Abstract
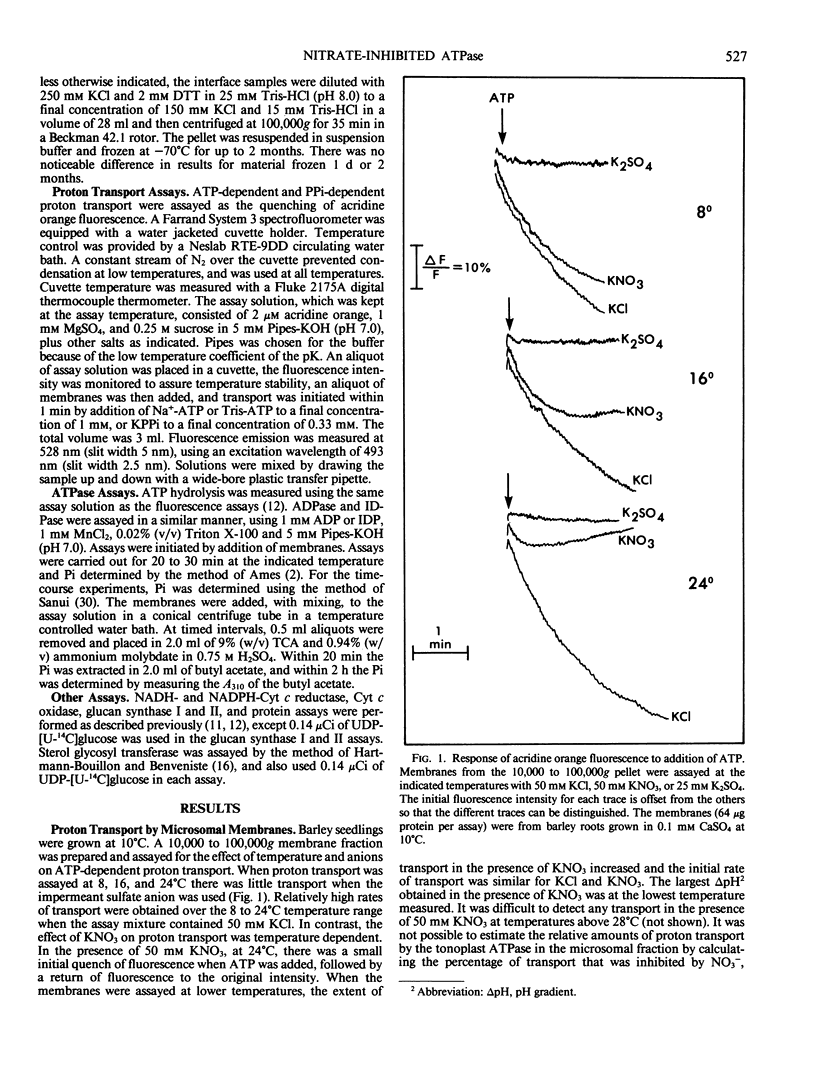
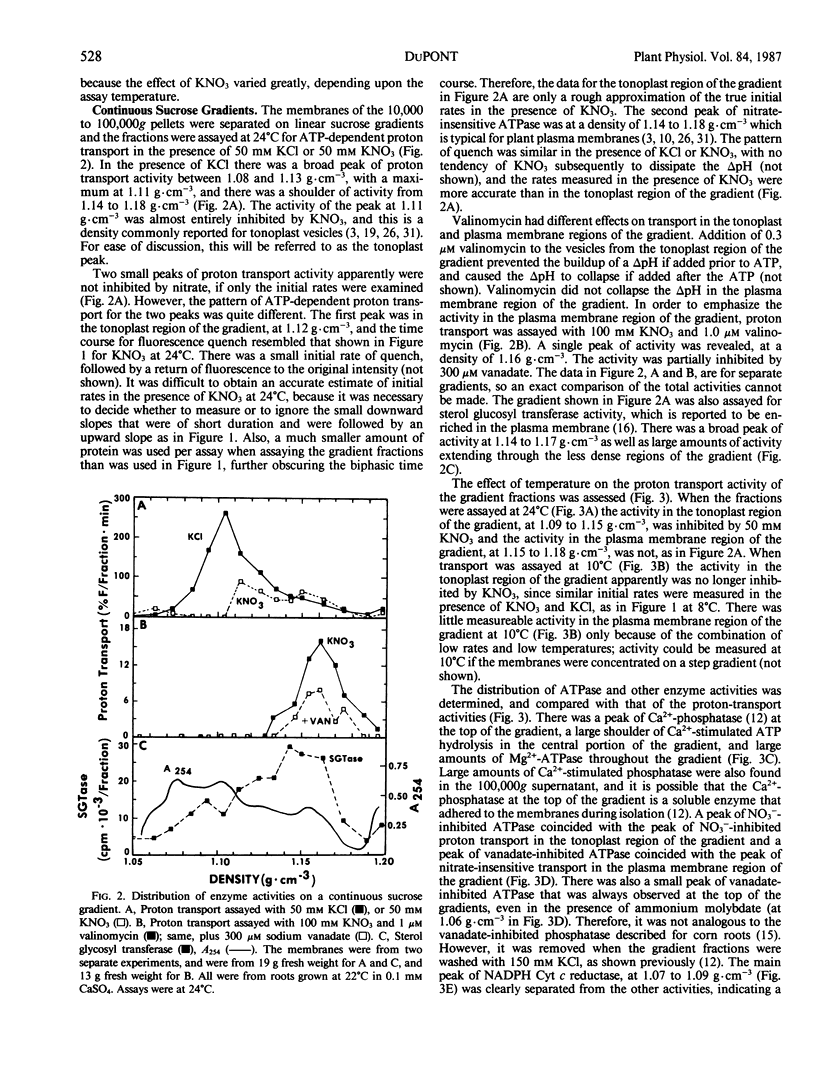
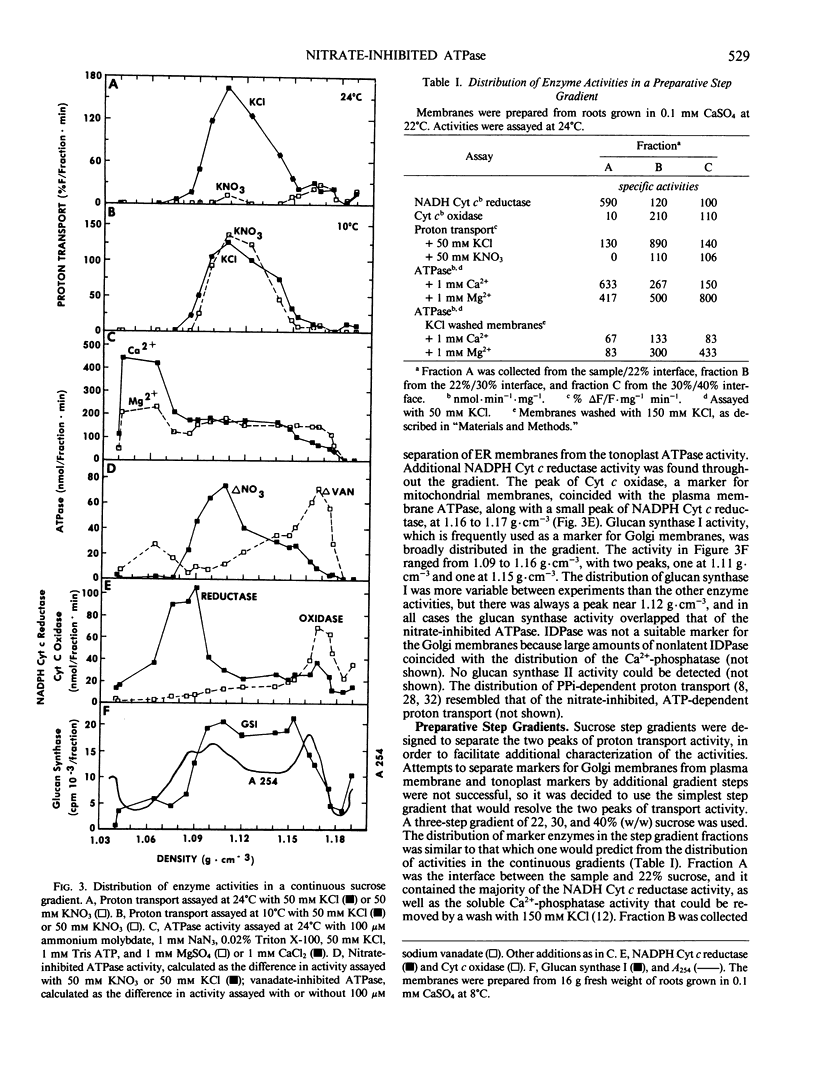
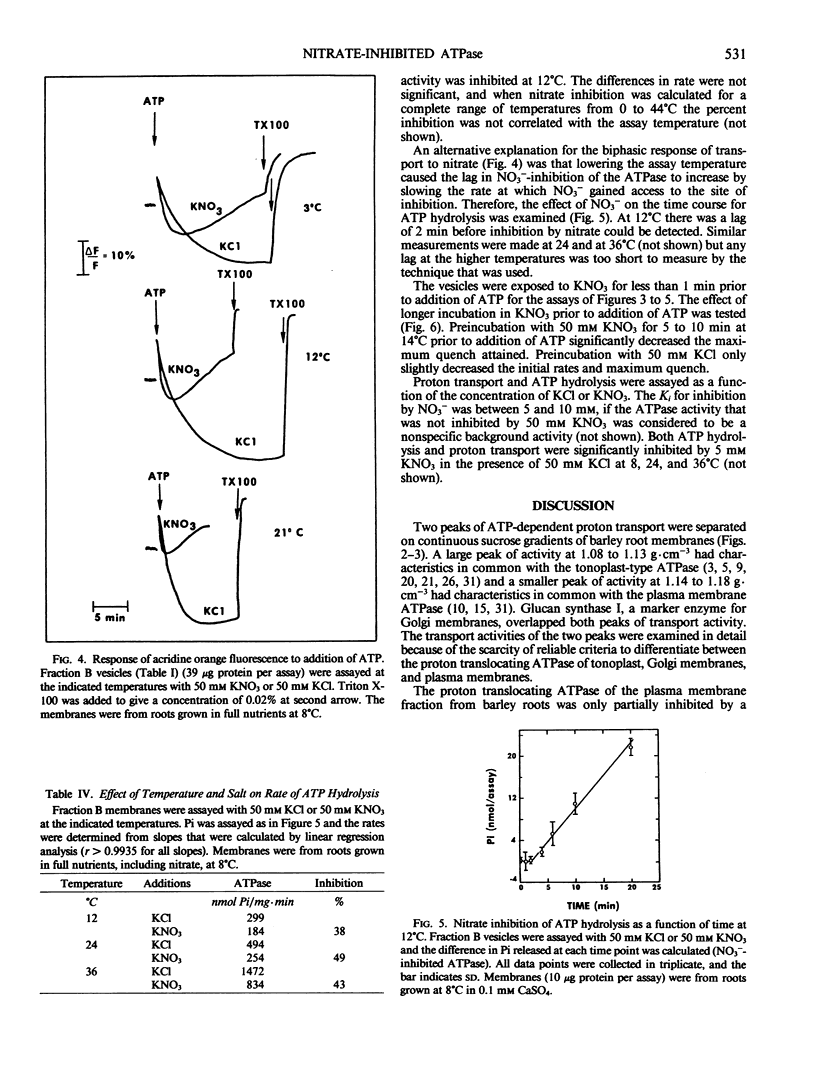
The effects of NO3− and assay temperature on proton translocating ATPases in membranes of barley (Hordeum vulgare L. cv California Mariout 72) roots were examined. The membranes were fractionated on continuous and discontinuous sucrose gradients and proton transport was assayed by monitoring the fluorescence of acridine orange. A peak of H+-ATPase at 1.11 grams per cubic centimeter was inhibited by 50 millimolar KNO3 when assayed at 24°C or above and was tentatively identified as the tonoplast H+-ATPase. A smaller peak of H+-ATPase at 1.16 grams per cubic centimeter, which was not inhibited by KNO3 and was partially inhibited by vanadate, was tentatively identified as the plasma membrane H+-ATPase. A step gradient gave three fractions enriched, respectively, in endoplasmic reticulum, tonoplast ATPase, and plasma membrane ATPase. There was a delay before 50 millimolar KNO3 inhibited ATP hydrolysis by the tonoplast ATPase at 12°C and the initial rate of proton transport was stimulated by 50 millimolar KNO3. The time course for fluorescence quench indicated that addition of ATP in the presence of KNO3 caused a pH gradient to form that subsequently collapsed. This biphasic time course for proton transport in the presence of KNO3 was explained by the temperature-dependent delay of the inhibition by KNO3. The plasma membrane H+-ATPase maintained a pH gradient in the presence of KNO3 for up to 30 minutes at 24°C.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali M. S., Akazawa T. Association of H-Translocating ATPase in the Golgi Membrane System from Suspension-Cultured Cells of Sycamore (Acer pseudoplatanus L.). Plant Physiol. 1986 May;81(1):222–227. doi: 10.1104/pp.81.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A. B., O'neill S. D., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: I. Identification and Characterization of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):538–544. doi: 10.1104/pp.74.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Poole R. J. Nitrate storage and retrieval in Beta vulgaris: Effects of nitrate and chloride on proton gradients in tonoplast vesicles. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3683–3687. doi: 10.1073/pnas.82.11.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. J., Slayman C. W. The effects of vanadate on the plasma membrane ATPase of Neurospora crassa. J Biol Chem. 1979 Apr 25;254(8):2928–2934. [PubMed] [Google Scholar]
- Chanson A., Taiz L. Evidence for an ATP-Dependent Proton Pump on the Golgi of Corn Coleoptiles. Plant Physiol. 1985 Jun;78(2):232–240. doi: 10.1104/pp.78.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill K. A., Sze H. Anion-Sensitive, H-Pumping ATPase of Oat Roots : Direct Effects of Cl, NO(3), and a Disulfonic Stilbene. Plant Physiol. 1984 Oct;76(2):490–497. doi: 10.1104/pp.76.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Michelis M. I., Spanswick R. M. H-pumping driven by the vanadate-sensitive ATPase in membrane vesicles from corn roots. Plant Physiol. 1986 Jun;81(2):542–547. doi: 10.1104/pp.81.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Bennett A. B., Spanswick R. M. Localization of a proton-translocating ATPase on sucrose gradients. Plant Physiol. 1982 Oct;70(4):1115–1119. doi: 10.1104/pp.70.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Hurkman W. J. Separation of the Mg-ATPases from the Ca-Phosphatase Activity of Microsomal Membranes Prepared from Barley Roots. Plant Physiol. 1985 Apr;77(4):857–862. doi: 10.1104/pp.77.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E., Norlyn J. D. Seawater-based crop production: a feasibility study. Science. 1977 Jul 15;197(4300):249–251. doi: 10.1126/science.197.4300.249. [DOI] [PubMed] [Google Scholar]
- Gallagher S. R., Leonard R. T. Effect of vanadate, molybdate, and azide on membrane-associated ATPase and soluble phosphatase activities of corn roots. Plant Physiol. 1982 Nov;70(5):1335–1340. doi: 10.1104/pp.70.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner L. E., Kjellbom P., Larsson C., Møller I. M. Surface properties of right side-out plasma membrane vesicles isolated from barley roots and leaves. Plant Physiol. 1985 Sep;79(1):72–79. doi: 10.1104/pp.79.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew R. R., Spanswick R. M. Characterization of Anion Effects on the Nitrate-Sensitive ATP-Dependent Proton Pumping Activity of Soybean (Glycine max L.) Seedling Root Microsomes. Plant Physiol. 1985 Feb;77(2):352–357. doi: 10.1104/pp.77.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala S., Mettler I. J., Taiz L. Localization of the proton pump of corn coleoptile microsomal membranes by density gradient centrifugation. Plant Physiol. 1982 Dec;70(6):1743–1747. doi: 10.1104/pp.70.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala S., Taiz L. Characterization of the subunit structure of the maize tonoplast ATPase. Immunological and inhibitor binding studies. J Biol Chem. 1986 Sep 25;261(27):12850–12855. [PubMed] [Google Scholar]
- Mandala S., Taiz L. Partial purification of a tonoplast ATPase from corn coleoptiles. Plant Physiol. 1985 Jun;78(2):327–333. doi: 10.1104/pp.78.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala S., Taiz L. Proton transport in isolated vacuoles from corn coleoptiles. Plant Physiol. 1985 May;78(1):104–109. doi: 10.1104/pp.78.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinoia E., Schramm M. J., Kaiser G., Kaiser W. M., Heber U. Transport of anions in isolated barley vacuoles : I. Permeability to anions and evidence for a cl-uptake system. Plant Physiol. 1986 Apr;80(4):895–901. doi: 10.1104/pp.80.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin D. S., Kasamo K., Brooker R. J., Slayman C. W. Electrogenic H+ translocation by the plasma membrane ATPase of Neurospora. Studies on plasma membrane vesicles and reconstituted enzyme. J Biol Chem. 1984 Jun 25;259(12):7884–7892. [PubMed] [Google Scholar]
- Poole R. J., Briskin D. P., Krátký Z., Johnstone R. M. Density gradient localization of plasma membrane and tonoplast from storage tissue of growing and dormant red beet : characterization of proton-transport and ATPase in tonoplast vesicles. Plant Physiol. 1984 Mar;74(3):549–556. doi: 10.1104/pp.74.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall S. K., Wang Y., Sze H. Purification and Characterization of the Soluble F(1)-ATPase of Oat Root Mitochondria. Plant Physiol. 1985 Dec;79(4):957–962. doi: 10.1104/pp.79.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea P. A., Poole R. J. Proton-Translocating Inorganic Pyrophosphatase in Red Beet (Beta vulgaris L.) Tonoplast Vesicles. Plant Physiol. 1985 Jan;77(1):46–52. doi: 10.1104/pp.77.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty T. W., Israel D. W., Volk R. J. Assimilation of NO(3) Taken Up by Plants in the Light and in the Dark. Plant Physiol. 1984 Nov;76(3):769–775. doi: 10.1104/pp.76.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanui H. Measurement of inorganic orthophosphate in biological materials: extraction properties of butyl acetate. Anal Biochem. 1974 Aug;60(2):489–504. doi: 10.1016/0003-2697(74)90259-0. [DOI] [PubMed] [Google Scholar]
- Wang Y., Leigh R. A., Kaestner K. H., Sze H. Electrogenic h-pumping pyrophosphatase in tonoplast vesicles of oat roots. Plant Physiol. 1986 Jun;81(2):497–502. doi: 10.1104/pp.81.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosin J. M., Forte J. G. Kinetic properties of the KCl transport at the secreting apical membrane of the oxyntic cell. J Membr Biol. 1983;71(3):195–207. doi: 10.1007/BF01875461. [DOI] [PubMed] [Google Scholar]


