SN36248 is a non-cleavable linker drug compound with a seco-CBI homodimer tethered to a maleimide linker. Anti-CD22-(LC:K149C)-SN36248 yielded a longer response duration than pinatuzumab vedotin and showed anti-lymphoma effects in several mouse models, including two resistant to pinatuzumab vedotin (Raji and WUS-DLCL2) [51].
Efficacy and safety of CD22 ADCs
The available clinical results [52–62] for CD22 ADCs in treating B-ALL and B-cell NHL are summarized in Table 2. Inotuzumab ozogamicin was evaluated in 90 patients with R/R B-ALL in a phase 2 study [54] and showed a complete response (CR) rate of 58% and a median duration of remission of 7 months. Seven patients (6.7%) experienced veno‐occlusive disease (VOD)/sinusoidal obstruction syndrome (SOS), among whom six developed VOD/SOS after transplantation. A two-arm, randomized phase 3 study [55, 56] compared the efficacy of inotuzumab ozogamicin (n = 164) and standard chemotherapy (n = 143) in adult patients with R/R B-ALL. The CR rate was also higher in the inotuzumab ozogamicin group (74% vs 31%). The median progression-free survival (PFS) and overall survival (OS) in the inotuzumab ozogamicin group were 5 and 7.7 months, respectively. Notably, the incidence of VOD/SOS was higher in the inotuzumab ozogamicin group (14% vs 2%). A phase 2 study assessed inotuzumab ozogamicin in 48 pediatric and adolescent patients with R/R B-ALL, among whom 10 (20.8%) underwent prior CD19 CAR-T cell therapy, 1 (2.1%) received CD22 CAR-T cell infusion, and 14 (29.2%) were treated with blinatumomab (a CD3/CD19 bispecific T cell engager). This study reported a CR rate of 58.3%, and minimal residual disease-negativity (< 0.01%) was achieved in 66.7% of patients. Twenty-one patients then proceeded to hematopoietic stem cell transplantation (HSCT) after ADC treatment, and six patients (28.6%) developed SOS after HSCT.
Table 2.
Results of clinical trials of CD22 ADCs in B-ALL, B-cell NHL and CLL (single-agent)
| Clinical trial information | Agent | Institution | Disease and patients (single agent cohort) |
Prior CD19-targeted therapy | ORR(≥ CR, best response) | Veno-occlusive disease | Median PFS | Median OS |
|---|---|---|---|---|---|---|---|---|
|
Phase 1 [52] |
Inotuzumab ozogamicin | Nagoya Daini Red Cross Hospital | R/R FL 13 pts | Rituximab 100% | 85% (54%) | - | - | - |
|
Phase 1 [53] |
Inotuzumab ozogamicin | Multicenter |
R/R B-cell NHL 79 pts |
- |
FL (MTD): 68% (-) DLBCL (MTD): 15% (-) |
1.30% |
FL: 317 days DLBCL: 49 days |
FL: not reached DLBCL: 193 days |
|
Phase 2 [54] |
Inotuzumab ozogamicin | MD Anderson Cancer Center |
R/R B-ALL 90 pts |
- | 58% (58%) | 6.7% | mDOR 7 mos | 6.2 mos |
|
Phase 3, 2-arm (INO-VATE) |
Inotuzumab ozogamicin | Multicenter |
R/R B-ALL 164 pts |
- | - (74%) | 14% | 5 mos | 7.7 mos |
|
Phase 2 [57] |
Inotuzumab ozogamicin | Multicenter |
Refractory indolent B-NHL 81 pts |
- | 67% (31%) | - | 12.7 mos | not reached |
|
Phase 1/2 [58] |
Inotuzumab ozogamicin | Multicenter |
R/R B-ALL 72 pts |
- | -(68%) | 5.6% | 3.9 mos | 7.4 mos |
|
Phase 1 EUDRA-CT 2016–000227-71 [59] |
Inotuzumab ozogamicin | Multicenter |
R/R B-ALL 25 pediatric pts |
Blinatumomab 24% CAR-T 4% |
80% (60%) | 8% | - |
DL1: 7.2 mos DL2: not reached |
|
Phase 2 EUDRA-CT 2016–000227-71 [60] |
Inotuzumab ozogamicin | Multicenter |
R/R B-ALL 28 pediatric pts |
Blinatumomab 25% | 82% (82%) in 27 evaluable pts | 25% | 1-year EFS 36.7% | 1-year OS 55.1% |
|
Phase 2 [61] |
Inotuzumab ozogamicin | Multicenter |
R/R B-ALL 48 pts |
CAR-T 23% Blinatumomab 29% |
65% (58%) | 13% | 2-year EFS 28.6% | 2-year OS 36% |
|
Phase 1 [62] |
Pinatuzumab vedotin |
Multicenter |
R/R DLBCL 25 pts indolent B-cell lymphoma 38 pts CLL 10 pts |
- |
DLBCL: 39% (18%) indolent B-cell lymphoma: 32% (12%) CLL: 0% (0%) |
- |
indolent B-cell lymphoma: 7.6 mos DLBCL (PR2D): 4 mos |
- |
ADC antibody–drug conjugate, ALL acute lymphocyte leukemia, NHL non-Hodgkin lymphoma, CLL chronic lymphocyte leukemia, FL follicular lymphoma, pts patients, DLBCL diffuse large B cell lymphoma, ORR overall response rate, CR complete response, MTD maximum tolerated dose, PFS progression-free survival, mos months, mDOR median duration of response, EFS event-free survival, PR2D recommended phase 2 dose, OS overall survival, DL dose level
Pinatuzumab vedotin, a novel anti-CD22 ADC, has rarely been used as a single agent in clinical settings. The efficacy of pinatuzumab vedotin with or without rituximab was tested in a phase 1 study of adult patients with diffuse large B-cell lymphoma (DLBCL), indolent B-cell NHL, and chronic lymphoblastic leukemia (CLL) [62]. At its recommended phase II dose, the overall response rate (ORR) was 36% in DLBCL and 50% in indolent B-cell NHL. Notably, no therapeutic effect was observed in CLL. The most common toxicity of pinatuzumab vedotin was peripheral neuropathy, especially peripheral sensory neuropathy.
Improving the clinical efficacy of CD22 ADCs
An in vitro study [63] indicated that internalization ability influenced the cytotoxicity of inotuzumab ozogamicin. Clinical data showed that CD22 density on the tumor cell surface correlated with clinical outcomes [55, 61, 64]. KMT2A translocations/rearrangement is a high-risk cytogenetic factor closely associated with low CD22 expression, and positive minimal residual disease status after treatment with inotuzumab ozogamicin [23, 65]. The patients with higher baseline CD22 expression and normal cytogenetics benefited most from inotuzumab ozogamicin. Patients treated with inotuzumab ozogamicin also showed decreased CD22 expression in blasts at relapse [61, 64]. The mechanisms are unknown. Bryostatin-1 is a natural substance that can specifically elevate CD22 surface distribution in a dose- and time-dependent manner [66]. Bryostatin-1 upregulates CD22 expression on CLL cells by activating protein kinase C [66] and on B-ALL cells through potential membrane trafficking [67]. Consequently, bryostatin-1 can be used for pretreatment or in combination with CD22 ADCs [66], though clinical effects need exploration.
Unlike CD22 CAR-T cell therapy, most patients receiving CD22 ADCs are naive to CD19 CAR-T cell therapy. Inotuzumab ozogamicin can effectively reduce the tumor burden and usually serves as a bridging therapy to HSCT or CAR-T cell therapy. Many clinical trials are also exploring the combination of CD22 ADCs with rituximab or other chemotherapies to increase the response depth. Inotuzumab ozogamicin plus mini-hyper-CVD chemotherapy, with or without blinatumomab, represents a feasible therapeutic regimen for elderly patients with newly diagnosed Ph- B-ALL [68–70]. Inotuzumab ozogamicin plus rituximab, with or without other chemotherapy agents, has also elicited encouraging clinical results in treating R/R B-cell NHL [71–73].
CD22 CAR-T cell structures and preclinical results
Unlike ADC, CAR consists of an extracellular antigen-recognizing single-chain variable fragment (scFv), a hinge and transmembrane domain, and an intracellular signaling domain. It can mimic the T-cells’ intrinsic activation mode and initiates their killing to tumor cells without the restriction of the major histocompatibility complex [74]. The investigation on CD22 CAR-T cell therapy is started with the scFvs from two recombinant immunotoxins, HA22 and BL22.
CAR-T cells using HA22-derived scFv did not exert enhanced cytolytic effects than that using BL22-derived scFv for the possible reason that HA22-derived scFv cannot produce a strong activation signal under sufficient antigen stimulation [75]. The novel fully human anti-CD22 antibody m971 has gradually become an appealing alternative to HA22. The epitope recognized by m971 is distinct from that by HA22 and BL22. HA22 and BL22 recognize IgG-like domain 3 of CD22, while m971 targets the most proximal three extracellular domains 5–7 with a relatively low avidity (Fig. 1A) [76]. Moreover, increasing the affinity of m971 scFv did not improve in vitro and in vivo CAR-T cell activity against CD22-low leukemia cells [67]. Second-generation CAR-T cells incorporating m971 scFv displayed a better anti-tumor activity than those with BL22 or HA22-derived scFv [37, 77]. This may be due to the decreased density of CD22 on the tumor cell surface caused by the HA22-mediated internalization, which was not observed with m971 antibody [76, 78]. The novel hCD22.7 scFv was shown to bind to the distal Ig-like domain 1 with high affinity without causing evident CAR-T cell-mediated antigen loss in a mouse model bearing the primary leukemia cells [79]. Several other CD22-targeted scFv structures have shown remarkable preclinical anti-tumor activity and have been adopted in clinical practice [21, 31]. Linkers between heavy and light chains [80] also affect the targeting capacity of CD22 CAR-T cells. Short linker facilitates the formation of immune synapse and spontaneous clustering of CARs without antigen stimulation, thus inducing a tonic activation and improved CAR-T cell function [81] (Fig. 1B).
Efficacy and safety of CD22 CAR-T cell therapy
The available clinical results on CD22 CAR-T cell therapy [20, 21, 24–31] are summarized in Table 3. The first in-human phase 1 trial of CD22 CAR-T cells, conducted at the National Cancer Institute [20, 24], reported an ORR of 72% (n = 57) and CR rate of 70%. At a median follow-up of 2 years, median OS and relapse-free survival in CR patients were 13.4 months and 6.0 months, respectively; 35% of patients relapsed, and the majority developed CD22-dim or negative disease. Cytokine release syndrome (CRS) and neurotoxicity occurred in 72% and 86% of patients, respectively. Two pilot studies recruited three adult and five pediatric patients with B-ALL [81]. The ORR was 50% and all of the responders achieved CR, with the longest CR being 7 months. CRS occurred in 75% of patients, and one developed grade 3 CRS.
Table 3.
Interim results of clinical trials of CD22 CAR-T cells
| Clinical trial information | Institution | Transduction/costimulatory domain/scFv (CAR-T product or manufacture procedure) |
Disease and patients | Prior CD19 CAR-T | CD19 negative or dim | Dosage | Pharmacokinetics | ORR(≥ CR, best response) | Prognosis | CRS at any grade (grade ≥ 3), evaluation criteria | Neurotoxicity at any grade (grade ≥ 3), evaluation criteria |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Phase 1 [20] |
NCI | Lentivirus/4-1BB/m971 |
R/R B-ALL 21 pts |
71.4% | 47.6% |
0.3 × 106 cells/kg 1 × 106 cells/kg 3 × 106 cells/kg |
Peak on D14, persist up to 18 mos | 57% (57%) | - |
76% (0%) Lee criteria |
37.5% (0%) in first 16 patients |
|
Phase 1 [24] |
NCI |
Lentivirus/4-1BB/m971 (CD4/CD8 TCS) |
R/R B-ALL 57 pts R/R DLBCL 1 pt |
62% | 56.9% |
0.3 × 106 cells/kg 1 × 106 cells/kg 3 × 106 cells/kg |
Peak on D14—D21, higher in those at CD4/CD8 TCS cohort | 71.9% (70.2%) in evaluable 57 pts |
mRFS (CR) 6.0 mos mOS (CR) 13.4 mos |
86.2% (8.6%) Lee criteria |
32.8% (1.7%) ASTCT criteria |
|
Phase 1 ChiCTR-OIC-17013523 [21] |
Beijing Boren Hospital |
Lentivirus/4-1BB/- (YK-CD22BB-002) |
R/R B-ALL 34 pts |
91% | 41.2% | 0.2 ~ 34.7 × 105 cells/kg |
Peak on D12—D15 median persistence time was 28 days by FCM |
81.3% (78.1%) in 32 evaluable pts |
- |
91.2% (2.9%) Lee criteria |
17.6% (0%) CTCAE criteria |
|
Phase 1 ChiCTR2000028793 [31] |
Beijing Boren Hospital |
Lentivirus/4-1BB/- (CD22-CARFH80) |
R/R B-ALL 8 pediatric pts |
100% | 12.5% | 0.68 ~ 9.4 × 106 cells/kg | Peak on D11- D15 | 87.5% (75%) | - |
87.5% (12.5%) ASTCT criteria |
ICANS 25% (12.5%) ASTCT criteria |
|
Two pilot studies [81] |
UPenn/Children’s Hospital of Philadelphia |
Lentivirus/4-1BB/m971 (CART22) |
R/R B-ALL 3 adult pts / 5 pediatric pts |
25% | 75% | 39.6 ~ 500 × 106 cells/pt |
2 CR pts showed significant CAR-T expansion within D20 |
50% (50%) | - |
75% (12.5%) Penn criteria |
- |
|
Phase 1 PLAT-07(NCT04571138) [25] |
Seattle Children's Hospital |
- /4-1BB/m971 (SCRI-CAR22v2) |
R/R B-ALL 3 pts |
100% | 66.7% | 2 × 105 cells/kg | - | 100% (100%) | - | - | - |
|
New Treatment Measure Clinical Study ChiCTR1800019298 [26] |
Tianjin First Central Hospital | -/4-1BB/- |
R/R B-ALL 6 pts R/R DLBCL 7 pts |
100% | 33.3% (B-ALL) |
DLBCL: 2.11 ± 0.24 × 106 cells/kg B-ALL: 2.07 ± 0.42 × 106 cells/kg |
Peak on D14 |
DLBCL: 85.7% (57.1%) B-ALL: 33.3% (33.3%) |
- |
DLBCL: 42.9% (0%) B-ALL: 100% (16.7%) Lee criteria |
ICANS 0% (0%) ASTCT criteria |
|
Phase 1 NCT04150497(BALLI-01) [27] |
Cellectis S.A |
Lentivirus/4-1BB/- (UCAR-T22, disruption of TRAC and CD52 genes using TALEN technology) |
R/R B-ALL 3 pts |
33.3% | - | ~ 1 × 106 cells/kg | Peak on D9—D14 | 66.7% (33.3%) | - | 33.3% (0%) | 0% (0%) |
|
Phase 1 [28] |
Stanford University School of Medicine |
Lentivirus/4-1BB/m971 (CD4/CD8 T selection) |
R/R LBCL 3 pts |
100% | 66.7% | 1 × 106 cells/kg |
Peak on D14, persist up to 3 mos by qPCR |
100% (100%) | - |
100% (0%) ASTCT criteria |
ICANS 0% (0%) ASTCT criteria |
|
Phase 1 (cohort expansion) [29] |
Stanford University School of Medicine | Lentivirus/4-1BB/m971 |
R/R LBCL 21 pts |
95% | - |
1 × 106 cells/kg 3 × 106 cells/kg |
Peak on D14 | 85.7% (66.7%) |
mPFS not reached mOS not reached |
100% (4.8%) ASTCT criteria |
ICANS 19% (0%) ASTCT criteria |
|
Phase 1 [30] |
UPenn |
Lentivirus/4-1BB/m971 (CART22-65 s) |
R/R B-ALL 17 pts |
94.1% | 100.0% |
0.8 ~ 10 × 106 cells/kg (3—day fractionated dosing) |
Peak on D20 | 76.5% (76.5%) |
mRFS 5.3 mos mEFS 5.8 mos mOS 16.5 mos |
88.2% (0%) | 35.3% (0%) |
NCI National Cancer Institute, UPenn University of Pennsylvania, TCS T-cell selection, UCAR-T universal chimeric antigen receptor T-cell, TRAC T- cell receptor alpha constant, TALEN transcription activator-like effector nuclease, R/R refractory or relapsed, ALL acute lymphocyte leukemia, LBCL large B cell lymphoma, FCM flow cytometry, ORR overall response rate, CR complete response, mos months, qPCR quantitative real-time polymerase chain reaction, mPFS median progression-free survival, mRFS median relapse-free suvival, mEFS median event-free survival, mo months, mOS median overall survival, NE not evaluated, CRS cytokine release syndrome, ASTCT American Society for Transplantation and Cellular Therapy, CTCAE Common Terminology Criteria for Adverse Events, ICANS immune effector cell-associated neurotoxicity syndrome
We conducted a subgroup analysis based on the available clinical results of CD22 CAR-T cell therapy (Table 4). While the pooled ORR did not differ with age, the CR rate was higher in children than that in adults (74% vs 57%, P = 0.05). Neurotoxicity tended to occur more frequently in children than adults (28% vs 11%, P = 0.04). In addition, CRS had a predilection for patients with B-ALL instead of those with B-cell lymphoma (87% vs 74%,P < 0.01). The relapse rate was higher in patients with B-ALL than that in patients with B-cell lymphoma (31% vs 8%, P = 0.04). Young patients also had a higher risk of relapse than adult patients (36% vs 6%, P = 0.01). Moreover, previous treatment with CD19 CAR-T cells did not influence the efficacy or safety of CD22 CAR-T cell therapy (CR 73% vs 79%, P = 0.63; CRS 76% vs 83%, P = 0.59; Neurotoxicity 13% vs 9%, P = 0.72).
Table 4.
Subgroup analysis and exploration of heterogeneity in CD22 CAR-T clinical trials
| Patients with available data | Overall response rate | Complete response rate |
negative Minimal residual disease | Cytokine release syndrome |
≥ Grade 3 cytokine release syndrome | Neurotoxicity | ≥ Grade 3 neurotoxicity | Relapse rate | CD22 dim/negative relapse rate |
|---|---|---|---|---|---|---|---|---|---|
| Age Group | |||||||||
| Children (N = 114) |
76% (68–83) |
74% (65–81) |
- |
87% (80–92) |
6% (3–12) |
28% (21–37) |
- |
36% (18–54) |
16% (0–32) |
| Adult (N = 37) |
75% (59–87) |
57% (41–72) |
- |
84% (68–93) |
5% (1–19) |
11% (4–25) |
- |
6% (0–20) |
3% (0–9) |
| p value | 0.94 | 0.05* | - | 0.6 | 0.9 | 0.04* | - | 0.01** | 0.14 |
| Bone marrow involvement (B-ALL) | |||||||||
| High burden (N = 98) |
74% (65–82) |
63% (43–83) |
66% (55–75) |
86% (78–92) |
7% (3–14) |
25% (17–34) |
1% (0–7) |
23% (6–58) |
8% (1–46) |
| Low burden (N = 25) |
81% (66–96) |
76% (59–93) |
68% (48–83) |
88% (69–96) |
4% (1–24) |
32% (17–52) |
4% (1–24) |
40% (23–60) |
12% (4–31) |
| p value | 0.22 | 0.54 | 0.82 | 0.81 | 0.6 | 0.46 | 0.32 | 0.37 | 0.73 |
| Disease | |||||||||
| B-ALL (N = 133) |
76% (69–83) |
72% (65–80) |
- |
87% (82–93) |
4% (1–8) |
20% (8–32) |
1% (0–3) |
31% (13–49) |
11% (0–23) |
| B cell lymphoma (N = 28) |
86% (73–99) |
64% (47–82) |
- |
74% (19–100) |
4% (0–12) |
10% (0–28) |
0% (0–6) |
8% (0–19) |
4% (0–12) |
| p value | 0.19 | 0.41 | - | < 0.01** | 0.87 | 0.36 | 0.75 | 0.04* | 0.35 |
| Prior CD19 CAR-T therapy | |||||||||
| Yes (N = 67) |
75% (65–87) |
73% (62–86) |
45% (16–74) |
76% (62–91) |
9% (3–30) |
0% (0–6) |
0% (0–6) |
19% (0–46) |
24% (9–66) |
| No (N = 15) |
76% (58–100) |
79% (58–100) |
62% (22–100) |
83% (62–100) |
13% (3–58) |
0% (0–6) |
0% (0–6) |
29% (5–52) |
20% (7–60) |
| p value | 0.76 | 0.64 | 0.5 | 0.59 | 0.72 | 1 | 1 | 0.59 | 0.55 |
Data are event rate, % (95% CI), p values, or number of patients. Adults were patients aged 20 years or older; children were patients aged younger than 20 years, CAR chimeric antigen receptor
Overcoming treatment failure of CD22 CAR-T cells
Lower CD22 density in blasts and a higher tumor burden are associated with poor outcomes. Zheng et al. [82] reported two CD22 splicing isoforms—a Δex5-6-splicing and Δex2-skipping isoform—from the RNA sequencing databases of newly diagnosed pediatric B-ALL patients. The former isoform causes epitope recognition failure in the IgG-like domain 3, while the latter is a CD22 Δex2 variant (Δex2 encodes mRNA containing the initiation codon AUG) that cannot be translated into any identifiable protein. The CD22 variants may partially explain the initial low CD22 density on tumor cell surface. In addition, prior CD22-targeted immunotherapy is a potential predictor of poor efficacy. Tumors can escape the killing of CAR-T cells by decreasing the antigen density on the surface, which has been observed in both CD19 and CD22 CAR-T cell therapy. Notably, Ramakrishna et al. [67] found that the genetic sequence and mRNA levels of CD22 remained unchanged in the tumor cells of relapsed patients, but the surface distribution of CD22 decreased. Moreover, CD22 CAR-T cells did not induce obvious trogocytosis of CD22 on tumor cells [83]. Membrane trafficking resulted in the internalization of CD22 molecules after antigen–antibody interactions [78], which might explain the downregulation of CD22 after CAR-T cell therapy. Notably, CD22 splicing isoforms were not detected after treatment failure with CD22 CAR-T cells.
CD22 downregulation is an important mechanism of treatment failure after CD22 CAR-T cell therapy [20, 21, 29–31, 81]. Bryostatin-1 can sensitize tumor cells to CD22 CAR-T cell therapy [67]. However, bryostatin-1 cannot affect CD22 expression on tumor cell lines with low primary CD22 expression. Direct exposure to bryostatin-1 can dampen interferon-γ production while enhancing granzyme B secretion in CD22 CAR-T cells. In a mouse model, bryostatin-1 effectively prolonged in vivo persistence and promoted the memory phenotype of CD22 CAR-T cells [67]. Epigenetic modifiers, such as 5-azacytidine and all-trans retinoic acid can also modulate CD22 expression in different cell lines [84]. Although these studies provide proof for combination therapies with CD22 CAR-T cells, further research is needed to determine the optimal timing and duration.
Dual-targeting effectively mitigates the antigen loss associated with CAR-T cell therapy and improves treatment outcomes. Many dual-targeting strategies have been reported, including sequential infusion [85–88] and co-administration [89] of two CAR-T cell products, co-transduction [90, 91] or sequential transduction [92] of T cells with two vectors conveying different CARs, and transduction of T cells with one vector encoding two separate CARs (bicistronic structure) [93, 94], using tandem [95–101] or loop [102, 103] scFv structures. Preclinical studies demonstrated that tandem CD19/CD22 CAR-T cells eradicated CD19 + tumor cells effectively but had a limited cytotoxicity on CD22 + tumor cellss compared to single-target CAR-T cells [104]. However, tandem CD19/CD22 CAR-T cell therapy resulted in a higher CR rate than single-target or sequential infusion of CD19 and CD22 CAR-T cells [105, 106]. Recently, Kokalaki et al. [107] screened out 9A8 as a better scFv in CAR design based on its sensitivity to CD22-low tumors after sequential antigen stimulation. They constructed two CAR vectors incorporating the FMC63 or 9AB scFv and developed a dual-target CAR-T cell product using a co-transduction method. CD19/CD22 dual-target CAR-T cell therapy showed relative better efficacy than single-target CAR-T cell therapy, with a CR rate ranging from 83%–100% in B-ALL and 50%–62.5% in B-cell lymphoma [85, 86, 90–101] (Table 5). Trispecific CAR-T cells targeting CD19, CD20 and CD22 also showed a promising ability for eliminating antigen-heterogeneous tumor cells [108].
Table 5.
Results of clinical trials of CD19/CD22 dual-targeting CAR-T cells
| Clinical trial information | Institution | Dual-targeting strategy | CAR structures | Disease and patients | Prior CD19 CAR-T treatment at baseline | ORR(≥ CR, best response) | Prognosis | CRS at any grade (grade ≥ 3), evaluation criteria | Neurotoxicity at any grade (grade ≥ 3), evaluation criteria |
|---|---|---|---|---|---|---|---|---|---|
|
Observational study ChiCTR-OPN-16008526 [86] |
Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology | Sequential infusion without interval (D0-D3) |
anti-CD19 scFv (Murine)/4-1BB anti-CD22 scFv (Murine)/4-1BB |
R/R B-ALL 51 pts R/R B-cell NHL 38 pts |
- |
B-ALL: 98% (96%) in 50 evaluable pts B-cell NHL: 72.2% (50%) in 36 evaluable pts |
B-ALL: mPFS 13.6 mos, mOS 31 mos B-cell NHL: mPFS 9.9 mos, mOS 18 mos |
95.5%(17.9%) Lee criteria |
CRES 13.5% (1.1%) CTCTE criteria |
|
Observational study ChiCTR-ONC-17013648 [87] |
Beijing Boren Hospital | Sequential infusion |
FMC63 scFv/4-1BB anti-CD22 scFv (human)/4-1BB |
R/R B-ALL 21 pts |
100% (16 CR, 3 PR, 2 relapsed) | 95% (95%) |
18-month OS rate 88.5% 18-month EFS rate 67.5% |
52% (0%) Penn criteria |
0% (0%) CTCTE criteria |
|
Phase 1 ChiCTR-OIB-17013670 [88] |
Beijing Boren Hospital | Sequential infusion |
anti-CD19 scFv/4-1BB anti-CD22 scFv/4-1BB |
R/R B-ALL 20 pts |
- | 100% (100%) |
mLFS/mOS not reached 1-year LFS rate 79.5% 1-year OS rate 92.3% |
CD19 CAR-T 90% (5%) CD22 CAR-T 75% (0%) |
CD19 CAR-T 15% (5%) CD22 CAR-T 15% (0%) |
|
Phase 2 ChiCTR2000032211 [89] |
Multicenter | Coadministration (1:1) |
anti-CD19 scFv/4-1BB anti-CD22 scFv/4-1BB |
B-ALL 6 pts R/R B-ALL 188 pts B-ALL with isolated EMD 31 pts |
- | 99% (99%) | 12-month EFS rate 73.5% |
88% (28.4%) ASTCT criteria |
20.9% (4.0%) ASTCT criteria |
|
Phase 1 [93] |
Autolus PLC | Bicistronic CAR-T |
FMC63 scFv/OX40 LT22 scFv-COMP/4-1BB |
R/R B-ALL 15 pts |
- | 86.7% ( 86.7%) | - |
80% (0%) Lee criteria |
ICANS 26.7% (0%) ASTCT criteria |
|
Phase 1 (UCAR-T) [95] |
The First Affiliated Hospital, School of Medicine, Zhejiang University | Tantem CAR-T | FMC63 scFv-m971 scFv/4-1BB |
R/R B-ALL 6 pts |
- | 83.3% (83.3%) | - | 100% (16.7%) | 0% (0%) |
|
Phase 1 ChiCTR1800015575 [96] |
The First Affiliated Hospital, School of Medicine, Zhejiang University | Tantem CAR-T | FMC63 scFv-anti-CD22 scFv(human)/4-1BB |
R/R B-cell lymphoma 16 pts |
- | 87.5% (62.5%) |
2-year OS rate 77.3% 2-year PFS rate 40.2% mPFS 246 days |
100% (6.3%) ASTCT criteria |
0% (0%) CTCAE criteria |
|
Phase 1 [97] |
Institute of Basic Medicine, Chinese PLA General Hospital | Tantem CAR-T | m971 scFv-FMC63 scFv/4-1BB |
R/R B-ALL 6 pts |
- | 100% (100%) | - |
100% (0%) Lee criteria |
ICANS 0% (0%) ASTCT criteria |
|
Phase 1 [102] |
Stanford University School of Medicine | Loop CAR-T | FMC63 VH-m971 VL-m971 VH-FMC63 VL/4-1BB |
R/R B-ALL 17 pts R/R LBCL 21 pts |
DLBCL 65% |
B-ALL: 100% (80%) LBCL: 62% (29%) |
- |
76% (5%) Lee criteria |
37% (10.5%) CTCAE criteria |
|
Phase 1 [103] |
Shanghai General Hospital, Shanghai Jiaotong University School of Medicine | Loop CAR-T | FMC63 VL-m971 VH-m971 VL-FMC63 VH/4-1BB |
B-ALL 15 pts |
- | 100% (100%) |
mRFS/mOS not reached 12-month RFS rate 77% 12-month OS rate 86% |
26.7% (0%) ASTCT criteria |
ICANS 0% (0%) ASTCT criteria |
PLA Liberation Army General, UCAR-T universal chimeric antigen receptor T-cell, R/R refractory or relapsed, ALL acute leukemia lymphocyte, CR complete response, pt patient, NHL non-Hodgkin lymphoma, PR partial remission, ORR overall response rate, mPFS median progression-free survival, mOS median overall survival, EFS median event-free survival, mos months, mLFS median leukemia-free survival, mRFS median relapse-free suvival, mos months, CRS cytokine release syndrome, ICANS immune effector cell-associated neurotoxicity syndrome, ASTCT American Society for Transplantation and Cellular Therapy, CTCAE Common Terminology Criteria for Adverse Events
Conclusion and future perspective
CD22 is a potential target especially for patients who have experienced treatment failure with CD19-targeted immunotherapies. CD22-targeted immunotherapies have shown promising efficacy and acceptable toxicities in hematologic malignancies. Despite the difference in patient characteristics, CD22 CAR-T cells outperformed CD22 ADCs in terms of clinical efficacy, reflecting the superiority of T-cell-mediated immunity over the cytotoxic agents. We also found that prior exposure to CD19 CAR-T cells did not profoundly affect the efficacy of CD22 CAR-T cells. Therefore, CD22 CAR-T cell therapy could either be an upfront choice if CD22 density is far higher than CD19 on the tumor cell surface or be a candidate for CD19 CAR-T cell therapy. CRS and neurotoxicity are typical toxicities caused by CAR-T cells. CRS could sometimes be intense and leads to multi-organ damage. But CD22 ADC provides a safer option for those at high risk to develop CAR-T-associated toxicities and the elders, at least as a bridging therapy. CD22 ADCs are not suitable for patients who relapsed after CD22 CAR-T treatment due to the associated antigen loss. Instead, CD22 ADCs can prepare the patients for subsequent CD19 CAR-T cell therapy, as the counts of CD3 + T cells are maintained after the treatment [61]. However, the prolonged B-cell aplasia caused by CD22 ADCs may be an unfavorable factor for subsequent CAR-T cell therapy [109], given that a lower percentage of CD19 + B cells (< 15%) in the bone marrow at infusion correlated with a shorter persistence of CD19 CAR-T cells [11]. One pediatric patient with B-ALL experienced a myeloid lineage switch after the infusion of CD19 CAR-T cells following the treatment with inotuzumab ozogamicin. Notably, KMT2A rearrangement was previously detected in the case, emphasizing the importance of detecting genetic abnormalities at enrollment [61].
Both CD22 ADCs and CAR-T cells were more effective in treating B-ALL than B-cell NHL. This was especially evident with CD22 ADCs, demonstrating a more suitable therapeutic profile against B-ALL. Therefore, both of CD22 ADC and CAR-T cell therapy are feasible for patients with B-ALL. But CD22 ADCs are not recommended to patients with DLBCL or large B-cell lymphoma. As for indolent lymphoma, limited data is accessible on CD22 CAR-T cell therapy, while CD22 ADCs demonstrate modest efficacy compared to other targeted therapies [110, 111].
Nevertheless, relapse after CD22-targeted immunotherapies remains an unsolved problem. Particularly, the antigen downregulation is apparent in CD22-targeted therapies, and the mechanism is unclear. The preclinical results [66, 67, 83, 84] of bryostatin-1 plus CD22-targeted therapies uphold the combination therapies, but how to schedule and administrate the use of bryoststin-1 still needs more clinical exploration. CD19/CD22 dual-targeting CAR-T cells demonstrated excellent efficacy and can avoid antigen escape to a great extent. Based on the different mechanisms of immunotherapies, CD22 ADC plus CD22 CAR-T, CD19 CAR-T, or CD19 bispecific T cell engager provide new directions for designing effective therapeutic strategies. However, whether this would produce a result greater than one plus one remains unexplored.
Collectively, optimization of CD22 CAR-T cells and ADCs, combination strategies and dual-targeting designs are future perspectives, which shed light on the better clinical application of CD22-targeted therapies.
Acknowledgements
The authors would like to thank all members of the study team.
Abbreviations
- ADC
Antibody–drug conjugate
- B-ALL
B-cell acute lymphoblastic leukemia
- CAR
Chimeric antigen receptor
- CLL
Chronic lymphoblastic leukemia
- CR
Complete response
- CRS
Cytokine release syndrome
- DLBCL
Diffuse large B-cell lymphoma
- HSCT
Hematopoietic stem cell transplantation
- NHL
Non-Hodgkin lymphoma
- ORR
Overall response rate
- OS
Overall survival
- R/R
Relapsed or refractory
- scFv
Single-chain fragment variable
- SOS
Sinusoidal obstruction syndrome
- VOD
Veno-occlusive disease
Author contributions
HM and CL raised the conceptualization; JX and CL structured and wrote the manuscript; WL conducted the subgroup analysis of clinical data. JX and WL designed and generated the figure and summarized the table; CL and WL revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Grants from the National Natural Science Foundation of China (No. 82070124 to Heng Mei), Natural Science Foundation of Hubei Province (No. 2020CFA065 to Heng Mei), and Fundamental Research Support Program of Huazhong University of Science and Technology (No. 5003530166 to Heng Mei).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chenggong Li, Email: chenggongli@hust.edu.cn.
Heng Mei, Email: hmei@hust.edu.cn.
References
- 1.Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377(26):2545–2554. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Westin JR, Kersten MJ, Salles G, et al. Efficacy and safety of CD19-directed CAR-T cell therapies in patients with relapsed/refractory aggressive B-cell lymphomas: observations from the JULIET, ZUMA-1, and TRANSCEND trials. Am J Hematol. 2021;96(10):1295–1312. doi: 10.1002/ajh.26301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abramson JS. Anti-CD19 CAR T-cell therapy for B-Cell non-Hodgkin lymphoma. Transfus Med Rev. 2020;34(1):29–33. doi: 10.1016/j.tmrv.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Martino M, Alati C, Canale FA, et al. A Review of Clinical Outcomes of CAR T-Cell Therapies for B-acute lymphoblastic leukemia. Int J Mol Sci. 2021;22(4):89. doi: 10.3390/ijms22042150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maude SL, Teachey DT, Rheingold SR, et al. Sustained remissions with CD19-specific chimeric antigen receptor (CAR)-modified T cells in children with relapsed/refractory ALL. J Clin Oncol. 2016;34(15):3011–3111. doi: 10.1200/JCO.2016.34.15_suppl.3011. [DOI] [Google Scholar]
- 6.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park JH, Rivière I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghorashian S, Kramer AM, Onuoha S, et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat Med. 2019;25(9):1408–1414. doi: 10.1038/s41591-019-0549-5. [DOI] [PubMed] [Google Scholar]
- 9.Schultz LM, Eaton A, Baggott C, et al. Outcomes after nonresponse and relapse post-tisagenlecleucel in children, adolescents, and young adults with B-cell acute lymphoblastic leukemia. J Clin Oncol. 2023;41(2):354–363. doi: 10.1200/JCO.22.01076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roddie C, Dias J, O'Reilly MA, et al. Durable responses and low toxicity after fast off-rate CD19 chimeric antigen receptor-T therapy in adults with relapsed or refractory B-cell acute lymphoblastic leukemia. J Clin Oncol. 2021;39(30):3352–3363. doi: 10.1200/JCO.21.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322–3331. doi: 10.1182/blood-2017-02-769208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J, Cho J, Yoon SE, et al. Efficacy of salvage treatments in relapsed or refractory diffuse large B-cell lymphoma including chimeric antigen receptor T-Cell therapy: a systematic review and meta-analysis. Cancer Res Treat. 2023;55(3):1031–1047. doi: 10.4143/crt.2022.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roschewski M, Longo DL, Wilson WH. CAR T-Cell therapy for large B-cell lymphoma - who, when, and how? N Engl J Med. 2022;386(7):692–696. doi: 10.1056/NEJMe2118899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuster SJ, Tam CS, Borchmann P, et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22(10):1403–1415. doi: 10.1016/S1470-2045(21)00375-2. [DOI] [PubMed] [Google Scholar]
- 15.Sotillo E, Barrett DM, Black KL, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5(12):1282–1295. doi: 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orlando EJ, Han X, Tribouley C, et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat Med. 2018;24(10):1504–1506. doi: 10.1038/s41591-018-0146-z. [DOI] [PubMed] [Google Scholar]
- 17.Hamieh M, Dobrin A, Cabriolu A, et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature. 2019;568(7750):112–116. doi: 10.1038/s41586-019-1054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu X, Sun Q, Liang X, et al. Mechanisms of relapse after CD19 CAR T-cell therapy for acute lymphoblastic leukemia and its prevention and treatment strategies. Front Immunol. 2019;10:2664. doi: 10.3389/fimmu.2019.02664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plaks V, Rossi JM, Chou J, et al. CD19 target evasion as a mechanism of relapse in large B-cell lymphoma treated with axicabtagene ciloleucel. Blood. 2021;138(12):1081–1085. doi: 10.1182/blood.2021010930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan J, Niu Q, Deng B, et al. CD22 CAR T-cell therapy in refractory or relapsed B acute lymphoblastic leukemia. Leukemia. 2019;33(12):2854–2866. doi: 10.1038/s41375-019-0488-7. [DOI] [PubMed] [Google Scholar]
- 22.Shor B, Gerber H-P, Sapra P. Preclinical and clinical development of inotuzumab-ozogamicin in hematological malignancies. Mol Immunol. 2015;67(2):107–116. doi: 10.1016/j.molimm.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Shah NN, Stevenson MS, Yuan CM, et al. Characterization of CD22 expression in acute lymphoblastic leukemia. Pediatr Blood Cancer. 2015;62(6):964–969. doi: 10.1002/pbc.25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah NN, Highfill SL, Shalabi H, et al. CD4/CD8 T-cell selection affects chimeric antigen receptor (CAR) T-cell potency and toxicity: updated results from a phase I anti-CD22 CAR T-cell trial. J Clin Oncol. 2020;38(17):1938–1950. doi: 10.1200/JCO.19.03279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Summers C, Baxter B, Annesley C, et al. CD22 CAR optimization for improved in-human activity following inadequate CD22 CAR activity in phase 1 clinical trial PLAT-04. Blood. 2021;138(Supplement 1):403–503. doi: 10.1182/blood-2021-147928. [DOI] [Google Scholar]
- 26.Zhu H, Deng H, Mu J, et al. Anti-CD22 CAR-T Cell therapy as a salvage treatment in B cell malignancies refractory or relapsed after anti-CD19 CAR-T therapy. Onco Targets Ther. 2021;14:4023–4037. doi: 10.2147/OTT.S312904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain N, Roboz GJ, Konopleva M, et al. Preliminary Results from the Flu/Cy/Alemtuzumab Arm of the Phase I BALLI-01 Trial of UCART22, an Anti-CD22 Allogeneic CAR-T Cell Product, in Adult Patients with Relapsed or Refractory (R/R) CD22+ B-Cell Acute Lymphoblastic Leukemia (B-ALL) Blood. 2021;138(Supplement 1):1746–1846. doi: 10.1182/blood-2021-150779. [DOI] [Google Scholar]
- 28.Baird JH, Frank MJ, Craig J, et al. CD22-directed CAR T-cell therapy induces complete remissions in CD19-directed CAR-refractory large B-cell lymphoma. Blood. 2021;137(17):2321–2325. doi: 10.1182/blood.2020009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frank MJ, Baird JH, Patel S, et al. CD22-CAR T-Cell therapy mediates high durable remission rates in adults with large B-cell lymphoma who have relapsed after CD19-CAR T-Cell Therapy. Blood. 2021;138(Supplement 1):741–841. doi: 10.1182/blood-2021-152145. [DOI] [Google Scholar]
- 30.Myers RM, DiNofia AM, Li Y, et al. CD22-Targeted CAR-Modified T-cells safely induce remissions in children and young adults with relapsed, CD19-Negative B-ALL after Treatment with CD19-Targeted CAR T-Cells. Blood. 2022;140(Supplement 1):2376–2377. doi: 10.1182/blood-2022-168139. [DOI] [Google Scholar]
- 31.Tan Y, Cai H, Li C, et al. A novel full-human CD22-CAR T cell therapy with potent activity against CD22low B-ALL. Blood Cancer J. 2021;11(4):71. doi: 10.1038/s41408-021-00465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldenberg DM. Epratuzumab in the therapy of oncological and immunological diseases. Expert Rev Anticancer Ther. 2006;6(10):1341–1353. doi: 10.1586/14737140.6.10.1341. [DOI] [PubMed] [Google Scholar]
- 33.Clark EA, Giltiay NV. CD22: a regulator of innate and adaptive B cell responses and autoimmunity. Front Immunol. 2018;9:2235. doi: 10.3389/fimmu.2018.02235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nitschke L. CD22 and Siglec-G: B-cell inhibitory receptors with distinct functions. Immunol Rev. 2009;230(1):128–143. doi: 10.1111/j.1600-065X.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- 35.Ramya TNC, Weerapana E, Liao L, et al. In situ trans ligands of CD22 identified by glycan-protein photocross-linking-enabled proteomics. Mol Cell Proteomics. 2010;9(6):1339–1351. doi: 10.1074/mcp.M900461-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.FitzGerald DJ, Wayne AS, Kreitman RJ, et al. Treatment of hematologic malignancies with immunotoxins and antibody-drug conjugates. Can Res. 2011;71(20):6300–6309. doi: 10.1158/0008-5472.CAN-11-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haso W, Lee DW, Shah NN, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013;121(7):1165–1174. doi: 10.1182/blood-2012-06-438002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raponi S, De Propris MS, Intoppa S, et al. Flow cytometric study of potential target antigens (CD19, CD20, CD22, CD33) for antibody-based immunotherapy in acute lymphoblastic leukemia: analysis of 552 cases. Leuk Lymphoma. 2011;52(6):1098–1107. doi: 10.3109/10428194.2011.559668. [DOI] [PubMed] [Google Scholar]
- 39.Shah NN, Sokol L. Targeting CD22 for the Treatment of B-Cell Malignancies. Immunotargets Ther. 2021;10:225–236. doi: 10.2147/ITT.S288546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olejniczak SH, Stewart CC, Donohue K, et al. A quantitative exploration of surface antigen expression in common B-cell malignancies using flow cytometry. Immunol Invest. 2006;35(1):78. doi: 10.1080/08820130500496878. [DOI] [PubMed] [Google Scholar]
- 41.Duan S, Paulson JC. Siglecs as immune cell checkpoints in disease. Annu Rev Immunol. 2020;38:365–395. doi: 10.1146/annurev-immunol-102419-035900. [DOI] [PubMed] [Google Scholar]
- 42.Fu Z, Li S, Han S, et al. Antibody drug conjugate: the "biological missile" for targeted cancer therapy. Signal Transduct Target Ther. 2022;7(1):93. doi: 10.1038/s41392-022-00947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin Y, Schladetsch MA, Huang X, et al. Stepping forward in antibody-drug conjugate development. Pharmacol Ther. 2022;229:107917. doi: 10.1016/j.pharmthera.2021.107917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kreitman RJ, Wilson WH, Bergeron K, et al. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N Engl J Med. 2001;345(4):241–247. doi: 10.1056/NEJM200107263450402. [DOI] [PubMed] [Google Scholar]
- 45.Bang S, Nagata S, Onda M, et al. HA22 (R490A) is a recombinant immunotoxin with increased antitumor activity without an increase in animal toxicity. Clin Cancer Res. 2005;11(4):1545–1550. doi: 10.1158/1078-0432.CCR-04-1939. [DOI] [PubMed] [Google Scholar]
- 46.Ho M, Kreitman RJ, Onda M, et al. In vitro antibody evolution targeting germline hot spots to increase activity of an anti-CD22 immunotoxin. J Biol Chem. 2005;280(1):607–617. doi: 10.1074/jbc.M409783200. [DOI] [PubMed] [Google Scholar]
- 47.Lamb YN. Inotuzumab ozogamicin: first global approval. Drugs. 2017;77(14):1603–1610. doi: 10.1007/s40265-017-0802-5. [DOI] [PubMed] [Google Scholar]
- 48.DiJoseph JF, Popplewell A, Tickle S, et al. Antibody-targeted chemotherapy of B-cell lymphoma using calicheamicin conjugated to murine or humanized antibody against CD22. Cancer Immunol Immunother. 2005;54(1):11–24. doi: 10.1007/s00262-004-0572-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li D, Poon KA, Yu S-F, et al. DCDT2980S, an anti-CD22-monomethyl auristatin E antibody-drug conjugate, is a potential treatment for non-Hodgkin lymphoma. Mol Cancer Ther. 2013;12(7):1255–1265. doi: 10.1158/1535-7163.MCT-12-1173. [DOI] [PubMed] [Google Scholar]
- 50.Yu S-F, Zheng B, Go M, et al. A novel anti-CD22 anthracycline-based antibody-drug conjugate (ADC) that overcomes resistance to auristatin-based ADCs. Clin Cancer Res. 2015;21(14):3298–3306. doi: 10.1158/1078-0432.CCR-14-2035. [DOI] [PubMed] [Google Scholar]
- 51.Yu S-F, Lee DW, Zheng B, et al. An Anti-CD22-seco-CBI-Dimer Antibody-Drug Conjugate (ADC) for the treatment of non-hodgkin lymphoma that provides a longer duration of response than auristatin-based ADCs in preclinical models. Mol Cancer Ther. 2021;20(2):340–346. doi: 10.1158/1535-7163.MCT-20-0046. [DOI] [PubMed] [Google Scholar]
- 52.Ogura M, Tobinai K, Hatake K, et al. Phase I study of inotuzumab ozogamicin (CMC-544) in Japanese patients with follicular lymphoma pretreated with rituximab-based therapy. Cancer Sci. 2010;101(8):1840–1845. doi: 10.1111/j.1349-7006.2010.01601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Advani A, Coiffier B, Czuczman MS, et al. Safety, pharmacokinetics, and preliminary clinical activity of inotuzumab ozogamicin, a novel immunoconjugate for the treatment of B-cell non-Hodgkin's lymphoma: results of a phase I study. J Clin Oncol. 2010;28(12):2085–2093. doi: 10.1200/JCO.2009.25.1900. [DOI] [PubMed] [Google Scholar]
- 54.Kantarjian H, Thomas D, Jorgensen J, et al. Results of inotuzumab ozogamicin, a CD22 monoclonal antibody, in refractory and relapsed acute lymphocytic leukemia. Cancer. 2013;119(15):2728–2736. doi: 10.1002/cncr.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N Engl J Med. 2016;375(8):740–753. doi: 10.1056/NEJMoa1509277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: Final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer. 2019;125(14):2474–2487. doi: 10.1002/cncr.32116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goy A, Forero A, Wagner-Johnston N, et al. A phase 2 study of inotuzumab ozogamicin in patients with indolent B-cell non-Hodgkin lymphoma refractory to rituximab alone, rituximab and chemotherapy, or radioimmunotherapy. Br J Haematol. 2016;174(4):571–581. doi: 10.1111/bjh.14094. [DOI] [PubMed] [Google Scholar]
- 58.DeAngelo DJ, Stock W, Stein AS, et al. Inotuzumab ozogamicin in adults with relapsed or refractory CD22-positive acute lymphoblastic leukemia: a phase 1/2 study. Blood Adv. 2017;1(15):1167–1180. doi: 10.1182/bloodadvances.2016001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brivio E, Locatelli F, Lopez-Yurda M, et al. A phase 1 study of inotuzumab ozogamicin in pediatric relapsed/refractory acute lymphoblastic leukemia (ITCC-059 study) Blood. 2021;137(12):1582–1590. doi: 10.1182/blood.2020007848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pennesi E, Michels N, Brivio E, et al. Inotuzumab ozogamicin as single agent in pediatric patients with relapsed and refractory acute lymphoblastic leukemia: results from a phase II trial. Leukemia. 2022;36(6):1516–1524. doi: 10.1038/s41375-022-01576-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O'Brien MM, Ji L, Shah NN, et al. Phase II trial of inotuzumab ozogamicin in children and adolescents with relapsed or refractory B-Cell acute lymphoblastic leukemia: children's oncology group protocol AALL1621. J Clin Oncol. 2022;40(9):956–967. doi: 10.1200/JCO.21.01693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Advani RH, Lebovic D, Chen A, et al. Phase I Study of the Anti-CD22 antibody-drug conjugate pinatuzumab vedotin with/without rituximab in patients with relapsed/refractory b-cell non-hodgkin lymphoma. Clin Cancer Res. 2017;23(5):1167–1176. doi: 10.1158/1078-0432.CCR-16-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Vries JF, Zwaan CM, De Bie M, et al. The novel calicheamicin-conjugated CD22 antibody inotuzumab ozogamicin (CMC-544) effectively kills primary pediatric acute lymphoblastic leukemia cells. Leukemia. 2012;26(2):255–264. doi: 10.1038/leu.2011.206. [DOI] [PubMed] [Google Scholar]
- 64.Kantarjian HM, Stock W, Cassaday RD, et al. Inotuzumab ozogamicin for relapsed/refractory acute lymphoblastic leukemia in the INO-VATE Trial: CD22 pharmacodynamics, efficacy, and safety by baseline CD22. Clin Cancer Res. 2021;27(10):2742–2754. doi: 10.1158/1078-0432.CCR-20-2399. [DOI] [PubMed] [Google Scholar]
- 65.Jabbour E, Advani AS, Stelljes M, et al. Prognostic implications of cytogenetics in adults with acute lymphoblastic leukemia treated with inotuzumab ozogamicin. Am J Hematol. 2019;94(4):408–416. doi: 10.1002/ajh.25394. [DOI] [PubMed] [Google Scholar]
- 66.Biberacher V, Decker T, Oelsner M, et al. The cytotoxicity of anti-CD22 immunotoxin is enhanced by bryostatin 1 in B-cell lymphomas through CD22 upregulation and PKC-βII depletion. Haematologica. 2012;97(5):771–779. doi: 10.3324/haematol.2011.049155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramakrishna S, Highfill SL, Walsh Z, et al. Modulation of target antigen density improves CAR T-cell functionality and persistence. Clin Cancer Res. 2019;25(17):5329–5341. doi: 10.1158/1078-0432.CCR-18-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kantarjian H, Ravandi F, Short NJ, et al. Inotuzumab ozogamicin in combination with low-intensity chemotherapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2018;19(2):240–248. doi: 10.1016/S1470-2045(18)30011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jabbour E, Short NJ, Senapati J, et al. Mini-hyper-CVD plus inotuzumab ozogamicin, with or without blinatumomab, in the subgroup of older patients with newly diagnosed Philadelphia chromosome-negative B-cell acute lymphocytic leukaemia: long-term results of an open-label phase 2 trial. Lancet Haematol. 2023;10(6):e433–e444. doi: 10.1016/S2352-3026(23)00073-X. [DOI] [PubMed] [Google Scholar]
- 70.Kantarjian H, Haddad FG, Jain N, et al. Results of salvage therapy with mini-hyper-CVD and inotuzumab ozogamicin with or without blinatumomab in pre-B acute lymphoblastic leukemia. J Hematol Oncol. 2023;16(1):44. doi: 10.1186/s13045-023-01444-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fayad L, Offner F, Smith MR, et al. Safety and clinical activity of a combination therapy comprising two antibody-based targeting agents for the treatment of non-Hodgkin lymphoma: results of a phase I/II study evaluating the immunoconjugate inotuzumab ozogamicin with rituximab. J Clin Oncol. 2013;31(5):573–583. doi: 10.1200/JCO.2012.42.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ogura M, Hatake K, Ando K, et al. Phase I study of anti-CD22 immunoconjugate inotuzumab ozogamicin plus rituximab in relapsed/refractory B-cell non-Hodgkin lymphoma. Cancer Sci. 2012;103(5):933–938. doi: 10.1111/j.1349-7006.2012.02241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ogura M, Tobinai K, Hatake K, et al. Phase I study of inotuzumab ozogamicin combined with R-CVP for relapsed/refractory CD22+ B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2016;22(19):4807–4816. doi: 10.1158/1078-0432.CCR-15-2488. [DOI] [PubMed] [Google Scholar]
- 74.Larson RC, Maus MV. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat Rev Cancer. 2021;21(3):145–161. doi: 10.1038/s41568-020-00323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Long AH, Haso WM, Orentas RJ. Lessons learned from a highly-active CD22-specific chimeric antigen receptor. Oncoimmunology. 2013;2(4):e23621. doi: 10.4161/onci.23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiao X, Ho M, Zhu Z, et al. Identification and characterization of fully human anti-CD22 monoclonal antibodies. MAbs. 2009;1(3):297–303. doi: 10.4161/mabs.1.3.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Long AH, Haso WM, Shern JF, et al. 4–1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21(6):581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Du X, Beers R, Fitzgerald DJ, et al. Differential cellular internalization of anti-CD19 and -CD22 immunotoxins results in different cytotoxic activity. Can Res. 2008;68(15):6300–6305. doi: 10.1158/0008-5472.CAN-08-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Velasco-Hernandez T, Zanetti SR, Roca-Ho H, et al. Efficient elimination of primary B-ALL cells in vitro and in vivo using a novel 4–1BB-based CAR targeting a membrane-distal CD22 epitope. J Immunother Cancer. 2020;8(2):7. doi: 10.1136/jitc-2020-000896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schirrmann T, Menzel C, Hust M, et al. Oligomeric forms of single chain immunoglobulin (scIgG) MAbs. 2010;2(1):73–76. doi: 10.4161/mabs.2.1.10784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh N, Frey NV, Engels B, et al. Antigen-independent activation enhances the efficacy of 4–1BB-costimulated CD22 CAR T cells. Nat Med. 2021;27(5):842–850. doi: 10.1038/s41591-021-01326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng S, Gillespie E, Naqvi AS, et al. Modulation of CD22 protein expression in childhood leukemia by pervasive splicing aberrations: implications for CD22-directed immunotherapies. Blood Cancer Discov. 2022;3(2):103–115. doi: 10.1158/2643-3230.BCD-21-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang L, Zhang Y, Anderson E, et al. Bryostatin Activates CAR T-cell antigen-non-specific killing (CTAK), and CAR-T NK-Like Killing for Pre-B ALL, while blocking cytolysis of a burkitt lymphoma cell line. Front Immunol. 2022;13:825364. doi: 10.3389/fimmu.2022.825364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang L, Zhang Y, Anderson E, et al. CD22 CAR-T induces both CD19 and CD22 surface down-modulation: defining a mechanism of generalized immune evasion and the effects of epigenetic modifiers. Blood. 2020;136(Supplement 1):22–23. [Google Scholar]
- 85.Zhang W, Yang J, Zhou C, et al. Early response observed in pediatric patients with relapsed/refractory Burkitt lymphoma treated with chimeric antigen receptor T cells. Blood. 2020;135(26):2425–2427. doi: 10.1182/blood.2019002008. [DOI] [PubMed] [Google Scholar]
- 86.Wang N, Hu X, Cao W, et al. Efficacy and safety of CAR19/22 T-cell cocktail therapy in patients with refractory/relapsed B-cell malignancies. Blood. 2020;135(1):17–27. doi: 10.1182/blood.2019000017. [DOI] [PubMed] [Google Scholar]
- 87.Liu S, Deng B, Yin Z, et al. Combination of CD19 and CD22 CAR-T cell therapy in relapsed B-cell acute lymphoblastic leukemia after allogeneic transplantation. Am J Hematol. 2021;96(6):671–679. doi: 10.1002/ajh.26160. [DOI] [PubMed] [Google Scholar]
- 88.Pan J, Zuo S, Deng B, et al. Sequential CD19-22 CAR T therapy induces sustained remission in children with r/r B-ALL. Blood. 2020;135(5):387–391. doi: 10.1182/blood.2019003293. [DOI] [PubMed] [Google Scholar]
- 89.Wang T, Tang Y, Cai J, et al. Coadministration of CD19- and CD22-directed chimeric antigen receptor T-cell therapy in childhood B-cell acute lymphoblastic leukemia: a single-arm, multicenter. Phase II Trial J Clin Oncol. 2023;41(9):1670–1683. doi: 10.1200/JCO.22.01214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Annesley C, Summers C, Pulsipher MA, et al. SCRI-CAR19x22v2 T cell product demonstrates bispecific activity in B-ALL. Blood. 2021;138(Supplement 1):470–570. doi: 10.1182/blood-2021-148881. [DOI] [Google Scholar]
- 91.Gardner R, Annesley C, Finney O, et al. Early Clinical Experience of CD19 x CD22 dual specific CAR T cells for enhanced anti-leukemic targeting of acute lymphoblastic leukemia. Blood. 2018;132(Supplement 1):278–378. doi: 10.1182/blood-2018-99-113126. [DOI] [Google Scholar]
- 92.Yang J, Li J, Zhang X, et al. A feasibility and safety study of CD19 and CD22 chimeric antigen receptors-modified T Cell Cocktail for therapy of B cell acute lymphoblastic leukemia. Blood. 2018;132(Supplement 1):277–377. doi: 10.1182/blood-2018-99-114415. [DOI] [Google Scholar]
- 93.Cordoba S, Onuoha S, Thomas S, et al. CAR T cells with dual targeting of CD19 and CD22 in pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia: a phase 1 trial. Nat Med. 2021;27(10):1797–1805. doi: 10.1038/s41591-021-01497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shalabi H, Qin H, Su A, et al. CD19/22 CAR T cells in children and young adults with B-ALL: phase 1 results and development of a novel bicistronic CAR. Blood. 2022;140(5):451–463. doi: 10.1182/blood.2022015795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu Y, Zhou Y, Zhang M, et al. CRISPR/Cas9-engineered universal CD19/CD22 dual-targeted CAR-T cell therapy for relapsed/refractory b-cell acute lymphoblastic leukemia. Clin Cancer Res. 2021;27(10):2764–2772. doi: 10.1158/1078-0432.CCR-20-3863. [DOI] [PubMed] [Google Scholar]
- 96.Wei G, Zhang Y, Zhao H, et al. CD19/CD22 Dual-Targeted CAR T-cell therapy for relapsed/refractory aggressive B-cell lymphoma: a safety and efficacy study. Cancer Immunol Res. 2021;9(9):1061–1070. doi: 10.1158/2326-6066.CIR-20-0675. [DOI] [PubMed] [Google Scholar]
- 97.Dai H, Wu Z, Jia H, et al. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. J Hematol Oncol. 2020;13(1):30. doi: 10.1186/s13045-020-00856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schultz LM, Muffly LS, Spiegel JY, et al. Phase I trial using CD19/CD22 bispecific CAR T cells in pediatric and adult acute lymphoblastic leukemia (ALL) Blood. 2019;134(1):744–844. doi: 10.1182/blood-2019-129411. [DOI] [Google Scholar]
- 99.Yang J, Jiang P, Zhang X, et al. Successful 24-Hours Manufacture of Anti-CD19/CD22 dual chimeric antigen receptor (CAR) T Cell therapy for B-Cell acute lymphoblastic leukemia (B-ALL) Blood. 2020;136(Supplement 1):2–3. [Google Scholar]
- 100.Tang X, Kang L, Qi W, et al. Tandem CAR T cells targeting CD19 and CD22 is a safe and highly efficacious treatment for relapse/ refractory ALL patients. Blood. 2019;134(1):1338–1438. doi: 10.1182/blood-2019-127890. [DOI] [Google Scholar]
- 101.Yang J, Jiang P, Zhang X, et al. Anti-CD19/CD22 Dual CAR-T therapy for refractory and relapsed B-cell acute lymphoblastic leukemia. Blood. 2019;134(1):284–384. doi: 10.1182/blood-2019-126429. [DOI] [Google Scholar]
- 102.Spiegel JY, Patel S, Muffly L, et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat Med. 2021;27(8):1419–1431. doi: 10.1038/s41591-021-01436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Niu J, Qiu H, Xiang F, et al. CD19/CD22 bispecific CAR-T cells for MRD-positive adult B cell acute lymphoblastic leukemia: a phase I clinical study. Blood Cancer J. 2023;13(1):44. doi: 10.1038/s41408-023-00813-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qin H, Ramakrishna S, Nguyen S, et al. Preclinical development of bivalent chimeric antigen receptors targeting both CD19 and CD22. Mol Ther Oncolytics. 2018;11:127–137. doi: 10.1016/j.omto.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu S, Zhang X, Dai H, et al. Tandem CD19/CD22 Dual Targets CAR-T cells therapy obtains superior CR Rate Than Single CD19 CAR-T cells infusion as well as sequential CD19 and CD22 CAR-T cells infusion for relapsed/refractory b-cell acute lymphoblastic leukemia patients. Blood. 2021;138(Supplement 1):1755–1855. doi: 10.1182/blood-2021-152927. [DOI] [Google Scholar]
- 106.Cui W, Zhang X, Dai H, et al. Tandem CD19/CD22 Dual targets CAR-T cells therapy acquires superior CR Rate Than CD19 CAR-T cells: a case controlled study. Blood. 2020;136(Supplement 1):44–44. doi: 10.1182/blood-2020-143474. [DOI] [Google Scholar]
- 107.Kokalaki E, Ma B, Ferrari M, et al. Dual targeting of CD19 and CD22 against B-ALL using a novel high-sensitivity aCD22 CAR. Mol Ther. 2023;23:7. doi: 10.1016/j.ymthe.2023.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schneider D, Xiong Y, Wu D, et al. Trispecific CD19-CD20-CD22-targeting duoCAR-T cells eliminate antigen-heterogeneous B cell tumors in preclinical models. Sci Transl Med. 2021;13:586. doi: 10.1126/scitranslmed.abc6401. [DOI] [PubMed] [Google Scholar]
- 109.Kansagra AJ, Frey NV, Bar M, et al. Clinical utilization of Chimeric Antigen Receptor T-cells (CAR-T) in B-cell acute lymphoblastic leukemia (ALL)-an expert opinion from the European Society for Blood and Marrow Transplantation (EBMT) and the American Society for Blood and Marrow Transplantation (ASBMT) Bone Marrow Transplant. 2019;54(11):1868–1880. doi: 10.1038/s41409-019-0451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rivero A, Mozas P, Magnano L, et al. Novel targeted drugs for follicular and marginal zone lymphoma: a comprehensive review. Front Oncol. 2023;13:1170394. doi: 10.3389/fonc.2023.1170394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jacobson CA, Chavez JC, Sehgal AR, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23(1):8. doi: 10.1016/S1470-2045(21)00591-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.