Abstract
Background
Preconception care (PCC) is the term used for activities and interventions designed to address and prevent problems related to pregnancy, the neonatal period and childhood. This study assessed maternal health status prior to conception in Trinidad by means of a screening tool, physical measurements, and laboratory samples.
Methods
A cross-sectional study was conducted among women aged 18–45 years at a primary care centre in Arima, Trinidad. A de novo PCC screening tool was used to assess 13 domains of high-risk pregnancy in participants. These domains included dietary details, gynaecological and obstetric histories, and genetic and vaccination histories, among others. Blood pressure, weight, height, and waist circumference were recorded, and a capillary blood sample was used to determine random blood glucose and HbA1c levels. All data were coded and entered into SPSS ver. 21.
Results
A total of 400 nongravid participants were recruited, of whom 366 were included in the final analysis. Most (96.7%) had one or more risk factors for adverse pregnancy outcomes. These included overweight (27%), obesity (35%), central obesity (69.4%), and impaired glucose tolerance/diabetes mellitus (IGT/DM) (26.2%). Additionally, a sedentary lifestyle and diet high in processed food/fats were self-reported by 74.9% and 88.8% of participants, respectively. Only 13.1% had planned to conceive, and of those who had no immediate plans to conceive, 76.4% were currently sexually active, and many (60.7%) did not use birth control techniques. More than half (57.1%) had never had a pap smear. On the other hand, 86.3% knew their HIV status. Self-reported percentages for vaccination were as follows: MMR (100%), tetanus (17.5%), hepatitis B (11.5%) and influenza (2.7%). The majority (82.8%) of participants had not visited the dentist in the past year, with 35.9% of these individuals reporting symptoms of periodontitis. Segments of the population had multiple risk factors; for example, 23.7% of participants were overweight or obese and had an elevated HbA1c level.
Conclusions
Unexpectedly, most participants had a risk factor for an adverse pregnancy outcome, and many had multiple risk factors. There is a strong case for enhanced preconception care for women in Trinidad.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12884-023-06017-2.
Keywords: Preconception care, Screening, Maternal health, Foetal health, Trinidad
Background
Preconception care (PCC) is the term used for a wide range of activities and interventions intended to address and prevent specific problems related to pregnancy, the neonatal period and childhood. PCC can be defined as “the provision of biomedical and behavioural interventions prior to pregnancy in order to optimize women’s wellness and subsequent pregnancy outcomes with the aim to improve not only foetal, infant, and maternal health but also the health of the whole family and the future well-being of the offspring” [1]. Internationally, preconception health promotion is at the forefront of improving maternal and child health.
There is emerging evidence that “early prenatal care is too late” [2]. By the time a pregnancy is recognized, or the woman makes it to her first prenatal visit, most foetal structures are already well advanced in their development. Interventions to improve the health of mothers and children at this stage may be too late to have any effect. Many international organizations now recommend routine PCC [2, 3]. The March of Dimes recommends that “the key physician/primary care provider must take advantage of every health encounter to provide preconception care and risk reduction before and between conceptions—the time when it really can make a difference” [2]. The American Academy of Pediatrics (AAP) and the American College of Obstetricians and Gynecologists (ACOG) offer similar recommendations [3].
To support these recommendations, the World Health Organization (WHO) convened a meeting to develop a global consensus [4]. The list of activities included programs for addressing tobacco use prevention and tobacco use cessation, nutrition, vaccination, infertility, HIV testing and counselling, mental health, alcohol and drug use, intimate partner abuse, genetic counselling, and occupational health. The WHO has recommended a more integrated approach to PCC within health systems and moving away from vertical programs [4].
Preconception health care in Trinidad and Tobago
It is a common clinical experience that antenatal health care in Trinidad and Tobago (T&T) does not commence until the pregnancy is well established and, in many cases, deep into the second trimester. Uche-Nwachi and colleagues in 2010 reported that 60.8% of antenatal patients had their initial visit after the first trimester [5]. This late-onset antenatal care may well contribute to the maternal and infant mortality rates in T&T. For example, PAHO reported that the maternal mortality rate (MMR) was 46.9 per 100,000 live births and the infant mortality ratio (IMR) was 12 per 1,000 live births in T&T in the early 2010s [6]. These rates are well above those of developed nations, where the MMR and IMR are estimated at 12 per 100,000 live births and 6 per 1,000 live births, respectively [6, 7].
Additionally, in T&T, many pregnancies are unplanned. In 2009, Ali and her colleagues reported a rate of unplanned pregnancies of 60.4% in North Central Trinidad [8]. These pregnancies are often accompanied by preexisting conditions such as sickle cell anaemia, thalassemia, and alcohol use [9]. Research suggests that drug use, mental health problems and chronic noncommunicable diseases such as obesity, diabetes, and hypertension often go unchecked and may therefore contribute to these statistics [6].
Currently, in T&T, there are existing programs that offer care to women of reproductive age, but they exist in silos, such as prenatal care, family planning clinics or well-infant checks, and do not adopt a PCC perspective. There is no formal service taking an integrated approach to identify and manage risk factors that affect maternal and foetal outcomes. As such, the current preconception health status of women of reproductive age is unknown. This study aimed to determine the prevalence of preconception health risks in a population of women aged 18–45 years in a Trinidadian primary care setting.
Methods
Study design
This was a cross-sectional study.
Setting
Health care in Trinidad and Tobago may be sourced via public or private health care facilities. The public sector, governed by the Ministry of Health, comprises 5 regional health authorities (RHAs) within which there are various hospitals and 9 district health facilities. The public health care system is financed by the government and taxpayers and is available free of charge at the point of service for all citizens on a walk-in basis [10]. The study was conducted at the Arima District Health Facility from December 4th, 2016, to January 14th, 2017.
The Arima Borough is semirural, with a catchment population of 33 606 people according to the 2011 census. Females in the reproductive age group make up 20% of the population of this region. The ethnic composition of the population is distributed among 3 major groups: African, South Asian and mixed ethnicity, with the former two groups being more common. Chinese, European and Middle Eastern populations together comprise a minority [11]. RB was an attending physician at the institution at the time of the study. The centre was chosen because of its convenience and because it is the major medical institution in the district.
Participants
Subjects were included if they were female and between the ages of 18 and 45 years. Pregnant women were not excluded. Exclusion criteria were women who had completed their families and were on contraception or those who were sterilized.
Following registration by a clerical officer, a de novo preconception care screening checklist (See Additional File 1) was administered by RB in English. Consecutive patients attending the walk-in service were invited to participate.
Data sources/Measurements
The patients’ blood pressure, weight, height, and waist circumference were recorded using the WHO STEPS Protocol [12].
Measurements were repeated three times, and the average value was used. A capillary blood sample was obtained for the determination of random blood glucose and HbA1c levels. Samples were analysed via a DCA 2000Ⓡ Analyser, which is very useful for point-of-care testing [13].
Variables: the preconception care screening tool
To ensure face and content validity, the preconception care screening tool was modelled after the recommendations published by the WHO in 2013 [14]. RB created the first version, and RGM and ST reviewed and edited the instrument for ease of use and item flow. The following domains were assessed [15–17]:
Intent of Pregnancy.
Gynaecological History.
Obstetric History.
Medical History.
Genetic History.
Immunization History.
Medication History.
Diet and Exercise.
Lifestyle.
Environmental Health.
Emotional Health.
Dental Health.
Partner’s History.
See Additional File 1 for an in-depth description of these domains and the survey instrument. The screening tool took the format of a checklist to aid in efficient patient screening. If a risk to maternal health was identified, steps to cue further management based upon the current guidelines were included on the form. In this study, impaired glucose tolerance (IGT) or prediabetes was defined by an HbA1c range of 5.7 to 6.5%, with diabetes (DM) being defined at an HbA1c level ≥ 6.5% [18, 19].
The Indo-Caribbean (originally from South Asia) population has a higher percentage of body fat than the Caucasian population of the same age, sex and BMI; therefore, in their expert consultation in 2004, the World Health Organization (WHO) highlighted the need for public health action to be taken in this subgroup at a lower cut-off limit [20]. For Indo-Caribbean people, the BMI categories suggested for public health actions are as follows: less than 18.5 kg/m2 (underweight); 18.5–23 kg/m2 (normal); 23–27.5 kg/m2 (overweight); and 27.5 kg/m2 or higher (obesity).
All data were coded and stored in a password-protected computer accessed solely by RB.
Bias
The main biases in a survey include sampling, nonresponse, question order, recall and desirability biases. In our limitations, we acknowledge that the final sample was not entirely representative of the overall Trinidad and Tobago population. Patients were invited by the administrative staff immediately upon presentation at the clinic. This could affect the willingness of those invited to favour participation in the study. It was stressed in the consenting process, however, that refusal would not affect their subsequent care. No special techniques were taken to reduce recall or desirability biases.
Study size determination
Sample size was determined using a margin of error of 5% and a confidence level of 95%. The following equation for estimating a population proportion with specified absolute precision, which is based on simple random sampling, was used to calculate the sample size:
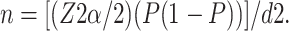 |
where “n” is the calculated sample size, “Z” is the critical value for a 95% confidence level (1.96), “P” is the anticipated population proportion and “d” is the margin of error. When “P” was unknown, the safest choice for the anticipated population proportion of 50% was used, and a sample size of 364 patients was calculated. Applying a 10% nonresponse rate gave a final sample size of 400 patients.
Ethics approval
Approvals for conducting this study were provided by the Institutional Review Boards of The University of the West Indies (UWI), St. Augustine, Trinidad and the NCRHA, Trinidad. An informed consent form was obtained from each patient, which was signed in the presence of a witness. The patient’s autonomy was respected; they were informed that they could discontinue the study at any time without affecting the care they would receive, and their participation was not coerced. Confidentiality was always maintained. The PCC screening tool did not gather any identification data. After the data were entered into statistical software and analysed, all physical records were destroyed.
Statistical methods
SPSS ver. 21.0 was used to construct various frequency tables. Demographic variables were described by standard subgroups, such as male and female, age, reported ethnicity, completed examination, parity and the presence of diabetes mellitus and hypertension, as well as by numbers and proportions. No analytic methods are presented. A sensitivity analysis was conducted to examine the rates of overweight and obesity in the Indo-Trinidadian population if the adjusted BMI cut-off points were applied. Estimates were also calculated for participants with multiple risk factors, such as being overweight and obese and having an elevated HbA1c level.
Results
A total of 400 consecutive subjects were invited to participate. There were no refusals. Thirty-four patients were excluded because they had completed their families or had undergone sterilization.
The majority (205; 56%) of participants were aged between 18 and 29 years, and 6.5% were over 40 years of age. Most (56.0%) self-identified as Afro-Trinidadian, followed by mixed ethnicity (26.8%) and Indo-Trinidadian (17.2%). More than half of the participants were multiparous (57.9%), and none were currently pregnant. Most (83.6%) had attained a secondary or higher level of completed education. Approximately 70% of participants had a positive family history (FH) of diabetes, and 54% had a FH of hypertension (see Table 1).
Table 1.
Demographic characteristics of a female population aged 18–45 years before pregnancy in Trinidad in 2016-17
| Age Group/years | Total | ||||||
| 18–24 | 25–29 | 30–34 | 35–39 | 40–45 | 18–45 | ||
|
N (%) 116 (100) |
N (%) 89 (100) |
N (%) 71 (100) |
N (%) 66 (100) |
N (%) 24 (100) |
N (%) 366 (100) |
||
| Single or Partnered | Single | 50 (43.1) | 34 (38.2) | 27 (38) | 21 (31.8) | 10 (41.7) | 142 (38.7) |
| Partnered | 66 (56.9) | 55 (61.8) | 44 (62) | 45 (61.8) | 14 (58.3) | 224 (60.6) | |
| Marital Status | Partnered | 63 (95.5) | 41 (74.5) | 21 (47.7) | 20 (44.4) | 7 (50.0) | 152 (67.8) |
| Married | 3 (4.5) | 14 (25.5) | 23 (52.3) | 25 (55.6) | 7 (50.0) | 72 (32.2) | |
| Divorced | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Other | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Ethnicity | Afro-Trinidadian | 61 (52.6) | 49 (55.1) | 41 (57.7) | 36 (54.5) | 18 (75.0) | 205 (56.0) |
| Indo-Trinidadian | 20 (17.2) | 19 (21.3) | 10 (14.1) | 12 (18.2) | 2 (8.3) | 63 (17.2) | |
| Mixed | 35 (30.2) | 21 (23.6) | 20 (28.2) | 18 (27.3) | 4 (16.7) | 98 (26.8) | |
| Highest level of completed education | None | 2 (1.7) | 1 (1.1) | 1 (1.4) | 0 (0) | 0 (0) | 4 (1.1) |
| Primary Level | 19 (16.4) | 9 (10.1) | 13 (18.3) | 8 (12.1) | 7 (29.2) | 56 (15.3) | |
| Secondary Level | 67 (57.8) | 55 (61.8) | 46 (64.8) | 51 (77.3) | 14 (58.3) | 233 (63.7) | |
| Tertiary Level | 28 (24.1) | 24 (30.0) | 11 (15.5) | 7 (10.6) | 3 (12.5) | 73 (19.9) | |
| Parity | 0 | 96 (82.8) | 40 (44.9) | 12 (16.9) | 9 (13.6) | 1(4.2) | 158 (43.2) |
| 1–2 | 17 (14.7) | 41 (46.1) | 41 (57.7) | 36 (54.5) | 11 (45.8) | 146 (39.9) | |
| 3+ | 3 (2.6) | 8 (9.0) | 18 (25.4) | 21 (31.8) | 12 (50.0) | 66 (16.9) | |
|
Current Frequency of Sexual Intercourse (times/wk) |
0 | 26 (22.4) | 22 (24.7) | 11 (15.5) | 11 (16.7) | 8 (33.3) | 78 (21.3) |
| < 1 | 49 (42.2) | 29 (32.6) | 27 (38.0) | 24 (36.4) | 9 (27.3) | 138 (37.7) | |
| 2–3 | 30 (25.9) | 33 (37.1) | 27 (38.0) | 25 (37.9) | 5 (20.8) | 120 (32.7) | |
| > 3 | 11 (9.5) | 5 (5.6) | 6 (8.5) | 6 (9.1) | 2 (8.3) | 30 (8.7) | |
| Family History of Diabetes Mellitus | Yes | 72 (62.1) | 61 (68.5) | 51 (71.8) | 49 (74.2) | 22 (91.7) | 255 (69.7) |
| No | 44 (37.9) | 28 (31.5) | 20 (28.2) | 17 (25.8) | 2 (8.3) | 111 (30.3) | |
| Family History of Hypertension | Yes | 50 (43.1) | 49 (55.1) | 40 (56.3) | 43 (65.2) | 16 (75.0) | 198 (54.1) |
| No | 66 (56.9) | 40 (44.9) | 31 (43.7) | 23 (34.8) | 8 (25.0) | 168 (49.5) | |
The clinical characteristics of the sample population are shown in Table 2. Body mass index (BMI) was in the normal range (BMI 18.5–25 kg/m2) in only 38% of patients, while 27% were overweight (BMI 25–30 kg/m2) and 35% were obese (BMI ≥ 30). Of the 366 patients, 69.4% were assessed as having central obesity with a waist circumference ≥ 80 cm. More than one-third (35.5%) exhibited acanthosis nigricans—a marker of insulin resistance. In terms of glycaemic status, 73.8% were normoglycaemic (HbA1c level < 5.7%), 21.6% were prediabetic/had impaired glucose tolerance (HbA1c level = 5.7–6.5%) and 4.6% fulfilled the criteria for diabetes (HbA1c level > 6.5%). While only 6% reported a personal history of hypertension, more than half of the subjects had an elevated blood pressure either in the pre- or hypertensive range [21]. When BMI was calculated for the Indo-Trinidadian population using the recommended cut-off points [20], the combined rate of overweight and obesity increased from 50 to 70%.
Table 2.
Clinical characteristics of a female population aged 18–45 years before pregnancy in Trinidad in 2016-17
| Age Group/years | Total | ||||||
| 18–24 | 25–29 | 30–34 | 35–39 | 40–45 | 18–45 | ||
|
N (%) 116 (100) |
N (%) 89 (100) |
N (%) 71 (100) |
N (%) 66 (100) |
N (%) 24 (100) |
N (%) 366 (100) |
||
|
BMI Overweight |
< 25 | 63 (54.3) | 38 (42.7) | 24 (33.8) | 11 (16.7) | 3 (12.5) | 139 (38) |
| > 25 | 53 (45.7) | 51 (57.3) | 47 (66.2) | 55 (83.3) | 24 87.5) | 227 (62) | |
| Waist Circumference Range | < 80 cm | 57 (49.1) | 26 (29.2) | 16 (22.5) | 11 (16.7) | 2 (8.3) | 112 (100) |
| ≥ 80 cm | 59 (50.9) | 63 (70.8) | 55 (77.5) | 55 (83.3) | 22 (91.7) | 254 (100) | |
| Presence of Acanthosis Nigricans | Yes | 22 (19.0) | 33 (37.1) | 27 (38.0) | 31 (47.0) | 17 (70.8) | 130 (35.5) |
| No | 94 (81.0) | 56 (62.9) | 44 (62.0) | 35 (53.0) | 7 (29.2) | 236 (64.5) | |
| Self-reported Hypertension | No | 109 (94.0) | 78 (87.6) | 59 (83.1) | 46 (69.7) | 17 (70.8) | 344 (94.0) |
| Yes | 7 (6.0) | 11 (12.4) | 12 (16.9) | 20 (30.3) | 7 (29.2) | 22 (6.0) | |
| Estimated BP Classification | Normo-tensive | 70 (60.3) | 44 (49.4) | 31 (43.7) | 14 (21.2) | 7 (29.2) | 116 (45.4) |
| Prehypertension | 39 (33.6) | 34 (38.2) | 28 (39.4) | 32 (48.5) | 10 (41.7) | 144 (39.3) | |
| Stage 1 Hypertension | 6 (5.2) | 10 (11.2) | 10 (14.1) | 15 (22.7) | 6 (25.0) | 46 (12.6) | |
| Stage 2 Hypertension | 1 (0.9) | 1 (1.1) | 2 (2.8) | 5 (7.6) | 1 (4.2) | 10 (2.7) | |
| Glycaemic status | HbA1c < 5.7% | 105 (90.5) | 66 (74.2) | 54 (76.1) | 37 (56.0) | 8 (33.3) | 270 (100) |
|
HbA1c ≥ 5.7–6.5% |
10 (8.6) | 21 (23.6) | 14 (19.7) | 24 (36.4) | 10 (41.7) | 79 (100) | |
| HbA1c ≥ 6.5% | 1 (0.9) | 2 (2.2) | 3 (4.2) | 5 (7.6) | 6 (25.0) | 17 (100) | |
Table 3 shows the lifestyle measures. A total of 8.2% of participants had a history of tobacco use, 25.1% reported exposure to second-hand tobacco smoke, 30.9% consumed alcohol, and 74.9% reported a sedentary lifestyle with no exercise. Current sexual activity was reported by 78.7% of participants. Most (86.9%) had no plans for an immediate pregnancy, yet 60.7% were not utilizing any contraceptive technique.
Table 3.
Lifestyle characteristics of a female population aged 18–45 years before pregnancy in Trinidad in 2016-17
| Age Group | TOTAL | ||||||
| 18–24 | 25–29 | 30–34 | 35–39 | 40–45 | 18–45 | ||
|
N (%) 116 (100) |
N (%) 89 (100) |
N (%) 71 (100) |
N (%) 66 (100) |
N (%) 24 (100) |
N (%) 366 (100) |
||
| Currently exercises | Yes | 30 (25.9) | 23 (25.8) | 22 (31.0) | 13 (19.7) | 4 (16.7) | 92 (25.1) |
| No | 86 (74.1) | 66 (74.2) | 49 (69.0) | 53 (80.3) | 20 (83.3) | 274 (74.9) | |
| Currently smokes cigarettes/tobacco products | Yes | 8 (6.9) | 7 (7.9) | 5 (7.0) | 9 (13.6) | 1 (4.2) | 30 (8.2) |
| No | 108 (93.1) | 82 (92.1) | 66 (93) | 57 (86.4) | 23 (95.8) | 336 (91.8) | |
| Current exposure to secondhand smoke | Yes | 29 (25.0) | 23 (25.8) | 15 (21.1) | 17 (25.8) | 8 (33.3) | 92 (25.1) |
| No | 87 (75.0) | 66 (74.2) | 56 (78.9) | 49 (74.2) | 16 (66.7) | 274 (74.9) | |
| Any current alcohol consumption | Yes | 32 (27.6) | 26 (29.2) | 27 (38.0) | 20 (30.3) | 8 (33.3) | 113 (30.9) |
| No | 84 (72.4) | 63 (70.8) | 44 (62.0) | 46 (69.7) | 16 (66.7) | 253 (69.1) | |
| Planning to get pregnant in 6 months | Yes | 10 (8.6) | 10 (11.2) | 13 (18.3) | 12 (18.2) | 3 (12.5) | 48 (13.1) |
| No | 106 (91.4) | 79 (88.8) | 58 (81.7) | 54 (81.8) | 21 (87.5) | 318 (86.9) | |
| Self-reported High-Fat Diet | Yes | 80 (70.0) | 56 (62.9) | 37 (52.1) | 29 (43.9) | 15 (62.5) | 217 (59.3) |
| No | 36 (30.0) | 33 (37.1) | 34 (47.9) | 37 (56.1) | 9 (37.5) | 149 (40.7) | |
| Self-reported High Processed Food Diet | Yes | 78 (67.2) | 49 (55.1) | 36 (50.7) | 31 (47.0) | 10 (41.7) | 204 (55.7) |
| No | 38 (32.8) | 40 (44.9) | 35 (49.3) | 35 (53.0) | 14 (58.3) | 162 (44.3) | |
| Alcohol History of the Partner | Yes | 33 (51.6) | 26 (46.4) | 20 (47.6) | 23 (51.1) | 5 (35.7) | 107 (48.4) |
| No | 31 (48.4) | 30 (53.6) | 22 (52.4) | 22 (48.9) | 9 (64.3) | 114 (51.2) | |
Table 4 highlights the frequency of contraception, immunization, and cervical cancer screening. Among 130 subjects who were utilizing contraception, a barrier method with male condoms was the most common (52%), followed by oral contraceptive pills (18%), IUDs (12%) and depot preparations (< 1%). More than half (57.1%) had never had a pap smear. Vaccination rates for MMR, tetanus, hepatitis B, varicella zoster and annual influenza vaccines were reported as follows: 100%, 17.5%, 11.5%, 4.6% and 2.7%, respectively. Most (82.8%) participants had not seen a dentist within the last year, and 35.9% of these individuals had symptoms of periodontitis.
Table 4.
Preventive measures practiced among a female population aged 18–45 years before pregnancy in Trinidad in 2016-17
| Age Group | TOTAL | ||||||
| 18–24 | 25–29 | 30–34 | 35–39 | 40–45 | 18–45 | ||
|
N (%) 116 (100) |
N (%) 89 (100) |
N (%) 71 (100) |
N (%) 66 (100) |
N (%) 24 (100) |
N (%) 366 (100) |
||
| Birth Control Usage | Yes | 45 (38.8) | 34 (38.2) | 23 (32.4) | 22 (33.3) | 9 (37.5) | 130 (35.5) |
| No | 71 (61.2) | 55 (61.8) | 48 (61.6) | 44 (66.7) | 15 (62.5) | 236 (64.5) | |
| Type of Birth Control | Oral Contraceptive Pills | 5 (11.1) | 6 (17.6) | 5 (22.7) | 5 (22.7) | 1 (11.1) | 22 (17.3) |
| Depot Preparations | 3 (6.7) | 5 (14.7) | 5 (22.7) | 0 (0.0) | 0 (0.0) | 13 (9.8) | |
| IUDs | 0 (0.0) | 3 (8.8) | 3 (13.6) | 6 (27.3) | 4 (36.4) | 16 (12.0) | |
| Condoms | 32 (71.1) | 15 (44.1) | 7 (31.8) | 10 (45.5) | 3 (33.3) | 67 (50.4) | |
| Other | 5 (11.1) | 5 (14.7) | 2 (9.1) | 1 (4.5) | 1 (11.1) | 14 (10.8) | |
| Pap Smear Done within the Past 3 Years | Yes | 8 (6.9) | 29 (32.6) | 33 (46.5) | 31 (47.0) | 10 (41.7) | 111 (30.3) |
| No | 3 (2.6) | 9 (10.1) | 11 (15.5) | 18 (27.3) | 5 (20.8) | 46 (12.6) | |
| Never | 105 (90.5) | 51 (57.3) | 27 (38.0) | 17 (25.8) | 9 (37.5) | 209 (57.1) | |
| Tested for HIV | Yes | 79 (68.1) | 81 (91.0) | 69 (97.2) | 65 (98.5) | 24 (100.0) | 318 (86.9) |
| No | 37 (31.9) | 8(9.0) | 2 (2.8) | 1 (1.5) | 0 (0.0) | 48 (13.1) | |
| Dental Visit within the Past 6 Months | Yes | 11 (18.6) | 7 (15.2) | 4 (11.8) | 10 (23.8) | 1 (9.1) | 33 (17.2) |
| No | 48 (81.4) | 39 (84.8) | 30 (88.2) | 32 (76.2) | 10 (90.9) | 159 (82.8) | |
| One or More Symptoms of Periodontitis | Yes | 18 (30.5) | 17 (40.0) | 15 (44.1) | 16 (38.1) | 3 (27.3) | 69 (35.9) |
| No | 41 (69.5) | 29 (60.0) | 19 (55.9) | 26 (61.9) | 8 (72.7) | 123 (64.1) | |
| MMR Vaccine | Yes | 116 (100) | 89 (100) | 71 (100) | 66 (100) | 24 (100) | 366 (100) |
| No | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0(0) | 0 (0) | |
| Hepatitis B Vaccine | Yes | 13 (11.2) | 13 (14.6) | 8 (11.3) | 6 (9.1) | 2 (8.3) | 42 (11.5) |
| No | 103 (88.8) | 76 (85.4) | 63 (88.7) | 60 (90.9) | 22 (91.7) | 324 (88.5) | |
| Varicella Zoster Vaccine | Yes | 5 (4.3) | 4 (4.5) | 5 (7.0) | 1 (1.5) | 2 (8.3) | 17 (4.6) |
| No | 111 (95.7) | 85 (95.5) | 66 (93.0) | 65 (98.5) | 22 (91.7) | 349 (95.4) | |
| Tetanus Vaccine | Yes | 21 (18.1) | 16 (20.0) | 16 (22.5) | 7 (10.6) | 4 (16.7) | 64 (17.5) |
| No | 95 (81.9) | 73 (80.0) | 55 (77.5) | 59 (89.4) | 20 (83.3) | 302 (82.5) | |
| Influenza Vaccine | Yes | 2 (1.7) | 2 (2.2) | 2 (2.8) | 3 (4.5) | 1 (4.2) | 10 (2.7) |
| No | 114 (98.3) | 87 (97.8) | 69 (97.2) | 63 (95.5) | 23 (95.8) | 356 (97.3) | |
Table 5 illustrates participant subgroups with multiple risk factors: 23.7% of participants were overweight or obese and had an elevated HbA1c level; 7.4% were overweight or obese and had an elevated HbA1c level and stage 1 or 2 hypertension.
Table 5.
Multiple risk factors among a female population aged 18–45 years before pregnancy in Trinidad in 2016-17
| Multiple risk factors | N (%) |
|---|---|
| Overweight or Obese with an elevated HbA1c level > 5.7% | 87 (23.7) |
| Overweight or Obese with an elevated HbA1c level > 5.7% AND Stage 1 or Stage 2 Hypertension | 27 (7.4) |
| Overweight or Obese with an elevated HbA1c level > 5.7% AND Stage 1 or Stage 2 Hypertension AND Alcohol use | 11 (3.0) |
Discussion
This study provided baseline statistics on preconception factors that can contribute to complications during pregnancy. Unexpectedly, most (96.7%) women aged 18–45 years in this Trinidadian primary care population had at least one risk factor for adverse pregnancy outcomes. Several participants had multiple risk factors, as illustrated in Table 5.
Overweight/obesity
Whether categorized by BMI or waist circumference, our results indicate that obesity is highly prevalent in the demographic under study. Maternal obesity is strongly associated with foetal macrosomia [22]. Furthermore, macrosomia is associated with a multitude of poor foetal outcomes, including musculoskeletal injuries such as shoulder dystocia and brachial plexus injury, respiratory complications including meconium aspiration and perinatal asphyxia, hypoglycaemia and foetal demise [23]. In addition, there is an association between foetal macrosomia and long-term health consequences in childhood as well as adulthood, such as obesity, diabetes, and heart disease [24–26]. A large proportion of the sample admitted to having a poor diet (88%) and not exercising (74.9%).
The recommended cut-off points for BMI in the Indo-Trinidadian population are lower than those for the other ethnic groups [20]. When we applied these cut-off points, the combined rate of overweight and obesity increased from 50 to 70%. This is a noteworthy increase, yet little attention and promotion are paid to these recommendations locally.
Impaired glucose tolerance and diabetes
More than two-thirds of participants indicated a positive family history of diabetes mellitus. Approximately one-third exhibited acanthosis nigricans—a marker of insulin resistance. Based on the participants’ glycaemic status, a total of 26% were prediabetic, had impaired glucose tolerance (HbA1c level 5.7–6.5%) or fulfilled the criteria for diabetes (HbA1c level > 6.5%). In contrast, internationally, the prevalence of gestational diabetes (GDM) varies between 1% and 14% in all pregnant women depending on the genetic characteristics of the population and the population prevalence of type 2 diabetes mellitus, the environment of the population under study and the tools used for screening and diagnosis [27].
GDM is associated with an elevated risk of maternal and perinatal mortality, obstructed labour, spontaneous abortion, congenital abnormalities and macrosomia [28]. There is evidence to show that the increasing prevalence of obesity globally, as discussed above, directly contributes to the increasing prevalence of impaired glucose tolerance and diabetes [29–31].
Substance use and the reproductive population
The detrimental effects of alcohol use, tobacco use and the use of other toxic substances during pregnancy are widely documented [32]. This includes foetal alcohol spectrum disorder (FASD), in which infants are at risk of preterm birth, low birth weight, abnormal facial features, and small head size [33–35].
After preconception counselling, it is possible for women to change their behaviour to reduce adverse outcomes during and after pregnancy, including reducing their alcohol and tobacco use [36]. For example, in one study after PCC counselling, significantly more women started using folic acid before pregnancy and reduced their alcohol use during the first three months of pregnancy [36]. This current study identified high levels of alcohol use and exposure to second-hand tobacco smoke before pregnancy among the female population. Given the low public health information available at the local level and the high levels of both alcohol and tobacco use by household members and the population under study, this finding is not unexpected.
Unplanned, unwanted pregnancies and the reproductive population
In this study, 86.9% of the sample did not plan to become pregnant in the near future; however, only 39.3% used contraception. This translates to a significant proportion of patients at risk for an unplanned and/or unwanted pregnancy. Available evidence shows that unplanned pregnancies can have a negative effect on women’s lives. Many women with unplanned and unwanted pregnancies may decide to have an abortion and are thus exposed to resulting social and medical complications. Additionally, women in this situation who give birth may have an increased risk of obstetric complications [37, 38]. There is therefore a compelling argument to strengthen current health education, skill building, counselling, and advocacy for long-acting methods of contraception.
Notably, short-acting contraception was the contraceptive method of choice in 78% of the sample, with barrier protection being utilized by 50% of those using contraception. Barrier protection plays a significant role in reducing the transmission of sexually transmitted infections and should be encouraged, especially for women who are at high risk. Preconception behavioural interventions significantly reduce reinfection or new STI rates by 35% [39].
Cervical cancer screening and the reproductive population
Cervical cancer is the second most common female cancer in the developing world, accounting for 84% of new cases worldwide (445,000 new cases in 2012) [40]. Despite the importance of having routine pap smears, 52.5% of our sample had never had a pap smear, and 13.9% had not had a recent pap smear. Enhanced PCC could improve health education about cervical screening.
Immunization status and the reproductive population
Less than one-fifth of the participants reported being vaccinated against tetanus in the past ten years, and 2.7% were vaccinated against influenza; however, 100% had received childhood MMR vaccines. Regarding the tetanus vaccination rate, this is most likely a case of underreporting since tetanus vaccination is given as part of the DPT vaccine to all primary school students as a compulsory prerequisite for attendance. It is likely that participants were unaware what the DPT acronym stands for. Vaccination against tetanus and rubella, among others, averts a significant number of neonatal deaths and diseases [41]. The results of this study suggest inadequate vaccine coverage for influenza and hepatitis B, which are recommended internationally for all women of reproductive age [42]. Opportunistic preconception screening would allow patients’ immunization status to be updated in adulthood, prior to pregnancy.
Periodontal disease and the reproductive population
Periodontitis has been linked to preterm labour and adverse pregnancy outcomes [43]. This study shows that more than one-third of the sample had one or more symptoms of periodontitis, and less than one-fifth had a dental visit in the past year. Therefore, there are opportunities to invest in dental health as a PCC intervention.
Limitations
There are several limitations to the study. First, this study was conducted in East Trinidad, where 56% of the sample was of Afro-Trinidadian descent and 16.9% was of Indo-Trinidadian descent. This would affect the generalizability to the wider national population, which consists of individuals of African descent (34.2%) and of Indo-Trinidadian descent (35.2%). Additionally, this study was conducted in a public health centre that provides services to individuals with lower to middle socioeconomic status. Moreover, several of the planned tests could not be completed because of a lack of funding. These included routine blood tests such as CBC, sickle cell, HIV and VDRL tests. Had these tests been done, the burden of risk might have been found to be higher.
Nevertheless, there are several strengths. The sample size was adequate. Additionally, this was the first such study in the English-speaking Caribbean population. Importantly, we were unaware as to how poorly T&T was doing in this area, and this study can help shape local policy.
What’s next?
These results suggest that there are real opportunities to improve the health of potential mothers and their foetuses in T&T through public health messages, physician education and the reorientation of services. A recent narrative review published in the Lancet established that “there is sufficient evidence from human and animal research showing that the periconceptional period is a key window during which poor maternal and paternal physiology, body composition, metabolism, and diet can induce increased risk of chronic disease in offspring—a lifetime legacy and major driver of the health burden in the 21st century” [44]. Others suggest that an integrated rather than a silo approach to preconception health promotion might be the most effective and efficient method [45–49]. Community physicians are strategically positioned to improve pregnancy-related outcomes through prenatal interventions and thus should be the foundation of such care [49]. A 2022 systematic review, while bemoaning the paucity of research, suggests that there can be favourable outcomes regarding social behaviours during pregnancy, disease activity and pregnancy outcomes. The authors conclude that the findings support the routine inclusion of PCC in preparation for pregnancy [50].
Despite overwhelming evidence that PCC has the potential to enhance the health and overall well-being of women, infants, and children globally, insufficient attention is given to the clinical reality of PCC research, service, and delivery, especially in low- and middle-income countries. The transfer of PCC guidelines from broad policy to on-the-ground practice requires a more detailed consideration of the practicalities of PCC implementation, especially within developing countries [48, 51].
Conclusions
This study unexpectedly revealed that as many as 97% of 18–45-year-old women in Trinidadian primary care practices present with factors that increase the chances of adverse pregnancy outcomes. Many women have multiple risk factors. In Small Island Developing States (SIDS) such as Trinidad, where our human resources are an important investment, these data suggest that there is much more that we can do to protect mothers and infants. There are opportunities for further research, the broadening of public health policy and reorientation of the health system.
Electronic supplementary material
Acknowledgements
This paper represents work submitted for the Doctor of Medicine (DM) degree in Family Medicine of the first author (RB) at the University of the West Indies in St. Augustine, Trinidad.
List of abbreviations
- BMI
Body Mass Index
- CBC
Complete Blood Count
- DM
Diabetes Mellitus
- DPT
Diphtheria, Pertussis and Tetanus
- GDM
Gestational Diabetes Mellitus
- HbA1c
Glycosylated Haemoglobin
- HIV
Human Immunodeficiency Virus
- IGT
Impaired Glucose Tolerance
- IMR
Infant Mortality Rate
- MMR
Maternal Mortality Rate
- MMR
Measles, Mumps and Rubella
- NCD
Noncommunicable Disease
- NCRHA
North Central Regional Health Authority
- PAHO
Pan American Health Organization
- PAP
Papanicolaou
- PCC
Preconception Care
- SCD
Sickle Cell Disease
- SD
Standard Deviation
- SPSS
Statistical Package for Social Sciences
- STEPS
STEPwise Approach to NCD Risk Factor Surveillance
- T&T
Trinidad and Tobago
- UWI
The University of the West Indies
- VDRL
Venereal Disease Research Laboratory
- WC
Waist Circumference
- WHO
World Health Organization
Authors’ contributions
RB conceptualized, designed the study and wrote the first draft of the manuscript. RGM and ST were the supervisors of the work; they assisted in designing the study, creating and reviewing the questionnaire, and edited various versions of the manuscript. LKTB assisted in data clean up and analysis and formatting the tables. All authors reviewed the final document and approved the submission.
Funding
This work was supported by the Helen Bhagwansingh Diabetes Education Research and Prevention Institute (DERPi), The University of The West Indies. St Augustine, Trinidad.
Data availability
The de novo questionnaire is provided in Additional File 1. The datasets used during the current study are not available to the public, as additional analysis is ongoing. It is, however, available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Approvals for conducting this study were provided by the Institutional Review Boards of The University of the West Indies, St. Augustine, Trinidad (CEC093/12/15) and the North Central Regional Health Authority, Trinidad. The approved protocols were carried out in strict adherence to the principles enunciated in the Declaration of Helsinki. All questionnaire respondents were informed of the study purpose; they all signed informed consent forms after it was explained to them and agreed to respond to the questionnaire. This was completed voluntarily and anonymously.
Consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Berglund A, Lindmark G. Preconception health and care (PHC)-a strategy for improved maternal and child health. Ups J Med Sci. 2016;121:216–21. doi: 10.1080/03009734.2016.1191564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.March of Dimes Birth Defects Foundation March of dimes updates: is early prenatal care too late? Contemp OB/GYN. 2002;12:54–72. [Google Scholar]
- 3.American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. Gilstrap L, Oh W. Guidelines for perinatal care. Washington, DC: American Academy of Pediatrics and the American College of Obstetricians and Gynecologists; 2002. [Google Scholar]
- 4.WHO. The world health report 2005. Make every mother and child count. 2005. https://reliefweb.int/report/world/world-health-report-2005-make-every-mother-and-child-count?gclid=Cj0KCQjwu-KiBhCsARIsAPztUF3bRYVKC5aAgj1d5GEI8S2Ybh6ODKKwt5FI3-OcPit7CZ1TLBg_vLwaApfpEALw_wcB. Accessed 8 May 2023.
- 5.Uche-Nwachi EO, Odekunle A, Jacinto S, Burnett M, Clapperton M, David Y, et al. Anaemia in pregnancy: associations with parity, abortions and child spacing in primary healthcare clinic attendees in Trinidad and Tobago. Afr Health Sci. 2010;10:66–70. [PMC free article] [PubMed] [Google Scholar]
- 6.PAHO. Country report. Trinidad and Tobago. In: Health in the Americas. https://hia.paho.org/en/countries-22/trinidad-tobago-country-profile Accessed 19 June 2023.
- 7.World Health Statistics 2014. WHO publication. http://apps.who.int/iris/bitstream/10665/112738/1/9789240692671_eng.pdf. Accessed 8 May 2023.
- 8.Ali S, Mohammed S, Mungrue K. The epidemiology of unplanned pregnancies in North-Central Trinidad. Int J Adolesc Med Health. 2009;21:73–7. doi: 10.1515/ijamh.2009.21.1.73. [DOI] [PubMed] [Google Scholar]
- 9.Ministry of Health, Government of the Republic of Trinidad and Tobago. Alcohol consumption: Women. In:Trinidad and Tobago chronic non-communicable disease, risk factor survey [Pan American STEPS] final report. 2012. https://extranet.who.int/ncdccs/Data/TTO_C7_PANAM%20STEPS%20REPORT_2012%20pdf.pdf Accessed 19 June 2023.
- 10.PAHO. Country Profiles. Trinidad and Tobago: Health situation and the health system. In: Health in the Americas.https://www.paho.org/salud-en-las-americas-2017/?p=4311. Accessed 19 June 2023.
- 11.The Government of the Republic of Trinidad and Tobago., Ministry of Planning and Development, Central Statistical Office. Ethnic composition. In: The 2011 population and housing census demographic report. https://cso.gov.tt/stat_publications/2011-population-and-housing-census-demographic-report/. Accessed 8 May 2023.
- 12.PAHO. Section 5: Collecting step 2 data: Physical measurements. In: STEPS manual. https://www.who.int/teams/noncommunicable-diseases/surveillance/systems-tools/steps. Accessed 8 May 2023.
- 13.Szymezak J, Leroy N, Lavalard E, Gillery P. Evaluation of the DCA vantage analyzer for HbA 1c assay. Clin Chem Lab Med. 2008;46:1195–8. doi: 10.1515/CCLM.2008.228. [DOI] [PubMed] [Google Scholar]
- 14.WHO . Meeting to develop a global consensus on preconception care to reduce maternal and childhood mortality and morbidity. Geneva: World Health Organization; 2013. [Google Scholar]
- 15.Johnson K, Posner SF, Biermann J, Cordero JF, Atrash HK, Parker CS, et al. Recommendations to improve preconception health and health care–United States. A report of the CDC/ATSDR preconception care work group and the select panel on preconception care. MMWR Recomm Rep. 2006;55:1–23. [PubMed] [Google Scholar]
- 16.Moos MK, Dunlop AL, Jack BW, Nelson L, Coonrod DV, Long R, et al. Healthier women, healthier reproductive outcomes: recommendations for the routine care of all women of reproductive age. Am J Obstet Gynecol. 2008;199:280–9. doi: 10.1016/j.ajog.2008.08.060. [DOI] [PubMed] [Google Scholar]
- 17.National Collaborating Centre for Women’s and Children’s Health, National Institute for Health and Clinical Excellence . Antenatal care: routine care for the healthy pregnant women. London, England: RCOG Press; 2008. [Google Scholar]
- 18.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 19.International Expert Committee International expert committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–34. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. [PubMed] [Google Scholar]
- 21.U.S. Department of Health and Human Services. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. 2003. https://www.nhlbi.nih.gov/files/docs/guidelines/express.pdf. Accessed 8 May 2023. [DOI] [PubMed]
- 22.Gaudet L, Ferraro ZM, Wen SW, Walker M. Maternal obesity and occurrence of fetal macrosomia: a systematic review and meta-analysis. Biomed Res Int. 2014;2014:640291. doi: 10.1155/2014/640291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boulet SL, Salihu HM, Alexander GR. Mode of delivery and birth outcomes of macrosomic infants. J Obstet Gynaecol. 2004;24:622–9. doi: 10.1080/01443610400007828. [DOI] [PubMed] [Google Scholar]
- 24.Barker DJ. In utero programming of cardiovascular disease. Theriogenology. 2000;53:555–74. doi: 10.1016/s0093-691x(99)00258-7. [DOI] [PubMed] [Google Scholar]
- 25.Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. BMJ. 1990;301:259–62. doi: 10.1136/bmj.301.6746.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113:1126–33. doi: 10.1111/j.1471-0528.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 27.Keshavarz M, Cheung NW, Babaee GR, Moghadam HK, Ajami ME, Shariati M. Gestational diabetes in Iran: incidence, risk factors and pregnancy outcomes. Diabetes Res Clin Pract. 2005;69:279–86. doi: 10.1016/j.diabres.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 28.WHO Expert Consultation Appropriate body-mass index for asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 29.WHO . Prevention of diabetes mellitus. Geneva: World Health Organization; 1994. [Google Scholar]
- 30.Bray GA. Risks of obesity. Endocrinol Metab Clin North Am. 2003;32:787–804. doi: 10.1016/s0889-8529(03)00067-7. [DOI] [PubMed] [Google Scholar]
- 31.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world–A growing challenge. N Engl J Med. 2007;356:213–5. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. Polysubstance use during pregnancy. In: Pregnancy. https://www.cdc.gov/pregnancy/polysubstance-use-in-pregnancy.html. Accessed 9 May 2023.
- 33.Riley EP, Mattson SN, Li TK, Jacobson SW, Coles CD, Kodituwakku PW, et al. Neurobehavioral consequences of prenatal alcohol exposure: an international perspective. Alcohol Clin Exp Res. 2003;27:362–73. doi: 10.1097/01.ALC.0000052703.38558.B2. [DOI] [PubMed] [Google Scholar]
- 34.Health Canada . Joint statement: prevention of fetal alcohol syndrome (FAS), fetal alcohol effects (FAE) in Canada (Rep. No. H39-348/1996E) Ottawa, ON: Health Canada; 1996. [Google Scholar]
- 35.Tolstrup JS, Kjaer SK, Munk C, Madsen LB, Ottesen B, Bergholt T, et al. Does caffeine and alcohol intake before pregnancy predict the occurrence of spontaneous abortion? Hum Reprod. 2003;18:2704–10. doi: 10.1093/humrep/deg480. [DOI] [PubMed] [Google Scholar]
- 36.Elsinga J, De Jong-Potjer LC, Van Pal-De Bruin D, Le Cessie KM, Assendelft S, Buitendijk WJ. The effect of preconception counselling on lifestyle and other behaviour before and during pregnancy. Womens Health Issues. 2008;18:117–25. doi: 10.1016/j.whi.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Mohllajee AP, Curtis KM, Morrow B, Marchbanks PA. Pregnancy intention and its relationship to birth and maternal outcomes. Obstet Gynecol. 2007;109:678–86. doi: 10.1097/01.AOG.0000255666.78427.c5. [DOI] [PubMed] [Google Scholar]
- 38.Gipson JD, Koenig MA, Hindin MJ. The effects of unintended pregnancy on infant, child, and parental health: a review of the literature. Stud Fam Plann. 2008;39:18–38. doi: 10.1111/j.1728-4465.2008.00148.x. [DOI] [PubMed] [Google Scholar]
- 39.Warner L, Stone KM, Macaluso M, Buehler JW, Austin HD. Condom use and risk of gonorrhea and Chlamydia: a systematic review of design and measurement factors assessed in epidemiologic studies. Sex Transm Dis. 2006;33:36–51. doi: 10.1097/01.olq.0000187908.42622.fd. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization. Human papilloma virus and cervical cancer. 2016. http://www.who.int/mediacentre/factsheets/fs380/en/. Accessed 8 May 2023.
- 41.Coonrod DV, Jack BW, Boggess KA, Long R, Conry JA, Cox SN, et al. The clinical content of preconception care: immunizations as part of preconception care. Am J Obstet Gynecol. 2008;199:290–5. doi: 10.1016/j.ajog.2008.08.061. [DOI] [PubMed] [Google Scholar]
- 42.Kim DK, Riley LE, Harriman KH, Hunter P, Bridges CB. Recommended immunization schedule for adults aged 19 years or older, United States, 2017. Ann Intern Med. 2017;166:209–19. doi: 10.7326/M16-2936. [DOI] [PubMed] [Google Scholar]
- 43.Sacco G, Carmagnola D, Abati S, Luglio PF, Ottolenghi L, Villa A, et al. Periodontal disease and preterm birth relationship: a review of the literature. Minerva Stomatol. 2008;57:233–46. [PubMed] [Google Scholar]
- 44.Fleming TP, Watkins AJ, Velazquez MA, Mathers JC, Prentice AM, Stephenson J, et al. Origins of lifetime health around the time of conception: causes and consequences. Lancet. 2018;391:1842–52. doi: 10.1016/S0140-6736(18)30312-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu MC, Halfon N. Racial and ethnic disparities in birth outcomes: a life-course perspective. Matern Child Health J. 2003;7:13–30. doi: 10.1023/a:1022537516969. [DOI] [PubMed] [Google Scholar]
- 46.Lu MC, Tache V, Alexander GR, Kotelchuck M, Halfon N. Preventing low birth weight: is prenatal care the answer? J Matern Fetal Neonatal Med. 2003;13:362–80. doi: 10.1080/jmf.13.6.362.380. [DOI] [PubMed] [Google Scholar]
- 47.Misra DP, Guyer B, Allston A. Integrated perinatal health framework. A multiple determinants model with a life span approach. Am J Prev Med. 2003;25:65–75. doi: 10.1016/s0749-3797(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 48.Moos MK. Preconceptional wellness as a routine objective for women’s health care: an integrative strategy. J Obstet Gynecol Neonatal Nurs. 2003;32:550–6. doi: 10.1177/0884217503255302. [DOI] [PubMed] [Google Scholar]
- 49.Moos MK. Preconceptional health promotion: opportunities abound. Matern Child Health J. 2002;6:71–3. doi: 10.1023/a:1015400322761. [DOI] [PubMed] [Google Scholar]
- 50.Nana M, Stannard MT, Nelson-Piercy C, Williamson C. The impact of preconception counselling on maternal and fetal outcomes in women with chronic medical conditions: a systematic review. Eur J Intern Med. 2023;108:52–9. doi: 10.1016/j.ejim.2022.11.003. [DOI] [PubMed] [Google Scholar]
- 51.Steel A, Lucke J, Reid R, Adams J. A systematic review of women’s and health professional’s attitudes and experience of preconception care service delivery. Fam Pract. 2016;33:588–95. doi: 10.1093/fampra/cmw094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The de novo questionnaire is provided in Additional File 1. The datasets used during the current study are not available to the public, as additional analysis is ongoing. It is, however, available from the corresponding author upon reasonable request.


